Abstract
The review addresses modern methods of electrical stimulation used to regulate the function of external respiration in humans. The methods include abdominal functional stimulation of respiratory muscles, diaphragmatic stimulation, phrenic nerve stimulation, epidural and transcutaneous spinal cord stimulation. The physiological rationale of their application is described along with the examples of their use in clinical practice, including stimulation parameters and electrode placement diagrams for each of the methods. We analyze the effectiveness of each of the methods in patients with respiratory muscle paresis and the features of their use depending on the level of spinal cord injury. Special attention is paid to the method of epidural spinal cord stimulation because this technique is widely used in electrophysiological studies on animal models, providing deeper insight into the spinal levels of the functional control of external respiration. The review substantiates the great potential of using the method of transcutaneous electrical spinal cord stimulation both in fundamental studies of external respiration and in clinical practice.
Similar content being viewed by others
INTRODUCTION
This review was conceived as an analysis of the ways to regulate human external respiration by the methods of electrical stimulation. Currently, electrical stimulation of breathing is implemented via both invasive and noninvasive methods [1]. The most studied of them include invasive phrenic nerve stimulation (PNS) and epidural spinal cord stimulation (SCS). Respiratory activation can be carried out by the methods of abdominal functional electrical stimulation (AFES) and transcutaneous electrical diaphragmatic stimulation (TEDS). Each of these methods has its own limitations, advantages and disadvantages. The method of transcutaneous electrical spinal cord stimulation (TESCS), developed in the last decade, is a perspective technique for the regulation of respiratory function, because it is noninvasive and allows activating several spinal centers of respiratory regulation, which is critical for the full-scale control of breathing.
The goal of the review was to critically analyze the frequency and advisability of using each of the methods of electrical stimulation of breathing in clinical practice, based on the works published within the last decade. Special attention was given to the methods of electrical SCS, including a new method of TESCS, as it can be equally successfully used both in basic research of the function of external respiration and in clinical practice.
The review was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) regulations [2]. The central question addressed in the review was “what does each of the electrical stimulation methods affect the respiratory system”. A systematic literature search in Russian and English was conducted among the publications that came out from May 2012 to September 2022 in the following electronic databases: Pubmed, Google Scholar, Scopus, Web of Science, RSCI. The review included studies carried out on animal models, healthy volunteers and patients with respiratory muscle paresis; also analyzed were retrospective clinical studies and literature reviews.
To select articles relevant to the goal of the review, we used “electrical stimulation” and “electrostimulation” as main keywords, as well as “respiratory system”, “breathing”, “diaphragm”, “phrenic nerve”, “inspiratory muscles”, “expiratory muscles”, “transcutaneous stimulation” as complementary terms. The inclusion criteria for clinical articles were the presentation of randomized and/or multicenter studies. To compare the effectiveness of the methods of electrical stimulation, exposure (treatment course) duration, as well as the factor of aftereffect duration, articles were selected by the presence of a control group. Retrospective studies, conducted to assess the quality of life of patients with implanted phrenic nerve stimulation devices and patients who underwent the course of epidural SCS, were analyzed separately. The method of epidural SCS received much attention in this review, as it is widely used both in clinical tasks on respiratory system regulation in respiratory muscle paresis and in electrophysiological studies of the topical localization of respiratory interneurons. Articles on the topical effects of epidural stimulation in animal models were only included in the review when the authors declared the observation of ethical standards in working with laboratory animals.
The divisions of the review present the results obtained in stimulation of different structures providing the function of external respiration: respiratory muscles, nerves innervating these muscles, and spinal respiratory centers.
ELECTRICAL STIMULATION OF RESPIRATORY MUSCLES
Abdominal functional electrical stimulation
Electrical stimulation of muscles has long been successfully used as a training and restorative method to improve the functional state of skeletal muscles. Abdominal functional electrical stimulation (AFES) of muscles is applied in respiratory system disorders of different etiology and pathogenesis, specifically, after spinal cord injury (SCI), tetraplegia, paraparesis. Transcutaneous AFES is able to elicit contractions of abdominal muscles even if they are paralyzed after SCI [3]. The authors showed that in patients with acute post-SCI tetraplegia, a course of AFES (20–40 min a day, 5 times a week for 4 weeks, 30 Hz, 30–105 mA, pulse duration 100–500 µs) reduced the duration of artificial lung ventilation (ALV) by 11 days compared to the control group whose patients received no AFES.
When using AFES, there has long been no standard protocol for placing stimulation electrodes. One or more anode/cathode pairs were typically placed within the limits of a single or several adjacent abdominal muscles (Fig. 1a). In a study on healthy volunteers, there have been analyzed the effects achieved with different electrode positions on the abdominal wall. It has been demonstrated that the maximum activation of expiratory muscles was achieved when the anode/cathode pair was applied to the posteriolateral position [4] (Fig. 1b). The electrodes were sized 4 × 18 or 4 × 14 cm; with their diagonal placing, multiple abdominal muscles were activated simultaneously.
