Abstract
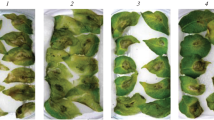
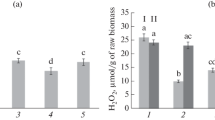
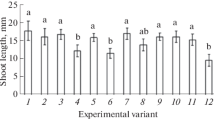
Late blight of potato (Solanum tuberosum L.) caused by the oomycete Phytophthora infestans (Mont.) de Bary was studied in respect to the disease resistance induced by Bacillus subtilis Cohn (strains 26D and 11VM) and B. thuringiensis Berliner (strains B-5351 and B-6066) bacteria. The content of hydrogen peroxide, activities of antioxidant (catalases and peroxidases) and hydrolytic (proteases and amylases) enzymes, together with the gene expression of inhibitors of hydrolases, as the parameters related to the resistance, were assessed. The 15-day-old plants of a cv. Rannyaya Rosa susceptible to late blight were derived from microtubers. The plants were sprayed with bacterial suspension (108 cells/mL) of one of the mentioned strains of B. subtilis or B. thuringiensis. After 5 days, part of the plants was inoculated with P. infestans zoospores (105 spores/mL). After 6, 24, or 48 h postinoculation, the plants were fixed for biochemical analyses. It was found that either B. subtilis or B. thuringiensis reduced the late blight severity on the potato leaves; the effect depended on the particular bacterial strain applied. This was apparently a consequence of H2O2 accumulation and increased expression of the genes encoding protease and amylase inhibitors. The transcriptional activity of the genes of hydrolase inhibitors was stimulated by B. subtilis and B. thuringiensis to different extents. This suggests the existence of different strain-dependent pathways of establishment of bacteria-induced potato resistance to P. infestans.






Similar content being viewed by others
REFERENCES
Novotel’nova, N.S., Pystina, K.A., and Golubeva, O.G., Fitoftorobye griby (Sem. Phytophthoraceae) (Phytophthora Fungi of Family Phytophthoraceae), Leningrad: Nauka, 1974.
Verma, P., Yadav, A.N., Kumar, V., Singh, D.P., and Saxena, A.K., Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement, in Plant-Microbe Interactions in Agro-Ecological Perspectives, Singh, D.P., Singh, H.B., and Prabha, R., Eds., Singapore: Springer-Verlag, 2017, p. 543.
Melent’ev, A.I., Aerobnye sporoobrazuyushchie bakterii Bacillus Cohn v agroekosistemakh (Aerobic Sporulating Bacteria Bacillus Cohn in Agricultural Ecosystems), Moscow: Nauka, 2007.
Yarullina, L.G., Kasimova, R.I., Kuluev, B.R., Surina, O.B., Yarullina, L.M., and Ibragimov, R.I., Comparative study of bunt pathogen resistance to the effects of fungicides in callus co-cultures Triticum aestivum with Tilletia caries, Agric. Sci., 2014, vol. 5, p. 906. https://doi.org/10.4236/as.2014.510098
Veselova, S.V., Nuzhnaya, T.V., and Maksimov, I.V., Role of jasmonic acid in interaction of plants with plant growth promoting rhizobacteria during fungal pathogenesis, in Jasmonic Acid: Biosynthesis, Functions and Role in Plant Development, Morrison, L., Ed., New York: Nova Science, 2015, ch. 3, p. 33.
Cawoy, H., Mariutto, M., Henry, G., Fisher, C., Vasilyeva, N., Thonart, P., Dommes, J., and Ongena, M., Plant defense stimulation by natural isolates of bacillus depends on efficient surfactin production, Mol. Plant Microbe Interact., 2014, vol. 27, p. 87. https://doi.org/10.1094/MPMI-09-13-0262-R
Bindschedler, L.V., Minibayeva, F., Gardner, S.L., Gerrish, C., Davies, D.R., and Bolwell, G.P., Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+, New Phytol., 2001, vol. 151, p. 185. https://doi.org/10.1046/j.1469-8137.2001.00170.x
Yarullina, L.G., Ibragimov, R.I., Tsvetkov, V.O., Yarullina, L.M., and Shpirnaya, I.A., Tsitokhimicheskie i biokhimicheskie metody issledovaniya mikroorganizmov–vozbuditelei boleznei rastenii: uchebnoe posobie (Cytochemical and Biochemical Study Methods of Microorganisms–Plant Pathogens: Manual), Ufa: Bashkir. Gos. Univ., 2016.
