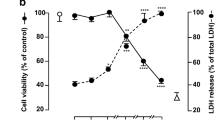
Abstract
Dipeptide carnosine (β-alanyl-L-histidine) is a natural antioxidant, but its protective effect under oxidative stress induced by neurotoxins is studied insufficiently. In this work, we show the neuroprotective effect of carnosine in primary cultures of rat cerebellar cells under oxidative stress induced by 1 mM 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH), which directly generates free radicals both in the medium and in the cells, and 20 nM rotenone, which increases the amount of intracellular reactive oxygen species (ROS). In both models, adding 2 mM carnosine to the incubation medium decreased cell death calculated using fluorescence microscopy and enhanced cell viability estimated by the MTT assay. The antioxidant effect of carnosine inside cultured cells was demonstrated using the fluorescence probe dichlorofluorescein. Carnosine reduced by half the increase in the number of ROS in neurons induced by 20 nM rotenone. Using iron-induced chemiluminescence, we showed that preincubation of primary neuronal cultures with 2 mM carnosine prevents the decrease in endogenous antioxidant potential of cells induced by 1 mM AAPH and 20 nM rotenone. Using liquid chromatographymass spectrometry, we showed that a 10-min incubation of neuronal cultures with 2 mM carnosine leads to a 14.5-fold increase in carnosine content in cell lysates. Thus, carnosine is able to penetrate neurons and exerts an antioxidant effect. Western blot analysis revealed the presence of the peptide transporter PEPT2 in rat cerebellar cells, which suggests the possibility of carnosine transport into the cells. At the same time, Western blot analysis showed no carnosine-induced changes in the level of apoptosis regulating proteins of the Bcl-2 family and in the phosphorylation of MAP kinases, which suggests that carnosine could have minimal or no side effects on proliferation and apoptosis control systems in normal cells.
Similar content being viewed by others
Abbreviations
- AAPH:
-
2,2'-azobis(2-amidinopropane)dihy-drochloride carnosine, ß-alanyl-L-histidine
- CNS:
-
central nervous system
- DCF:
-
dichlorofluorescein
- MAPK:
-
mitogen-activated protein kinases
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTT:
-
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- PEPT1 and PEPT2:
-
peptide transporters 1 and 2
- ROS:
-
reactive oxygen species
- rotenone:
-
(2R,6aS,12aS)-1,2,6,6a,12,12a-hexahydro-2-iso-propenyl-8,9-dimethoxychromeno[3,4-b]furo(2,3-h)chromen-6-one
References
Warner, D. S., Sheng, H., and Batinic-Haberle, I. (2004) Oxidants, antioxidants and the ischemic brain, J. Exp. Biol., 207, 3221–3231.
Zuo, L., and Motherwell, M. S. (2013) The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease, Gene, 532, 18–23.
Boldyrev, A., Bulygina, E., and Makhro, A. (2004) Glutamate receptors modulate oxidative stress in neuronal cells, a mini-review, Neurotox. Res., 6, 581–587.
Boldyrev, A. A., and Kukley, M. L. (1996) Free radicals in normal and ischemic brain, Neirokhimiya, 13, 271–278.
Illarioshkin, S. N. (2003) Conformational Diseases of Brain [in Russian], Janus-K, Moscow.
Lu, Y. M., Yin, H. Z., Chiang, J., and Weiss, J. H. (1996) Ca2+-permeable AMPA/kainate and NMDA channels: high rate of Ca2+ influx underlies potent induction of injury, J. Neurosci., 16, 5457–5465.
Nicholls, D. G., and Scott, I. D. (1980) The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membranepotential-dependent calcium-ion efflux mechanisms, Biochem. J., 186, 833–839.
Mark, L. P., Prost, R. W., Ulmer, J. L., Smith, M. M., Daniels, D. L., Strottmann, J. M., Brown, W. D., and Hacein-Bey, L. (2001) Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging, Am. J. Neuroradiol., 22, 1813–1824.
Rafalowska, J. (2002) Experimental and human ischemia: is the penumbra present in human ischaemic stroke? Folia Neuropathol., 40, 211–217.
Rajendran, P., Nandakumar, N., Rengarajan, T., Palaniswami, R., Gnanadhas, E. N., Lakshminarasaiah, U., Gopas, J., and Nishigaki, I. (2014) Antioxidants and human diseases, Clin. Chim. Acta, 436, 332–347.