Exactly this placement layout of stimulating electrodes is used in modern AFES studies. For example, Butler et al. [5] found that superficial stimulation of the posteriolateral abdominal muscles in patients with SCI at the spinal cord segmental level T6 or upward leads to an increase in peak and mean expiratory flows by 36 and 80% respectively, increases tidal volumes by 41%, and reduces the risk of respiratory complications.
Analysis of clinical studies conducted in 2019 showed that in patients with SCI-induced respiratory muscle paresis, undergoing a course of AFES for 6 weeks or more, independent breathing is facilitated, respiratory airway clearance is normalized, lung secretory function improves [6].
Thus, the main purpose, for which AFES can be used in a clinic, is to reduce the risk of respiratory complications caused by poor mucus detachment in the respiratory airways of patients with respiratory muscle paresis.
Transcutaneous electrical diaphragmatic stimulation
Transcutaneous electrical diaphragmatic stimulation (TEDS) is aimed at activating and training of intact muscle fibers that elicit diaphragmatic contractions. TEDS prevents muscular hypotrophy, including in patients with neuromuscular diseases and those dependent on artificial lung ventilation [7, 8].
In a retrospective study, there were analyzed the 2007–2016 medical records of intensive care patients with cervical SCI [7]. Out of 13 tracheostomized patients, 4 received a 7-week course of TEDS (30 Hz, 60 mA, pulse duration 1 ms), 6 were run along a standard ALV protocol, while the remaining patients were excluded from the analysis due to data incompleteness or death. TEDS electrodes were placed bilaterally along the midaxillary line between the 6–7th and 7–8th ribs and above the xiphoid process. A course of TEDS led to an increase in inspiratory muscle strength, reduction in the number of days spent in intensive care, and decrease in the need for ALV. No need for ALV was a key indication for TEDS completion; the whole course of treatment included an average of 47 sessions. All 4 patients were weaned from the ventilator, with the latter circumstance being most important during respiratory resuscitation.
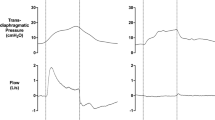
TEDS was also used in intensive care COVID-19 patients on ALV (bipolar waves with a 30 Hz stimulation frequency, 400 µs pulse duration, and 0.7 s rise time). TEDS intensity was gradually increased until visible muscle contraction was observed. TEDS duration and frequency were 30 min a day and 5 days a week, respectively. During each session, rectangular electrodes were placed in the parasternal region near the xiphoid process, in the area of the 6th and 7th intercostals, at the midaxillary line. In TEDS-treated ALV patients, at the end of the study, minute volume increased by 0.64 (0.67–2.3) L, whereas in the group of patients with ALV alone, it decreased by 1.2 (2.5–0.78) L. The differences were statistically significant (p = 0.01) [8].
As Postma et al. [9] believe, the TEDS protocols are not standardized, no recommendations have been formulated for the treatment of patients in a critical condition. Moreover, the sample size of patients in the studies of TEDS effects is rather small (n = 4–12), while the persistence of these effects in retrospective studies has not been proved [9].
ELECTRICAL PHRENIC NERVE STIMULATION
Electrical stimulation of breathing in ALV-dependent patients with traumas and other cervical SCI has a long history that goes back more than two centuries. Since ALV is accompanied by a non-physiological increase in transpulmonary pressure and as non-physiological change in pleural pressure, lung ventilatory capacity decreases during prolonged ventilation, so different techniques are applied to stimulate nerves and muscles involved in the act of breathing [10].
Invasive phrenic nerve stimulation (PNS) in the cervical and thoracic regions entails the activation of diaphragmatic contractions. A direct PNS is well known to elicit synchronous axonal activation in the electric field, followed by a rhythmic respiratory pattern generation [11]. This method is considered a safe and efficient way to provide respiratory support to ALV-dependent patients with cervical SCI [12, 13]. Using endoscopy, implantable electrodes were inserted at the level of the 2nd, 3rd or 4th intercostals (Fig. 2), attached to the phrenic nerves toward the upper mediastinum, and connected to an external stimulator [14]. This approach can be used at an early stage of treatment to reduce the need for ALV in intensive care units.
In a retrospective study conducted in 2011, it was shown that after 37 patients with a SCI at the cervical level were implanted with diaphragmatic nerve stimulators, in 98% of the patients, tidal volume became 15% larger during stimulation compared to that before stimulation [16].
Recently, numerous preclinical and clinical studies aimed at restoring voluntary breathing in patients with a SCI above the phrenic nuclei have been analyzed and concluded that complete respiratory recovery through PNS is possible [17].
In a 2018 study, it has been shown that 5 of 92 patients with SCI, who received PNS, had their respiratory function fully recovered, with 2 of them being able to have their stimulators removed [18]. In the subgroup of individuals, who received PNS in the first year after SCI, 73% of patients were successfully weaned from ALV the throughout whole day (24 h), while in the subgroup of patients, who were stimulated 2 years after SCI, this result was achieved in 51% of cases. The authors concluded that early use of PNS leads to favorable outcomes and improves the quality of life in people with SCI-induced respiratory dysfunction.
