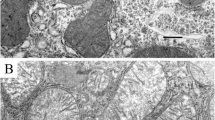
Abstract—The characteristics of the ultrastructure and functioning of mitochondria in the liver of Sprague Dawley rats in the experimental model of type I diabetes mellitus have been investigated. It has been found that diabetes mellitus induced in response to streptozotocin administration at a dose of 75 mg/kg body weight was accompanied by disturbances in the structural organization of mitochondrial cristae and a decrease in the size of the organelles compared to the control. It has been also demonstrated that in type I diabetes the respiratory rates of liver mitochondria in metabolic states 2, 3, and 4 increase. This may be associated with an increase in the total content of fatty acids in liver mitochondria of diabetic rats. At the same time, streptozotocin-induced diabetes mellitus in rats did not affect the indices of oxidative phosphorylation efficiency (ADP/O, respiratory control ratio, and phosphorylation time) in liver mitochondria.



Similar content being viewed by others
REFERENCES
American Diabetes Association, in Diabetes Care (American Diabetes Association, 2011), pp. 62–69.
L. C. Groop and J. G. Eriksson, Ann. Med. 24, 483 (1992).
W. H. Gispen and G. J. Biessels, Trends Neurosci. 23, 542 (2000).
D. M. D’Souza, D. Al-Sajee, and T. J. Hawke, Front. Physiol. 4, 379 (2013).
M. A. Liu, H. B. Cao, Y. A. Hou, et al., Cell Physiol. Biochem. 45 (4), 1423 (2018).
D. E. Frances, M. T. Ronco, J. A. Monti, et al., J. Endocrinol. 205 (2), 187 (2010).
F. M. Ferreira, C. M. Palmeira, R. Seica, et al., J. Biochem. Mol. Toxicol. 17 (4), 214 (2003).
M. K. Montgomery and N. Turner, Endocr. Connect. 4 (1), R1 (2015).
M. P. Murphy and R. A. Smith, Annu. Rev. Pharmacol. Toxicol. 47, 629 (2007).
J. Chen, S. E. Stimpson, G. A. Fernandez-Bueno, et al., Antioxid. Redox Signal. 29 (14), 1361 (2018).
K. Morino, K. F. Petersen, and G. I. Shulman, Diabetes 55 (2), S9 (2006).
K. F. Petersen, D. Befroy, S. Dufour, et al., Science 300, 1140 (2003).
F. Malka, O. Guillery, C. Cifuentes-Diaz, et al., EMBO Rep. 6, 853 (2005).
H. Chen, A. Chomyn, and D. C. Chan, J. Biol. Chem. 280, 26185 (2005).
H. Chen, M. Vermulst, Y. E. Wang, et al., Cell 141, 280 (2010).
J. A. Baur, K. J. Pearson, N. L. Price, et al., Nature 444, 337 (2006).
T. Nishikawa, D. Kukidome, K. Sonoda, et al., Diabetes Res. Clin. Pract. 77, 161 (2007).
B. Rodrigues, P. Poucheret, M. L. Battell, et al., in Experimental Models of Diabetes, Ed by J. H. McNeill (Boca Raton, FL: CRC Press, 1999), pp 3–17.
K. N. Belosludtsev, N. V. Belosludtseva, A. V. Agafonov, et al., Biochim. Biophys. Acta 1838 (10), 2600 (2014).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, et al., J. Biol.- Chem. 193 (1), 265 (1951).
N. I. Venediktova, O. S. Gorbacheva, N. V. Beloslud-tseva, et al., J. Bioenerg. Biomembr. 49 (2), 149 (2017).
B. Chance and G. R. Williams, Nature 176 (4475), 250 (1955).
E. Bligh and W. Dyer, Can. J. Biochem. Physiol. 37, 911 (1959).
W. I. Sivitz and M. A. Yorek, Antioxid. Redox Signal. 12 (4), 537 (2010).
M. Patti and S. Corvera, Endocr. Rev. 31 (3), 364 (2010).
R. N. Remedioa, A. Castellar, R. A. Barbosa, et al., Micron 42 (5), 484 (2011).
D. A. Hood, L. D. Tryon, H. N. Carter, et al., Biochem. J. 473, 2295 (2016).
A. T. Erlich, L. D. Tryon, M. J. Crilly, et al., Integr. Med. Res. 5, 187 (2016).
P. A. Parone, S. Da Cruz, D. Tondera, et al., PLoS One 3, 3257 (2008).
R. S. Kaplan, D. L. Oliveira, and G. L. Wilson, Arch. Biochem Biophys. 280 (1), 181 (1990).
V. N. Samartsev, Biochemistry (Moscow) 65 (9), 991 (2000).
Funding
The work was supported by the Russian Foundation for Basic Research, project no. 19-015-00117-а.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed in accordance with the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (Strasbourg, 1986) and the ethical principles of the Declaration of Helsinki (2000). All the experimental protocols were approved by the Ethics Committee of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences (order no. 173 / from October 3, 2011, protocol no. 04/2019 from March 5, 2019).
Additional information
Translated by I. Matiulko
Abbreviations: DNP, 2,4-dinitrophenol.
Rights and permissions
About this article
Cite this article
Starinets, V.S., Lebedeva, E.V., Mikheeva, I.B. et al. Ultrastructural and Functional Changes in Liver Mitochondria in a Rat Model of Type I Diabetes Mellitus. BIOPHYSICS 64, 755–760 (2019). https://doi.org/10.1134/S0006350919050221
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350919050221