Abstract
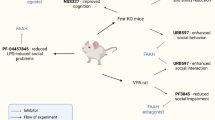
The mechanisms of autism are of extreme interest due to the high prevalence of this disorder in the human population. In this regard, special attention is given to the transcription factor Freud-1 (encoded by the Cc2d1a gene), which regulates numerous intracellular signaling pathways and acts as a silencer for 5-HT1A serotonin and D2 dopamine receptors. Disruption of the Freud-1 functions leads to the development of various psychopathologies. In this study, we found an increase in the expression of the Cc2d1a/Freud-1 gene in the hippocampus of BTBR mice (model of autistic-like behavior) in comparison with C57Bl/6J mice and examined how restoration of the Cc2d1a/Freud-1 expression in the hippocampus of BTBR mice affects their behavior, expression of 5-HT1A serotonin and D2 dopamine receptors, and CREB and NF-κB intracellular signaling pathways in these animals. Five weeks after administration of the adeno-associated viral vector (AAV) carrying the pAAV_H1-2_shRNA-Freud-1_Syn_EGFP plasmid encoding a small hairpin RNA (shRNA) that suppressed expression of the Cc2d1a/Freud-1 gene, we observed an elevation in the anxiety levels, as well as the increase in the escape latency and path length to the platform in the Morris water maze test, which was probably associated with a strengthening of the active stress avoidance strategy. However, the Cc2d1a/Freud-1 knockdown did not affect the spatial memory and phosphorylation of the CREB transcription factor, although such effect was found in C57Bl/6J mice in our previous study. These results suggest the impairments in the CREB-dependent effector pathway in BTBR mice, which may play an important role in the development of the autistic-like phenotype. The knockdown of Cc2d1a/Freud-1 in the hippocampus of BTBR mice did not affect expression of the 5-HT1A serotonin and D2 dopamine receptors and key NF-κB signaling genes (Nfkb1 and Rela). Our data suggest that the transcription factor Freud-1 plays a significant role in the pathogenesis of anxiety and active stress avoidance in autism.






Similar content being viewed by others
Abbreviations
- AAV:
-
adeno-associated viral vector
- ASD:
-
autism spectrum disorder
- Cc2d1a/Freud-1:
-
Cc2d1a gene encoding the transcription factor Freud-1
- EGFP:
-
enhanced green fluorescent protein
References
Christensen, D. L., Baio, J., Van Naarden Braun, K., Bilder, D., Charles, J., et al. (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 Sites, United States, 2012, Morbid. Mortal. Weekly Rep. Surveill. Summaries, 65, 1-23, https://doi.org/10.15585/mmwr.ss6503a1.
Alexander, A. L., Lee, J. E., Lazar, M., Boudos, R., DuBray, M. B., et al. (2007) Diffusion tensor imaging of the corpus callosum in autism, NeuroImage, 34, 61-73, https://doi.org/10.1016/j.neuroimage.2006.08.032.
Ecker, C., Suckling, J., Deoni, S. C., Lombardo, M. V., Bullmore, E. T., et al. (2012) Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study, Arch. Gen. Psychiatry, 69, 195-209, https://doi.org/10.1001/archgenpsychiatry.2011.1251.
Hoischen, A., Krumm, N., and Eichler, E. E. (2014) Prioritization of neurodevelopmental disease genes by discovery of new mutations, Nat. Neurosci., 17, 764-772, https://doi.org/10.1038/nn.3703.
Lainhart, J. E. (2006) Advances in autism neuroimaging research for the clinician and geneticist, Am. J. Med. Genet. C Semin. Med. Genet., 142C, 33-39, https://doi.org/10.1002/ajmg.c.30080.
Basel-Vanagaite, L., Attia, R., Yahav, M., Ferland, R. J., Anteki, L., et al. (2006) The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation, J. Med. Genet., 43, 203-210, https://doi.org/10.1136/jmg.2005.035709.
Nakamura, A., Naito, M., Tsuruo, T., and Fujita, N. (2008) Freud-1/Aki1, a novel PDK1-interacting protein, functions as a scaffold to activate the PDK1/Akt pathway in epidermal growth factor signaling, Mol. Cell. Biol., 28, 5996-6009, https://doi.org/10.1128/MCB.00114-08.
Zamarbide, M., Mossa, A., Munoz-Llancao, P., Wilkinson, M. K., Pond, H. L., et al. (2019) Male-specific cAMP signaling in the hippocampus controls spatial memory deficits in a mouse model of autism and intellectual disability, Biol. Psychiatry, 85, 760-768, https://doi.org/10.1016/j.biopsych.2018.12.013.