The limitations of the method include the following. PNS is suitable for patients with SCI at the level above C4 because in this case, phrenic nerve integrity at levels C3 to C5 is remains intact [19]. In patients with SCI-induced tetraplegia, implantation of a diaphragmatic stimulator to restore respiratory function is impossible due to insufficient functional activity of the phrenic nerves [20]. It is also important to consider the fact that PNS initiates inspiration but not expiration.
Phrenic nerve stimulation (from [15]).
ELECTRICAL SPINAL CORD STIMULATION
There is great interest in the results of studies of the effect of electrical spinal cord stimulation (SCS) on physiological functions (cardiorespiratory, motor, excretory) and the possibility to actively manipulate these results by changing the site and parameters of stimulation. By now, there is a great experience of using SCS in the treatment of chronic incurable pain of various etiology [21, 22]. It is shown on thousands of patients all over the world that the method of high-frequency stimulation (> 10 kHz) is effective for the treatment of the neck and upper limb pain syndrome [23, 24], while low-frequency SCS (< 100 Hz) can be used in lower limb pain [25].
In the last 15 years, it has been demonstrated that a combination of both invasive and noninvasive electrical SCS with motor stimulation is effective in restoring voluntary movements and independent walking in patients paralyzed due to SCI [26–29]. The use of SCS for motor rehabilitation is based on the proven effect of activation of the spinal locomotor neural network and motor pools during stimulation of the lumbar thickening of the spinal cord [30–32]. The noninvasive transcutaneous electrical spinal cord stimulation (TESCS) method has been increasingly used in recent years to control locomotion [33].
One of the advantages of TESCS compared to epidural SCS is the possibility to exert a multisegmental effect on the spinal cord [34]. Recently, a noninvasive spinal neuroprosthetic device has been developed that activates leg flexors and extensors depending on step phases; it has been designed to facilitate walking in stroke-induced hemiparesis [35]. The neuroprosthesis provides continuous TESCS of the stepping pattern generator at the T11–T12 level to facilitate locomotion; in addition, it realizes a continuous stimulation of the cervical generator at the C5–C6 level to activate hand movements that accompany walking and increase excitability of the descending tracts of the spinal cord. Against the background of continuous activation of the central generators, phase-dependent stimulation of the spinal cord roots on the side of paresis occurs at the level of the T11 vertebra to activate paretic flexor muscles during the step transfer phase and L1 to activate paretic extensor muscles during the support phase.
A network of respiratory interneurons is localized in the spinal cord which provides respiratory pattern shaping into the final efferent motor output from the spinal respiratory nerves, coordinates respiratory muscle activity, as well as respiratory, locomotor, and postural movements [36] (Fig. 3).
The respiratory rhythm generated in the brainstem is transmitted to spinal neurons via bulbospinal premotor neurons. The superior cervical interneurons reside within the C1–C2 segments and can generate a respiratory rhythm. Cervical and lumbar central locomotor pattern generators can reproduce rhythmic patterns recorded on respiratory nerves. Respiratory interneurons modulate the bulbospinal rhythm and coordinate activity between motor pools. Interneurons can switch between tasks, and it is quite likely that the classification of interneurons is not static. Respiratory and limb muscle afferents project to spinal motoneurons and can modify respiratory pattern generation.
Layout of respiratory and motor centers and nerves in the spinal cord (from [36]).
EPIDURAL SPINAL CORD STIMULATION
Electrode localization and SCS frequency are critical determinants of the epidural SCS outcome. In studies of the respiratory system, the method of epidural SCS has primarily been used as the only electrophysiological method to detect respiratory interneurons (by an action potential).
Epidural spinal cord stimulation in animal models
Most of the works aimed at the topical diagnosis of epidural SCS respiratory effects have been carried out on animal models. For example, it has been shown in rats that the activation of cervical and thoracic interneurons is associated with respiration [37]. It has been demonstrated that the cat propriospinal C1–C3 neurons receive phasic excitation during inhalation and inhibition during exhalation [38]. The neurons of this region project within the diaphragmatic motor pool limits [39]. In decerebrated cats, C3–C6 interneurons are also activated depending on the respiratory cycle [40, 41]. In 35 rabbits, 63 interneurons were recorded at the C3–C6 level in the ventral horns dorsally and dorsomedially from the diaphragmatic motor pool, of which 54% were inspiratory and 46% were expiratory [42]. These electrophysiological results underlay further studies allowing the control of breathing in spinalized dogs. For instance, high-frequency stimulation of the ventral surface of the T2 spinal cord segment (300 Hz, 0–6 mA, 0.2 ms) in 11 dogs activated local pools of the diaphragmatic motoneurons through spinal pathways located in the lateral funiculus, whereas the upper cervical interneurons remained silent [43]. Current studies on dogs (2017–2019) have shown that the effect of inspiratory muscle activation is achieved by the stimulation of the ventral segments at the thoracic level [44, 45], whereas the effect of expiratory muscle activation can be achieved by the stimulation of the lower thoracic and lumbar regions [46–48].