Maksimov, I.V., Sorokan’, A.V., Burkhanova, G.F., and Abizgildina, P.P., Regulation of peroxidase activity under the influence of signaling molecules and Bacillus subtilis 26D in potato plants infected with Phytophthora infestans, Appl. Biochem. Microbiol., 2014, vol. 50, p. 173. https://doi.org/10.1134/S0003683814020136
Tsvetkov, V.O., Shpirnaya, I.A., Maksutova V.O., and Ibragimov, R.I., Determination of amylase and protease activity using substrates immobilized in polyacrylamide gel, Izv. Ufimsk. Nauchn. Tsentra, Ross. Akad. Nauk, 2018, no. 3-5, p. 81.
Yarullina, L.G., Akhatova, A.R., and Kasimova, R.I., Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant–pathogen interactions, Russ. J. Plant Physiol., 2016, vol. 63, p. 193. https://doi.org/10.1134/S1021443716020151
Yarullina, L.G., Sorokan, A.V., Burkhanova, G.F., and Tsvetkov, V.O., Signal regulation of activity of protective proteins in potato plants in vitro with the defeat potato late blight, Theor. Appl. Ecol., 2019, vol. 4, p. 136. https://doi.org/10.25750/1995-4301-2019-4-136-141
Berg, G., Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture, Appl. Microbiol. Biotehnol., 2009, vol. 84, p. 11. https://doi.org/10.1007/s00253-009-2092-7
Chanda, B., Xia, Y., Mandal, M.K., Sekine, K.T., Gao, Q.M., and Selote, D., Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants, Nat. Genet., 2011, vol. 43, p. 421. https://doi.org/10.1038/ng.798
White, J.F. and Torres, M.S., Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol. Plant, 2010, vol. 138, p. 440. https://doi.org/10.1111/j.1399-3054.2009.01332.x
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, p. 405. https://doi.org/10.1016/S1360-1385(02)02312-9
Bakalova, S., Nikolova, A., and Wedera, D., Isoenzyme profiles of peroxidase catalase and superoxide dismutase as affected by dehydration stress and ABA during germination of wheat seeds, J. Plant Physiol., 2004, vol. 30, p. 64.
Kolupaev, Yu.E. and Karpets, Yu.V., Formirovanie adaptivnykh reaktsii rastenii na deistvie abioticheskikh stressorov (Development of Adaptive Plant Reactions on the Effect of Abiotic Stress), Kyiv: Osnova, 2010.