Oeckinghaus, A., and Ghosh, S. (2009) The NF-kappaB family of transcription factors and its regulation, Cold Spring Harb. Perspect. Biol., 1, a000034, https://doi.org/10.1101/cshperspect.a000034.
Liao, X., and Li, Y. (2020) Nuclear factor kappa B in autism spectrum disorder: a systematic review, Pharmacol. Res., 159, 104918, https://doi.org/10.1016/j.phrs.2020.104918.
El-Ansary, A., and Al-Ayadhi, L. (2012) Neuroinflammation in autism spectrum disorders, J. Neuroinflamm., 9, 265, https://doi.org/10.1186/1742-2094-9-265.
Matta, S. M., Hill-Yardin, E. L., and Crack, P. J. (2019) The influence of neuroinflammation in autism spectrum disorder, Brain Behav. Immun., 79, 75-90, https://doi.org/10.1016/j.bbi.2019.04.037.
Theoharides, T. C., Asadi, S., and Patel, A. B. (2013) Focal brain inflammation and autism, J. Neuroinflamm., 10, 815, https://doi.org/10.1186/1742-2094-10-46.
Young, A. M., Chakrabarti, B., Roberts, D., Lai, M. C., Suckling, J., et al. (2016) From molecules to neural morphology: understanding neuroinflammation in autism spectrum condition, Mol. Autism, 7, 9, https://doi.org/10.1186/s13229-016-0068-x.
Impey, S., McCorkle, S. R., Cha-Molstad, H., Dwyer, J. M., Yochum, G. S., et al. (2004) Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions, Cell, 119, 1041-1054, https://doi.org/10.1016/j.cell.2004.10.032.
Zamarbide, M., Oaks, A. W., Pond, H. L., Adelman, J. S., and Manzini, M. C. (2018) Loss of the intellectual disability and autism gene Cc2d1a and its homolog Cc2d1b differentially affect spatial memory, anxiety, and hyperactivity, Front. Genet., 9, 65, https://doi.org/10.3389/fgene.2018.00065.
Ou, X. M., Lemonde, S., Jafar-Nejad, H., Bown, C. D., Goto, A., et al. (2003) Freud-1: A neuronal calcium-regulated repressor of the 5-HT1A receptor gene, J. Neurosci., 23, 7415-7425.
Al-Tawashi, A., Jung, S. Y., Liu, D., Su, B., and Qin, J. (2012) Protein implicated in nonsyndromic mental retardation regulates protein kinase A (PKA) activity, J. Biol. Chem., 287, 14644-14658, https://doi.org/10.1074/jbc.M111.261875.
Zhao, M., Raingo, J., Chen, Z. J., and Kavalali, E. T. (2011) Cc2d1a, a C2 domain containing protein linked to nonsyndromic mental retardation, controls functional maturation of central synapses, J. Neurophysiol., 105, 1506-1515, https://doi.org/10.1152/jn.00950.2010.
Chen, K. R., Chang, C. H., Huang, C. Y., Lin, C. Y., Lin, W. Y., et al. (2012) TBK1-associated protein in endolysosomes (TAPE)/CC2D1A is a key regulator linking RIG-I-like receptors to antiviral immunity, J. Biol. Chem., 287, 32216-32221, https://doi.org/10.1074/jbc.C112.394346.
Vahid-Ansari, F., Daigle, M., Manzini, M. C., Tanaka, K. F., Hen, R., et al. (2017) Abrogated Freud-1/Cc2d1a repression of 5-HT1A autoreceptors induces fluoxetine-resistant anxiety/depression-like behavior, J. Neurosci., 37, 11967-11978, https://doi.org/10.1523/JNEUROSCI.1668-17.2017.
Kondaurova, E. M., Plyusnina, A. V., Ilchibaeva, T. V., Eremin, D. V., Rodnyy, A. Y., et al. (2021) Effects of a Cc2d1a/Freud-1 knockdown in the hippocampus on behavior, the serotonin system, and BDNF, Int. J. Mol. Sci., 22, 13319 https://doi.org/10.3390/ijms222413319.
Farook, M. F., DeCuypere, M., Hyland, K., Takumi, T., LeDoux, M. S., et al. (2012) Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models, PLoS One, 7, e43030, https://doi.org/10.1371/journal.pone.0043030.
Faraji, J., Karimi, M., Lawrence, C., Mohajerani, M. H., and Metz, G. A. S. (2018) Non-diagnostic symptoms in a mouse model of autism in relation to neuroanatomy: the BTBR strain reinvestigated, Translat. Psychiatry, 8, 234, https://doi.org/10.1038/s41398-018-0280-x.