As is known, because of the similar organization of the corticospinal tracts in primates and the respiratory bulbospinal tracts in rodents, the latter can also be suitable for studying the functional respiratory muscle recovery after the reproduction of the SCI effect (partial or complete hemisection) [49]. Intraspinal microstimulation at the C3 level (within the diaphragmatic motor pool) both in intact rats and in those with SCI, enables a direct activation of diaphragmatic motoneurons [50]. This, in turn, makes it possible to resuscitate the damaged diaphragm and modulate its activity in order to increase inspiratory volume. In rats with incomplete SCI, high-frequency epidural SCS at C4 and T2 levels leads to asynchronous activation of respiratory motoneurons, which causes spontaneous breathing [51].
Epidural spinal cord stimulation in clinical practice
In studies involving patients with SCI-induced tetraplegia, it has been shown that combined stimulation of the T9, T11 and L1 segments elicits the act of coughing due to expiratory muscle contraction [52]. A retrospective study demonstrated that all patients continued to use the epidural stimulation device regularly for 4.6 years. As a result, the control indices of life quality and respiratory function remained at a high level: the mean value of maximum airway pressure during breathing induced by epidural stimulation remained at 108 ± 23 cm H2O. At the same time, the need for a professional caregiver support significantly decreased, which allowed 5 patients out of 9 to move independently [53].
A clinical case has been described, in which an electrode was inserted into the epidural space at the level of C2–C4 vertebrae during minimally invasive surgery to a patient (aged 9 years) with complete anatomical spinal cord interruption at the C2 level. The patient was all the time connected to an ALV apparatus, with the duration of independent breathing episodes not exceeding 2 min. The process of expanding the patient’s abilities to breathe independently was carried out in four consecutive stages. The first stage was the development and implementation of electrical stimulation regimens, corresponding to respiratory patterns, with a repetition rate of 12, 15 and 20 cycles per min (10 Hz, pulse duration 2500 µs); during this stage, the duration of independent breathing episodes increased. During the second period (5 days), the patient’s ability to breathe independently increased from 9 to 16.5 min. At the third stage (4 weeks), electrical stimulation was run 4 times a day (by 15 min each), when the patient was breathing on his/her own simultaneously with an audiovisual support. At the onset of the fourth stage, the patient was implanted with a device for chronic electrical stimulation. Six months after having been discharged from the hospital, the patient carried on breathing independently with a concurrent stimulation (3 times a day by 20 min) [54].
Table 1 summarizes the effects of epidural SCS, obtained on decerebrated animal models and in clinical studies on patients with SCI. It has been proved that stimulation of the cervical region (upper and middle cervical) leads to activation of diaphragmatic breathing; with combined stimulation of several levels, the effect of spontaneous breathing is achieved, while the stimulation of the thoracolumbar region is accompanied by expiratory muscle activation and initiates the act of coughing.
In a small number of clinical studies, it has been shown that regular SCS is accompanied by the improvement of respiratory function, an increase in the patients’ quality of life, and normalization of lung secretory activity. However, the available data are insufficient to unambiguously correlate the stimulation site, stimulation parameters and effects that invasive electrical SCS exerts on breathing. The method of epidural SCS for the purposes of respiratory regulation has not been widely used in clinical practice, probably due to the risks associated with surgical intervention, as well as incompleteness of data on the effectiveness of this exposure.
TRANSCUTANEOUS ELECTRICAL SPINAL CORD STIMULATION
At present, studies of the effect of TESCS on external respiratory function are usually performed on healthy volunteers.
Minyaeva et al. [57], in a study involving 10 healthy volunteers, have shown that dynamic changes in the lung ventilation and gas exchange parameters during TESCS at the level of T11–T12 vertebrae over the spinal cord segments, where the central stepping pattern generator is located (Fig. 3), are associated with stimulation-induced stepping movements.
In the following study conducted on healthy volunteers, the data have been obtained on the regulation of respiratory muscle activity by TESCS (30 Hz, modulated with a 5 kHz frequency) [58]. The subjects were in a half reclining position, and TESCS was performed at the level of T12–L1 vertebrae. TESCS intensity was selected individually, using the intensity of single monopolar pulses (pulse duration 1 ms, pulse repetition frequency 1 pulse per 2–3 s) that elicited motor response in lower limb muscles, as a reference. The working range of currents was 30–90 mA. Noninvasive stimulation at a resting state caused a significant decrease in respiratory depth by 0.10 ± 0.03 L due to a synchronous decrease in the duration and rate of both inhalation and exhalation.
In a 2020 study, a 39-year-old patient with tetraplegia, developed due to diving injury at the C5 level, was treated with TESCS [59]. Before treatment, the patient reported impaired respiratory function and decreased ability to cough and expectorate sputum, especially in the supine position. During two weeks, high-frequency stimulation (10 kHz) was alternated with low-frequency stimulation (30 Hz), also alternated was the electrode localization at C3–C4, C5–C6 and T1–T2 levels. As a result, inhalation and exhalation volume increased, and the loudness of the act of coughing rose from 80 to 86 dB.
