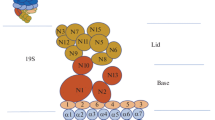
Abstract
Recent proteomic profiling of mouse brain preparations using the ubiquitin receptor, Rpn10 proteasome subunit, as an affinity ligand revealed a representative group of proteins bound to this sorbent (Medvedev, A. E., et al. (2017) Biochemistry (Moscow), 82, 330-339). In the present study, we investigated interaction of the Rpn10 subunit of proteasomes with some of these identified proteins: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase, and histones H2A and H2B. The study revealed: (i) quantitative affinity interaction of the proteasome subunit immobilized on a Biacore-3000 optical biosensor cuvette with both the GAPDH (K d = 2.4·10–6 M) and pyruvate kinase (K d = 2.8·10–5 M); (ii) quantitative high-affinity interaction of immobilized histones H2A and H2B with the Rpn10 subunit (Kd values of 6.5·10–8 and 3.2·10–9 M, respectively). Mass spectrometric analysis revealed the presence of the ubiquitin signature (GG) only in a highly purified preparation of GAPDH. We suggest that binding (especially high-affinity binding) of non-ubiquitinated proteins to the Rpn10 proteasome subunit can both regulate the functioning of this proteasomal ubiquitin receptor (by competing with ubiquitinated substrates) and promote activation of other pathways for proteolytic degradation of proteins destined to the proteasome.
Similar content being viewed by others
Abbreviations
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- K d :
-
equilibrium dissociation constant
References
Hershko, A., and Ciechanover, A. (1998) The ubiquitin system, Annu. Rev. Biochem., 67, 425–479.
Hershko, A., Ciechanover, A., and Varshavsky, A. (2000) The ubiquitin system, Nature Med., 6, 1073–1081.
Schwartz, A. L., and Ciechanover, A. (2009) Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology, Annu. Rev. Pharmacol. Toxicol., 49, 73–96.
Kravtsova-Ivantsiv, Y., and Ciechanover, A. (2012) Noncanonical ubiquitin-based signals for proteasomal degradation, J. Cell Sci., 125, 539–548.
Buneeva, O. A., and Medvedev, A. E. (2016) The role of atypical ubiquitination in cell regulation, Biomed. Khim., 62, 496–509.
Deveraux, Q., Ustrell, V., Pickart, C., and Rechsteiner, M. (1994) A 26S protease subunit that binds ubiquitin conjugates, J. Biol. Chem., 269, 7059–7061.
Hamazaki, J., Sasaki, K., Kawahara, H., Hisanaga, S., Tanaka, K., and Murata, S. (2007) Rpn10-mediated degradation of ubiquitinated proteins is essential for mouse development, Mol. Cell. Biol., 19, 6629–6638.
Medvedev, A. E., Buneeva, O. A., Kopylov, A. T., Tikhonova, O. V., Medvedeva, M. V., Nerobkova, L. N., Kapitsa, I. G., and Zgoda, V. G. (2017) Brain mitochondrial subproteome of Rpn10-binding proteins and its changes induced by the neurotoxin MPTP and the neuroprotector isatin, Biochemistry (Moscow), 82, 330–339.
Ivanov, A., Medvedev, A., Ershov, P., Molnar, A., Mezentsev, Y., Yablokov, E., Kaluzhsky, L., Gnedenko, O., Buneeva, O., Haidukevich, I., Sergeev, G., Lushchyk, A., Yantsevich, A., Medvedeva, M., Kozin, S., Popov, I., Novikova, S., Zgoda, V., Gilep, A., Usanov, S., Lisitsa, A., and Archakov, A. (2014) Protein interactomics based on direct molecular fishing on paramagnetic particles: practical realization and further SPR validation, Proteomics, 14, 2261–2274.
Buneeva, O., Gnedenko, O., Zgoda, V., Kopylov, A., Glover, V., Ivanov, A., Medvedev, A., and Archakov, A. (2010) Isatin binding proteins of rat and mouse brain: proteomic identification and optical biosensor validation, Proteomics, 10, 23–37.
Medvedev, A. E., Buneeva, O. A., Kopylov, A. T., Gnedenko, O. V., Medvedeva, M. V., Kozin, S. A., Ivanov, A. S., Zgoda, V. G., and Makarov, A. A. (2015) The effects of an endogenous non-peptide molecule isatin and hydrogen peroxide on proteomic profiling of rat brain amyloidbeta binding proteins: relevance to Alzheimer’s disease? Int. J. Mol. Sci., 16, 476–495.
Scopes, R. K., and Stoter, A. (1982) Purification of all glycolytic enzymes from one muscle extract, Methods Enzymol., 90 (Pt. E), 479–490.
Wang, H., Walsh, S. T. R., and Parthun, M. R. (2008) Expanded binding specificity of the human histone chaperone NASP, Nucleic Acids Res., 36, 5763–5772.
Blumenfeld, N., Gonen, H., Mayer, A., Smith, C. E., Siegel, N. R., Schwartz, A. L., and Ciechanover, A. (1994) Purification and characterization of a novel species of ubiquitin-carrier protein, E2, that is involved in degradation of non-“N-end rule” protein substrates, J. Biol. Chem., 269, 9574–9581.
Jeon, H. B., Choi, E. S., Yoon, J. H., Hwang, J. H., Chang, J. W., Lee, E. K., Choi, H. W., Park, Z. Y., and Yoo, Y. J. (2007) A proteomics approach to identify the ubiquitinated proteins in mouse heart, Biochem. Biophys. Res. Commun., 357, 731–736.
Schrader, E. K., Harstad, K. G., and Matouschek, A. (2009) Targeting proteins for degradation, Nat. Chem. Biol., 10, 815–822.
Elsasser, S., Chandler-Militello, D., Muller, B., Hanna, J., and Finley, D. (2004) Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome, J. Biol. Chem., 279, 26817–26822.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2017, Vol. 82, No. 9, pp. 1338-1344.
Rights and permissions
About this article
Cite this article
Buneeva, O.A., Gnedenko, O.V., Kopylov, A.T. et al. Quantitative affinity interaction of ubiquitinated and non-ubiquitinated proteins with proteasome subunit Rpn10. Biochemistry Moscow 82, 1042–1047 (2017). https://doi.org/10.1134/S0006297917090073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917090073