Lemonde, S., Turecki, G., Bakish, D., Du, L., Hrdina, P. D., et al. (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide, J. Neurosci., 23, 8788-8799, https://doi.org/10.1523/JNEUROSCI.23-25-08788.2003.
Rogaeva, A., Ou, X. M., Jafar-Nejad, H., Lemonde, S., and Albert, P. R. (2007) Differential repression by freud-1/CC2D1A at a polymorphic site in the dopamine-D2 receptor gene, J. Biol. Chem., 282, 20897-20905, https://doi.org/10.1074/jbc.M610038200.
Ford, C. P. (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission, Neuroscience, 282, 13-22, https://doi.org/10.1016/j.neuroscience.2014.01.025.
Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Dopamine receptors: from structure to function, Physiol. Rev., 78, 189-225, https://doi.org/10.1152/physrev.1998.78.1.189.
Stephenson, D. T., O’Neill, S. M., Narayan, S., Tiwari, A., Arnold, E., et al. (2011) Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis, Mol. Autism, 2, 7, https://doi.org/10.1186/2040-2392-2-7.
Gould, G. G., Burke, T. F., Osorio, M. D., Smolik, C. M., Zhang, W. Q., et al. (2014) Enhanced novelty-induced corticosterone spike and upregulated serotonin 5-HT1A and cannabinoid CB1 receptors in adolescent BTBR mice, Psychoneuroendocrinology, 39, 158-169, https://doi.org/10.1016/j.psyneuen.2013.09.003.
Gould, G. G., Hensler, J. G., Burke, T. F., Benno, R. H., Onaivi, E. S., et al. (2011) Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior, J. Neurochem., 116, 291-303, https://doi.org/10.1111/j.1471-4159.2010.07104.x.
Guo, Y. P., and Commons, K. G. (2017) Serotonin neuron abnormalities in the BTBR mouse model of autism, Autism Res., 10, 66-77, https://doi.org/10.1002/aur.1665.
Wirth, A., Chen-Wacker, C., Wu, Y. W., Gorinski, N., Filippov, M. A., et al. (2013) Dual lipidation of the brain-specific Cdc42 isoform regulates its functional properties, Biochem. J., 456, 311-322, https://doi.org/10.1042/BJ20130788.
Grimm, D., Kay, M. A., and Kleinschmidt, J. A. (2003) Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6, Mol. Ther., 7, 839-850, https://doi.org/10.1016/s1525-0016(03)00095-9.
Slotnick, B. M., and Leonard, C. M. (1975) A Stereotaxic Atlas of the Albino Mouse Forebrain, U.S. Dept. of Health, Education and Welfare, Rockville, Maryland.
Khotskin, N. V., Plyusnina, A. V., Kulikova, E. A., Bazhenova, E. Y., Fursenko, D. V., et al. (2019) On association of the lethal yellow (A(Y)) mutation in the agouti gene with the alterations in mouse brain and behavior, Behav. Brain Res., 359, 446-456, https://doi.org/10.1016/j.bbr.2018.11.013.
Kulikov, A. V., Fursenko, D. V., Khotskin, N. V., Bazovkina, D. V., Kulikov, V. A., et al. (2014) Spatial learning in the Morris water maze in mice genetically different in the predisposition to catalepsy: the effect of intraventricular treatment with brain-derived neurotrophic factor, Pharmacol. Biochem. Behav., 122, 266-272, https://doi.org/10.1016/j.pbb.2014.04.009.
Kulikov, A. V., Tikhonova, M. A., and Kulikov, V. A. (2008) Automated measurement of spatial preference in the open field test with transmitted lighting, J. Neurosci. Methods, 170, 345-351, https://doi.org/10.1016/j.jneumeth.2008.01.024.
Kulikov, A. V., Naumenko, V. S., Voronova, I. P., Tikhonova, M. A., and Popova, N. K. (2005) Quantitative RT-PCR assay of 5-HT1A and 5-HT2A serotonin receptor mRNAs using genomic DNA as an external standard, J. Neurosci. Methods, 141, 97-101, https://doi.org/10.1016/j.jneumeth.2004.06.005.
Naumenko, V. S., and Kulikov, A. V. (2006) Quantitative assay of 5-HT(1A) serotonin receptor gene expression in the brain, Mol. Biol. (Mosk), 40, 37-44.