Guan, L.M. and Scandalios, J.G., Hydrogen-peroxide-mediated catalase gene expression in response to wounding, Free Radical Biol. Med., 2000, vol. 28, p. 1182. https://doi.org/10.1016/S0891-5849(00)00212-4
Minibayeva, F., Kolesnikov, O., Chasov, A., Beckett, R.P., Lüthje, S., Vylegzhanina, N., Buck, F., and Bottger, M., Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species, Plant, Cell Environ., 2009, vol. 32, p. 497. https://doi.org/10.1111/j.1365-3040.2009.01944.x
Maksimov, I.V., Sorokan’, A.V., Cherepanova, E.A., Surina, O.B., Troshina, N.B., and Yarullina, L.G., Effects of salicylic and jasmonic acids on the components of pro/antioxidant system in potato plants infected with late blight, Russ. J. Plant Physiol., 2011, vol. 58, p. 299. https://doi.org/10.1134/S1021443711010109
Yarullina, L.G., Kasimova, R.I., Akhatova, A.R., Maksimov, I.V., Ibragimov, R.I., and Umarov, I.A., Qualitative and quantitative changes of potato tuber proteome under the influence of signal molecules and infection with Phytophthora infestans, Appl. Biochem. Microbiol., 2016, vol. 52, p. 71. https://doi.org/10.1134/S0003683816010154
Lastochkina, O.V., Aliniaeifard, S., Seifikalhor, M., Yuldashev, R., Pusenkova, L., and Garipova, S., Plant growth-promoting bacteria: biotic strategy to cope with abiotic stresses in wheat, in Wheat Production in Changing Environments. Responses, Adaptation and Tolerance, Hasanuzzaman, M., Nahar, K., and Hossain, A., Eds., Singapore: Springer-Verlag, 2019, p. 579. https://doi.org/10.1007/978-981-13-6883-7_23
Gappa-Adachi, R., Yano1, K., Takeuchi, S., Morita, Y., and Uematsu, S., Phytophthora blight of southern star (Oxypetalum caeruleum) caused by Phytophthora palmivora in Japan, J. Gen Plant Pathol., 2012, vol. 78, p. 39. https://doi.org/10.1007/s10327-011-0351-9
Feng, T., Nyffenegger, C., Højrup, P., Vidal-Melgosa, S., Yan, K., Fangel, J.U., Meyer, A.S., and Kirpekar, F., Characterization of an extension-modifying metalloprotease: N-terminal processing and substrate cleavage pattern of Pectobacterium carotovorum, Appl. Microbiol. Biotechnol., 2014, vol. 98. P. 10077. https://doi.org/10.1007/s00253-014-5877-2
Gancheva, M.S., Malovichko, Y.V., Poliushkevich, L.O., Dodueva, I.E., and Lutova, L.A., Plant peptide hormones, Russ. J. Plant Physiol., 2019, vol. 66, p. 171. https://doi.org/10.1134/S1021443719010072
Vasyukova, N.I. and Ozeretskovskaya, O.L., Jasmonate-dependent defense signaling in plant tissues, Russ. J. Plant Physiol., 2009, vol. 56, p. 581. https://doi.org/10.1134/S102144370905001X
Schoonbeek, H.-J., Jacquat-Bovet, A.C., Mascher, F., and Métraux, J.P., Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea, Mol. Plant Microbe Interact., 2007, vol. 20, p. 1535. https://doi.org/10.1094/MPMI-20-12-1535
Yang, J.W., Yu, S.H., and Ryu, C.-M., Priming of defense related genes confers root-colonizing Bacilli-elicited induced systemic resistance in pepper, Plant Pathol. J., 2009, vol. 25, p. 389. https://doi.org/10.5423/PPJ.2009.25.4.389
Valenzuela-Soto, J.H., Estrada-Hernández, M.G., Laclette, E.I., and Délano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development, Planta, 2010, vol. 231, p. 397. https://doi.org/10.1007/s00425-009-1061-9
ACKNOWLEDGMENTS
The equipment of Biomika Center of Collective Use (Department of Biochemical Research and Nanobiotechnology of the Agidel Regional Center of Collective Use) and that of the CODINK Unique Scientific Installation was used.
Funding
The work was partially carried out through State Task no. AAA-A21-121011990120-7 with support of the Russian Foundation for Basic Research and the Belarusian Republican Foundation for Fundamental Research (project no. 20-516-00005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by A. Aver’yanov
Abbreviations: CAT—catalase; ISR—induced systemic resistance; PB—phosphate buffer; PGPB—plant growth-promoting bacteria; PI—proteinase inhibitors; PO—peroxidase; PR-proteins—pathogenesis-related proteins; SAR—systemic acquired resistance.
Supplementary Information
Rights and permissions
About this article
Cite this article
Yarullina, L.G., Tsvetkov, V.O., Burkhanova, G.F. et al. Effect of Bacillus Bacteria on Hydrogen Peroxide Content and Gene Expression of Hydrolase Inhibitors in Potato Plants Infected with Phytophthora infestans (Mont.) de Bary. Russ J Plant Physiol 68, 1257–1264 (2021). https://doi.org/10.1134/S1021443721060194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721060194