Thus, the possibility of using the method of TESCS for breathing regulation in principle has been shown. This method, being unrelated with the risks of surgical intervention, is a convenient tool to dissect the role of the spinal cord centers in human external respiration control. The issue of whether it is possible to regulate the activity of motor nuclei of respiratory muscles and “respiratory generator”, which are localized in thoracic and cervical segments of the spinal cord, through TESCS remains relevant [60].
It is of interest to design a “respiratory neuroprosthesis” by analogy with the noninvasive spinal neuroprosthesis that facilitates walking [35]. Using TESCS, it is possible to affect different segments of the spinal cord and thus initiate breathing, enhance the activity of the diaphragm, as well as inspiratory and expiratory muscles, during the respiratory cycle phases corresponding to their activity. Most spinal cord segments innervate both inspiratory and expiratory muscles (Fig. 3), however, by placing TESCS electrodes at the same position, different effects on breathing can hopefully be obtained due to the use of different stimulation parameters. Over many years of using SCS for pain management, it has been shown that the main stimulation parameters (amplitude, pulse duration, frequency) affect the therapeutic effect, as they determine the delivery of electrical charge to different loci of the spinal cord [61–63].
Thus, for the time being, TESCS is a potent and convenient tool for unraveling the functions of the spinal cord in the regulation of breathing. In the future, this technique may become an affordable and effective method to restore breathing in patients with respiratory muscle paresis.
CONCLUSIONS
Among the above-addressed methods of electrical stimulation, most data bear on the phrenic nerve stimulation, which is used in clinics to restore breathing in patients with high tetraplegia causing respiratory paralysis. It is a method with a highly predictable result, because the physiological mechanism behind its effect is well known. Moreover, it is a little invasive method. One of the major limitations of diaphragmatic nerve electrical stimulation is that it is only suitable for patients with SCI above the C4 segment. Another serious disadvantage of this method is that it activates inspiration, while having no effect on exhalation, and can also cause muscle fatigue, as it synchronously activates all axons in the electric field. Electrical stimulation of respiratory muscles is applied in respiratory system disorders of different etiology. Although these methods are noninvasive, they are, at the same time, nonstandardized, and, therefore, rarely used. It is obvious, that the study of the physiology of respiratory muscles, specifically, the relationships between the activity of the diaphragm, abdominal and intercostal respiratory muscles, with respiratory cycle phases are prospective research trends, because this knowledge will allow the existing clinical protocols for electrical muscle stimulation aimed at regulating the function of external respiration to be substantially improved.
Spinal cord stimulation has a potential advantage over muscle and phrenic nerve stimulation, based on the fact that the spinal cord contains neural structures that control the whole respiratory cycle. Therefore, by stimulating different regions of the spinal cord, it is possible to control different phases of the respiratory cycle and to use this method with a great variety of causes behind respiratory disorders, as convincingly demonstrated by numerous SCS studies conducted on animals. In clinical practice, epidural SCS is used extremely rarely: just sporadic clinical cases using this method have been published to date. Quite evidently, the serious risks of complications associated with the implantation of an SCS system hamper the clinical use of epidural stimulation. It is also obvious that the current level of knowledge about the role of spinal cord structures in respiratory cycle regulation is still not sufficient enough to disregard the risks for the sake of a predictable result, as it happens in the case of using SCS for pain management.
In the last few years, the fundamental possibility of using the TESCS method for breathing regulation has been clearly demonstrated. This noninvasive method has good prospects of clinical use in restoring respiratory function in patients with disorders of external respiration, concomitant to numerous pathologies. It is also a convenient tool to investigate the role of spinal centers in human external respiration control. In addition, the specific technical features of TESCS enable different regions of the spinal cord to be affected simultaneously. Thus, the development of a respiratory spinal neuroprosthesis, which will control the function of external respiration by modulating the activity of respiratory centers, enhancing inspiratory and expiratory muscle contractions during the appropriate respiratory cycle phases, is getting high on the agenda. We pin great hopes on the use of TESCS for gaining a deeper insight into the regulatory mechanisms of external respiration in humans.
LIMITATIONS
In this review, we considered the clinical application of respiratory electrical stimulation techniques in the context of a single nosology, spinal cord injury.