Naumenko, V. S., Osipova, D. V., Kostina, E. V., and Kulikov, A. V. (2008) Utilization of a two-standard system in real-time PCR for quantification of gene expression in the brain, J. Neurosci. Methods, 170, 197-203, https://doi.org/10.1016/j.jneumeth.2008.01.008.
Grove, J., Ripke, S., Als, T. D., Mattheisen, M., Walters, R. K., et al. (2019) Identification of common genetic risk variants for autism spectrum disorder, Nat. Genet., 51, 431-444, https://doi.org/10.1038/s41588-019-0344-8.
Manzini, M. C., Xiong, L., Shaheen, R., Tambunan, D. E., Di Costanzo, S., et al. (2014) CC2D1A regulates human intellectual and social function as well as NF-kappaB signaling homeostasis, Cell Rep., 8, 647-655, https://doi.org/10.1016/j.celrep.2014.06.039.
Oaks, A. W., Zamarbide, M., Tambunan, D. E., Santini, E., Di Costanzo, S., et al. (2017) Cc2d1a loss of function disrupts functional and morphological development in forebrain neurons leading to cognitive and social deficits, Cereb. Cortex, 27, 1670-1685, https://doi.org/10.1093/cercor/bhw009.
Sener, E. F., Uytun, M. C., Bayramov, K. K., Zararsiz, G., Oztop, D. B., et al. (2016) The roles of CC2D1A and HTR1A gene expressions in autism spectrum disorders, Metab. Brain Dis., 31, 613-619, https://doi.org/10.1007/s11011-016-9795-0.
White, S. W., Oswald, D., Ollendick, T., and Scahill, L. (2009) Anxiety in children and adolescents with autism spectrum disorders, Clin. Psychol. Rev., 29, 216-229, https://doi.org/10.1016/j.cpr.2009.01.003.
Defensor, E. B., Pearson, B. L., Pobbe, R. L., Bolivar, V. J., Blanchard, D. C., et al. (2011) A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice, Behav. Brain Res., 217, 302-308, https://doi.org/10.1016/j.bbr.2010.10.033.
Kida, S., Josselyn, S. A., Pena de Ortiz, S., Kogan, J. H., Chevere, I., et al. (2002) CREB required for the stability of new and reactivated fear memories, Nat. Neurosci., 5, 348-355, https://doi.org/10.1038/nn819.
Sun, S. C. (2011) Non-canonical NF-kappaB signaling pathway, Cell Res., 21, 71-85, https://doi.org/10.1038/cr.2010.177.
Gavalda, N., Gutierrez, H., and Davies, A. M. (2009) Developmental switch in NF-kappaB signalling required for neurite growth, Development, 136, 3405-3412, https://doi.org/10.1242/dev.035295.
Gutierrez, H., O’Keeffe, G. W., Gavalda, N., Gallagher, D., and Davies, A. M. (2008) Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA, J. Neurosci., 28, 8246-8256, https://doi.org/10.1523/JNEUROSCI.1941-08.2008.
Acknowledgments
The study was conducted at the Center for Genetic Resources of Laboratory Animals; Institute of Cytology and Genetics (RFMEFI62119X0023).
Funding
This article was supported by the Russian Science Foundation (project no. 22-15-00028). Animal maintenance was provided by the Budget Project FWNR-2022-0023.
Author information
Authors and Affiliations
Contributions
V. S. Naumenko, N. K. Popova, and E. M. Kondaurova developed the study concept and supervised the study; E. A. Kulikova, T. V. Ilchibaeva, V. S. Naumenko, and I. I. Belokopytova conducted the experiments; V. S. Naumenko and E. M. Kondaurova discussed the results; I. I. Belokopytova and V. S. Naumenko wrote the article; V. S. Naumenko, N. K. Popova, and E. M. Kondaurova edited the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. All procedures with experimental animals were conducted in compliance with the international protocols on the treatment of laboratory animals (Directive 2010/63/EU EC) and the Order of the Ministry of Health of the Russian Federation from 01.04.2016 no. 199n “On the establishment of Rules for appropriate laboratory practice” (registered 15.08.2016, no. 43232).
Rights and permissions
About this article
Cite this article
Belokopytova, I.I., Kondaurova, E.M., Kulikova, E.A. et al. Effects of the Cc2d1a/Freud-1 Knockdown in the Hippocampus of BTBR Mice on the Autistic-Like Behavior, Expression of Serotonin 5-HT1A and D2 Dopamine Receptors, and CREB and NF-kB Intracellular Signaling. Biochemistry Moscow 87, 1206–1218 (2022). https://doi.org/10.1134/S0006297922100145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922100145