Abstract
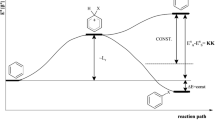
The structures of (E)-1-styrylnaphthalene (1SN) and its aza derivatives 1-styrylisoquinoline (1SiQ) and 4- and 8-styrylquinolines (4SQ and 8SQ, respectively) in the neutral and protonated forms were calculated by the semiempirical (PM3) and DFT (B3LYP/6-31G*) methods. It follows from the DFT data that, in the ground state (S0), 1SiQ and 8SQ are planar, whereas 1SN, neutral 4SQ, and all protonated azastyrylnaphthalenes are nonplanar with aromatic cores twisted by 5 to 40° out of the plane of the double bond and with linear correlation between the torsion angles of the two cores. The calculated adiabatic excitation energy (E ad) varies within 61–64 kcal mol−1 for the neutral compounds and decreases for the protonated forms to 48, 45, and 33 kcal mol−1 for 1SiQH+, 4SQH+, and 8SQH+, respectively. The lower E ad value for 8SQH+ is in qualitative agreement with a lower photoisomerization quantum yield for this compound as compared with that for other protonated azastyrylnaphthalenes.
Similar content being viewed by others
References
Sugimoto, H., Kimura, T., and Inoue, S., J. Am. Chem. Soc., 1999, vol. 121, p. 2325.
Leaustic, A., Riviere, E., and Clement, R., Chem. Mater., 2003, vol. 15, p. 4784.
Otsuki, J., Suka, A., Yamazaki, K., Abe, H., Araki, Y., and Ito, O., Chem. Commun., 2004, p. 1290.
Gust, D., Moore, T.A., and Moore, A.L., Chem. Commun., 2006, p. 1169.
Qu, D.H., Ji, F.Y., Wang, Q.C., and Tian, H., Adv. Mater., 2006, vol. 18, p. 2035.
Mazzucato, U., Pure Appl. Chem., 1982, vol. 54, p. 1705.
Galiazzo, G., Bortolus, P., and Gennari, G., Gazz. Chim. Ital., 1990, vol. 120, p. 581.
Gennari, G., Bortolus, P., and Galiazzo, G., J. Mol. Struct., 1991, vol. 249, p. 189.
Gennari, G., Galiazzo, G., and Bortolus, P., J. Photochem. Photobiol. A: Chem., 1988, vol. 43, p. 293.
Bartocci, G., Bortolus, P., and Mazzucato, U., J. Phys. Chem., 1973, vol. 77, p. 605.
Marconi, G., Bartocci, G., Mazzucato, U., Spalletti, A., Abbate, F., Angeloni, L., and Castellucci, E., Chem. Phys., 1995, vol. 196, p. 383.
Mazzucato, U. and Spalletti, A., Photochem. Photobiol. Sci., 2003, vol. 2, p. 282.
Stewart, J.J.P., J. Comput. Chem., 1989, vol. 10, p. 208, 221.
Gaussian 03, Revision B.02, Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Pittsburgh: Gaussian Inc., 2003.
Catalan, J., Chem. Phys. Lett., 2006, vol. 421, p. 134.
Arai, T. and Tokumaru, K., Chem. Rev., 1993, vol. 93, p. 32.
Gusten, H. and Schulte-Frohlinde, D., Z. Naturforsch., A: Phys. Sci., 1979, vol. 34b, p. 1556.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.F. Budyka, I.V. Oshkin, 2007, published in Khimiya Vysokikh Energii, 2007, Vol. 41, No. 6, pp. 503–509.
Rights and permissions
About this article
Cite this article
Budyka, M.F., Oshkin, I.V. A quantum-chemical study of aza derivatives of (E)-1-styrylnaphthalene in the ground and lowest electronically excited states. High Energy Chem 41, 444–450 (2007). https://doi.org/10.1134/S0018143907060100
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0018143907060100