Abbreviations
- ALV:
-
artificial lung ventilation
- SCS:
-
spinal cord stimulation
- PNS:
-
phrenic nerve stimulation
- SCI:
-
spinal cord injury
- AFES:
-
abdominal functional electrical stimulation
- TESCS:
-
transcutaneous electrical spinal cord stimulation
- TEDS:
-
transcutaneous electrical diaphragmatic stimulation
REFERENCES
Safonov VA, Tarasova NN (2010) Electrical Stimulation оf Breathing. Human Physiol 36: 483–494.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration J Сlin Epidemiol 62(10): e1–e34. https://doi.org/10.1136/bmj.b2700
McCaughey EJ, Berry HR, McLean AN, Allan DB, Gollee H (2015) Abdominal functional electrical stimulation to assist ventilator weaning in acute tetraplegia: A cohort study. PLoS One10 (6): e0128589. https://doi.org/10.1371/journal.pone.0128589
Lim J, Gorman RB, Saboisky JP, Gandevia SC, Butler JE (2007) Optimal electrode placement for noninvasive electrical stimulation of human abdominal muscles. J Appl Physiol 102(4): 1612–1617. https://doi.org/10.1152/japplphysiol.00865.2006
Butler JE, Lim J, Gorman RB, Boswell-Ruys C, Saboisky JP, Lee BB, Gandevia SC (2011) Posterolateral surface electrical stimulation of abdominal expiratory muscles to enhance cough in spinal cord injury. Neurorehabil Neural Repair 25: 158–167. https://doi.org/10.1177/1545968310378509
McCaughey EJ, Butler JE, McBain RA, Boswell-Ruys CL, Hudson AL, Gandevia SC, Lee BB (2019) Abdominal functional electrical stimulation to augment respiratory function in spinal cord injury. Top Spinal Cord Inj Rehabil 25(2): 105–111. https://doi.org/10.1310/sci2502-105
Duarte GL, Bethiol AL, Ratti LD, Franco G, Moreno R, Tonella RM, Falcão AL (2021) Transcutaneous electrical diaphragmatic stimulation reduces the duration of invasive mechanical ventilation in patients with cervical spinal cord injury: retrospective case series. Spinal Cord Series Cases 7(1): 1–6. https://doi.org/10.1038/s41394-021-00396-4
Hsin YF, Chen SH, Yu TJ, Huang CC, Chen YH (2022) Effects of transcutaneous electrical diaphragmatic stimulation on respiratory function in patients with prolonged mechanical ventilation. Ann Thorac Med 17(1): 14–20. https://doi.org/10.4103/atm.atm_158_21
Postma K, Haisma JA, Hopman MT, Bergen MP, Stam HJ, Bussmann JB (2014) Resistive inspiratory muscle training in people with spinal cord injury during inpatient rehabilitation: a randomized controlled trial. Phys Therapy 94: 1709–1719. https://doi.org/10.2522/ptj.20140079
Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, Vasques F, Quintel M (2017) The future of mechanical ventilation: lessons from the present and the past. Crit Care 21(1): 183. https://doi.org/10.1186/s13054-017-1750-x
Levy M, Mizrahi J, Susak Z (1990) Recruitment, force and fatigue characteristics of 768 quadriceps muscles of paraplegics isometrically activated by surface functional 769 electrical stimulation. J Engin 12: 150–156. https://doi.org/10.1016/0141-5425(90)90136-B
DiMarco AF (2001) Neural prostheses in the respiratory system. J Rehabil Res Develop 38: 601–607.
Glenn WW, Brouillette RT, Dentz B, Fodstad H, Hunt CE, Keens TG, Marsh HM, Pande S, Piepgras DG, Vanderlinden RG (1988) Fundamental considerations in pacing of the diaphragm for chronic ventilatory insufficiency. A multi-center study. Pacing Clin Electrophysiol 11: 2121–2127. https://doi.org/10.1111/j.1540-8159.1988.tb06360.x
Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, Bass B, Dunkin B, Ingvarsson PE, Oddsdóttir M (2009) Complete Worldwide Operative Experience in Laparoscopic Diaphragm Pacing: Results and Differences in Spinal Cord Injured Patients and Amyotrophic Lateral Sclerosis Patients. Surg Endoscopy 23: 1433–1440. https://doi.org/10.1007/s00464-008-0223-3
DiMarco AF (2009) Phrenic nerve stimulation in patients with spinal cord injury. Respirat Physiol Neurobiol 169(2): 200–209. https://doi.org/10.1016/j.resp.2009.09.008
Romero-Ganuza FJ, Gambarrutta-Malfatti C, Diez de la Lastra-Buigues E, Marín-Ruiz MÁ, Merlo-González VE, Sánchez-Aranzueque Pantoja AM, García-Moreno FJ, Mazaira-Álvarez J (2011) Diaphragmatic pacemaker as an alternative to mechanical ventilation in patients with cervical spinal injury. Med Intensiva (Engl Edition) 35: 13–21. https://doi.org/10.1016/j.medin.2010.10.003
Locke KC, Randelman ML, Hoh DJ, Zholudeva LV, Lane MA (2022) Respiratory plasticity following spinal cord injury. Neural Regen Res 17: 2141–2148. https://doi.org/10.4103/1673-5374.335839
Onders RP, Elmo M, Kaplan C, Schilz R, Katirji B, Tinkoff G (2018) Long-term experience with diaphragm pacing for traumatic spinal cord injury: early implantation should be considered. Surgery 164(4): 705–711. https://doi.org/10.1016/j.surg.2018.06.050
Son BC, Kim DR, Kim IS, Hong JT (2013) Phrenic nerve stimulation for diaphragm pacing in a quadriplegic patient. J Korean Neurosurg Soc 54(4): 359–362. https://doi.org/10.3340/jkns.2013.54.4.359
