Abstract
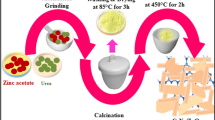
In this work, the Fe-doped mixed crystal TiO2 photocatalyst which can utilize visible light was prepared by sol-gel and heat-treated methods. During heat-treatment, the phase transformation of Fe-doped TiO2 powder occurs and the process is characterized by XRD and TG-DTA technologies. Otherwise, the sizes and shapes of Fe-doped and undoped TiO2 powders were also compared using TEM images. The azo fuchsine in aqueous solutions, as a model compound, was treated under visible light irradiation using Fe-doped mixed crystal TiO2 powders as photocatalyst. The results showed that, under visible light irradiation, the (0.25%) Fe-doped mixed crystal TiO2 powder heat-treated at 600°C for 3.0 h behaved very high photocatalytic activities for degradation of azo fuchsine. The remarkable improvement of the photocatalytic activity of TiO2 powder was elucidated through the cooperative effects of iron doping and phase transformation. The iron doping can restrain the recombination of photogenerated electron-hole pairs and the phase transformation can enhance the absorption of visible light. Furthermore, other influence factors such as azo fuchsine concentration, solution acidity, Fe3+ ion content and irradiation time were also studied. Thus, this method is applicable for the treatment of wastewater.
Similar content being viewed by others
References
A. B. Prevot, C. Baiocchi, M. C. Brussino, et al., Environ. Sci. Technol. 35, 971 (2001).
P. A. Pekakis, N. P. Xekoukoulotakis, and D. Mantzavinos, Water Res. 40, 1276 (2006).
Y. Bessekhouad, N. Chaoui, M. Trzpit, et al., J. Photochem. Photobiol. A: Chem. 183, 218 (2006).
Y. Liu, X. Chen, J. Li, and C. Burda, Chemophere 61, 11 (2005).
S. Al-Qaradawi and S. R. Salman, J. Photochem. Photobiol. A: Chem. 148, 161 (2002).
K. Tetsuro, O. Toshiaki, I. Mitsunobu, et al., J. Colloid. Interf. Sci. 267, 377 (2003).
H. E. Chao, Y. U. Yun, and X. F. Hu, J. Eur. Ceram. Soc. 23, 1457 (2003).
L. Q. Jing, X. J. Sun, B. F. Xin, et al., J. Solid. State. Chem. 177, 3375 (2004).
J. C. Yu, L. Wu, J. Lin, et al., Chem. Commun. 13, 1552 (2003).
T. Y. Timothy, M. Z. Tan, B. Donia, and A. Rose, J. Nanopar. Res. 4, 541 (2002).
U. Diebold, Surf. Sci. Rep. 48, 53 (2003).
K. Nagaoka, K. Takanabe, and K. Aika, Chem. Commun. 9, 1006 (2002).
Z. Ambrus, N. Balázs, T. Alapi, et al., Appl. Catal. B: Environ. 81, 27 (2008).
W. C. Hung, S. H. Fu, J. J. Tseng, et al., Chemosphere 66, 2142 (2007).
S. H. Nahar, K. Hasegawa, and S. Kaga, Chemosphere 65, 1976 (2006).
M. H. Zhou, J. G. Yu, and B. Cheng, J. Hazard. Mater. 137, 1838 (2006).
D. H. Kima, H. S. Honga, S. J. Kimb, et al., J. Alloy Compd. 375, 259 (2004).
M. H. Zhou, J. G. Yu, B. Cheng, and H. G. Yu, Mater. Chem. Phys. 93, 159 (2005).
C. Y. Wang, C. Bottcher, D. W. Bahnemann, and J. K. Dohrmann, J. Mater. Chem. 13, 2322 (2003).
L. Hinda, P. Eric, H. Ammar, K. Mohamed, et al., Appl. Catal. B: Environ. 39, 75 (2002).
R. A. Spurr and H. Myers, Anal. Chem. 29, 760 (1957).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, J., Li, R., Zhang, L. et al. Sol-gel preparation of Fe-doped mixed crystal TiO2 powder and its photocatalytic activity for degradation of azo fuchsine under visible light irradiation. Russ. J. Inorg. Chem. 55, 692–699 (2010). https://doi.org/10.1134/S0036023610050074
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023610050074