Vázquez RG, Sedes PR, Fariña MM, Marqués AM, Velasco MEF (2013) Respiratory Management in the Patient with Spinal Cord Injury. Biomed Res Int 2013: 168757. https://doi.org/10.1155/2013/168757
Verrills P, Sinclair C, Barnard A (2016) A review of spinal cord stimulation systems for chronic pain. J Pain Res 9: 481. https://doi.org/10.2147/JPR.S108884
Pérez JT (2021) Spinal cord stimulation: beyond pain management. Neurología (Engl Edition) 37(7): 586-595. https://doi.org/10.1016/j.nrleng.2019.05.007
Stauss T, El Majdoub F, Sayed D, Surges G, Rosenberg WS, Kapural L, Bundschu R, Lalkhen A, Patel N, Gliner B, Subbaroyan J (2019) A multicenter real‐world review of 10 kH z SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Translat Neurol 6: 496–507. https://doi.org/10.1002/acn3.720
Baranidharan G, Bretherton B, Montgomery C, Titterington J, Crowther T, Vannabouathong C, Inzana JA, Rotte A (2021) Pain Relief and Safety Outcomes with Cervical 10 kHz Spinal Cord Stimulation: Systematic Literature Review and Meta-analysis. Pain and Therapy 10: 849–874. https://doi.org/10.1007/s40122-021-00269-6
Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E (2007) Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 132: 179–188. https://doi.org/10.1016/j.pain.2007.07.028
Indications, Safety and Warnings for Spinal Cord Stimulation. Medtronic. Accessed April 4th, 2022.
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ (2014) Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409. https://doi.org/10.1093/brain/awu038
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947. https://doi.org/10.1016/S0140-6736(11)60547-3
Hachmann JT, Yousak A, Wallner JJ, Gad PN, Edgerton VR, Gorgey AS (2021) Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J Neurophysiol 126(6): 1843–1859. https://doi.org/10.1152/jn.00020.2021
Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR (2015) Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 32: 1968–1980. https://doi.org/10.1089/neu.2015.4008
Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR (2015) Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol 113: 834–842. https://doi.org/10.1152/jn.00609.2014
Gorodnichev RM, Pivovarova EA, Pukhov A, Moiseev SA, Savokhin AA, Moshonkina TR, Shcherbakova NA, Kilimnik VA, Selionov VA, Kozlovskaia IB, Edgerton VR, Gerasimenko YP (2012) Transcutaneous electrical stimulation of the spinal cord: non-invasive tool for activation of locomotor circuitry in human. Human Physiol 38: 46–56.
Megia Garcia A, Serrano-Muñoz D, Taylor J, Avendaño-Coy J, Gómez-Soriano J (2020) Transcutaneous spinal cord stimulation and motor rehabilitation in spinal cord injury: a systematic review. Neurorehabil Neural Repair 34(1): 3–12. https://doi.org/10.1177/1545968319893298
Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR (2015) Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol 113(3): 834–842. https://doi.org/10.1152/jn.00609.2014
Grishin AA, Bobrova EV, Reshetnikova VV, Moshonkina TR, Gerasimenko YP (2021) A system for detecting stepping cycle phases and spinal cord stimulation as a tool for controlling human locomotion. Biomed Engineer 54(5): 312–316. https://doi.org/10.1007/s10527-021-10029-7
Sunshine MD, Sutor TW, Fox EJ, Fuller DD (2020) Targeted activation of spinal respiratory neural circuits. Exp Neurol 328: 113256. https://doi.org/10.1016/j.expneurol.2020.113256
Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ (2008) Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Compar Neurol 511(5): 692–709. https://doi.org/10.1002/cne.21864
Duffin J, Hoskin RW (1987) Intracellular recordings from upper cervical inspiratory neurons in the cat. Brain Res 435: 351–354. https://doi.org/10.1016/0006-8993(87)91623-4
Lipski J, Duffin J (1986) An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res 61: 625–637. https://doi.org/10.1007/BF00237589
Bellingham MC, Lipski J (1990) Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533(1): 141–146. https://doi.org/10.1016/0006-8993(90)91807-S
Duffin J, Iscoe S (1996) The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res 112(1): 35–40. https://doi.org/10.1007/BF00227175
Palisses R, Persegol L, Viala D (1989) Evidence for respiratory interneurones in the C3-C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res 78(3): 624–632. https://doi.org/10.1007/BF00230250
DiMarco AF, Kowalski KE (2013) Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Resp Physiol Neurobiol 186: 1–6. https://doi.org/10.1016/j.resp.2012.12.003
Kowalski KE, Romaniuk JR, Kirkwood PA, DiMarco AF (2019) Inspiratory muscle activation via ventral lower thoracic high-frequency spinal cord stimulation. J Appl Physiol (1985) 126: 977–983. https://doi.org/10.1152/japplphysiol.01054.2018
DiMarco AF, Kowalski KE (2013) Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol 189: 438–449. https://doi.org/10.1016/j.resp.2013.06.001
DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G (1999) Pattern of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol 86: 1881–1889. https://doi.org/10.1152/jappl.1999.86.6.1881
DiMarco AF, Kowalski KE (2008) Effects of chronic electrical stimulation on paralyzed expiratory muscles. J Appl Physiol 104(6): 1634–1640. https://doi.org/10.1152/japplphysiol.01321.2007
Kowalski KE, Romaniuk JR, Kowalski T, DiMarco AF (2017) Effects of expiratory muscle activation via high-frequency spinal cord stimulation. J Appl Physiol 123(6): 1525–1531. https://doi.org/10.1152/japplphysiol.00402.2017
Kastner A, Gauthier P (2008) Are rodents an appropriate pre-clinical model for treating spinal cord injury? Examples from the respiratory system. Exp Neurol 213(2): 249–256. https://doi.org/10.1016/j.expneurol.2008.07.008
Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM (2017) Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury. J Neurophysiol 117: 767. https://doi.org/10.1152/jn.00721.2016
Gonzalez-Rothi EJ, Streeter KA, Hanna MH, Stamas AC, Reier PJ, Baekey DM, Fuller DD (2017) High-frequency epidural stimulation across the respiratory cycle evokes phrenic short-term potentiation after incomplete cervical spinal cord injury. J Neurophysiol 118(4): 2344–2357. https://doi.org/10.1152/jn.00913.2016
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR (2006) Spinal cord stimulation: a new method to produce an effective cough in patients with spinal cord injury. Am J Respir Crit Care Med 173: 1386–1389. https://doi.org/10.1164/rccm.200601-097CR
DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT (2014) Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med 37: 380–388. https://doi.org/10.1179/2045772313Y.0000000152
Erokhin AN, Kobizev AE, Sergeenko OM, Turovinina EF (2020) Phrenic nerve stimulation in complex rehabilitation for cervical spinal cord injury using modified implantable device (case report). Geniy Ortopedii 26(1): 89–94. https://doi.org/10.18019/1028-4427-2020-26-1-89-94
Duffin J, Li YM (2006) Transmission of respiratory rhythm: midline-crossing connections at the level of the phrenic motor nucleus? Resp Physiol Neurobiol 153(2): 139–147. https://doi.org/10.1016/j.resp.2005.09.011
Tian GF, Duffin J (1996) Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111(2): 178–186. https://doi.org/10.1007/BF00227296
Minyaeva AV, Moiseev SA, Pukhov AM, Savokhin AA, Gerasimenko YP, Moshonkina TR (2017) Response of external inspiration to the movements induced by transcutaneous spinal cord stimulation. Human Physiol 43(5): 524–531. https://doi.org/10.1134/S0362119717050115
Moshonkina TR, Scherbakova NA, Moiseev SA, Minyaeva AV, Gerasimenko YP (2020) Regulation of respiration during electrical stimulation of the lumbar spinal cord in humans. Integrat Physiol 1: 108–115. https://doi.org/10.1016/j.mayocp.2017.04.011
Gad P, Kreydin E, Zhong H, Edgerton VR (2020) Spinal Networks and Spinal Cord Injury: A Tribute to Reggie Edgerton: Enabling respiratory control after severe chronic tetraplegia: an exploratory case study. J Neurophysiol 124(3): 774. https://doi.org/10.1152/jn.00320.2020
Del Negro CA, Funk GD, Feldman JL (2018) Breathing matters. Nat Rev Neurosci 19(6): 351–367. https://doi.org/10.1038/s41583-018-0003-6
Miller J, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B (2016) Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation: Technology at the Neural Interface 19(4): 373–384. https://doi.org/10.1111/ner.12438
Joosten EA, Franken G (2020) Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain 161(1): S104. https://doi.org/10.1097/j.pain.0000000000001854
Shandybina ND, Kuropatenko MV, Moshonkina TR (2022) Relevance of the transcutaneous spinal cord stimulation for regulation of the external respiration. Motor Control 2022: 122.
ACKNOWLEDGMENT
The authors are grateful to Ksenia Belaya for the design of the figures.
Funding
This study was implemented within the State budget funded assignment; registration no. 0113-2019-0006 (63.4.).
Author information
Authors and Affiliations
Contributions
Conceptualization, collection and analysis of the literature sources, manuscript preparation (N.D.Sh.); literature data analysis, involvement in manuscript writing (M.V.K.); article design, literature data analysis, involvement in the finalizing of the manuscript (T.M.M.). The authors attest that their authorship meets the ICMJE international criteria (all authors have made substantial contributions to conceptualization, database search and analysis, article preparation, and also have read and approved the final version of the article before publication).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 11, pp. 1410–1425https://doi.org/10.31857/S0869813922110115.
Rights and permissions
About this article
Cite this article
Shandybina, N.D., Kuropatenko, M.V. & Moshonkina, T.R. Regulation of Human Respiration by Electrical Stimulation. J Evol Biochem Phys 58, 1879–1891 (2022). https://doi.org/10.1134/S0022093022060175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060175