Boldyrev, A. A., Stvolinsky, S. L., and Fedorova, T. N. (2007) Carnosine: endogenous physiological corrector of antioxidative system activity, Usp. Fiziol. Nauk, 38, 57–71.
Boldyrev, A., and Abe, H. (1999) Metabolic transformation of neuropeptide carnosine modifies its biological activity, Cell. Mol. Neurobiol., 19, 163–175.
Boldyrev, A. A. (2012) Carnosine: new concept for the function of an old molecule, Biochemistry (Moscow), 77, 313–326.
Boldyrev, A. A., Stvolinsky, S. L., Fedorova, T. N., and Suslina, Z. A. (2010) Carnosine as a natural antioxidant and geroprotector: from molecular mechanisms to clinical trials, Rejuv. Res., 13, 156–158.
Dobrota, D., Fedorova, T. N., Stepanova, M. S., Babusikova, E., Statelova, D., Tatarkova, Z., Stvolinsky, S. S., and Boldyrev, A. A. (2010) Oxidative stress induced in rat brain by a combination of 3-nitropropionic acid and global ischemia, Int. J. Clin. Exp. Med., 3, 144–151.
Afshin-Majd, S., Khalili, M., Roghani, M., Mehranmehr, N., and Baluchnejadmojarad, T. (2015) Carnosine exerts neuroprotective effect against 6-hydroxydopamine toxicity in hemiparkinsonian rat, Mol. Neurobiol., 51, 1064–1070.
Fedorova, T. N., Stvolinsky, S. L., Bagyeva, G. H., Ivanova-Smolenskaia, I. A., and Illarioshkin, S. N. (2005) Neurodegenerative alterations induced by MPTP neurotoxin in senescence accelerated mice essay, Usp. Fiziol. Nauk, 36, 94–101.
Pavlov, A. R., Revina, A. A., Dupin, A. M., Boldyrev, A. A., and Iaropolov, A. I. (1990) Interactions of carnosine and superoxide radicals in aqueous solutions, Byul. Eksp. Biol. Med., 10, 391–393.
Boldyrev, A. A., Kurella, E. G., Rubtsov, A. M., Tiulina, O. V., Shara, M., and Shentiurts, M. (1992) Direct measurement of the interaction of carnosine and its analogs with free radicals, Biokhimiya, 57, 1360–1365.
Gorbunov, N. V., and Erin, A. N. (1991) Mechanism of antioxidant action of carnosine, Byul. Eksp. Biol. Med., 111, 477–478.
Tamba, M., and Torreggiani, A. (1998) A pulse radiolysis study of carnosine in aqueous solution, Int. J. Radiat. Biol., 74, 333–340.
Boldyrev, A. A., Johnson, P., Wei, Y., Tan, Y., and Carpenter, D. O. (1999) Carnosine and taurine protect rat cerebellar granular cells from free radical damage, Neurosci. Lett., 263, 169–172.
Xiang, J., Hu, Y., Smith, D. E., and Keep, R. F. (2006) PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes, Brain Res., 1122, 18–23.
Sofic, E., Sapcanin, A., Tahirovic, I., Gavrankapetanovic, I., Jellinger, K., Reynolds, G. P., Tatschner, T., and Riederer, P. (2006) Antioxidant capacity in postmortem brain tissues of Parkinson’s and Alzheimer’s diseases, J. Neural Transm. Suppl., 39–43.
Lakshminarayana, R., Aruna, G., Sathisha, U. V., Dharmesh, S. M., and Baskaran, V. (2013) Structural elucidation of possible lutein oxidation products mediated through peroxyl radical inducer 2,2'-azobis(2-methylpropionamidine)dihydrochloride: antioxidant and cytotoxic influence of oxidized lutein in HeLa cells, Chem. Biol. Interact., 203, 448–455.
Li, N., Ragheb, K., Lawler, G., Sturgis, J., Rajwa, B., Melendez, J. A., and Robinson, J. P. (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production, J. Biol. Chem., 278, 8516–8525.
Gao, H. M., Liu, B., and Hong, J. S. (2003) Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons, J. Neurosci., 23, 6181–6187.
Voronkov, D. N., Dikalova, Y. V., Khudoerkov, R. M., and Yamshikova, N. G. (2013) Brain nigrostriatal system changes in rotenone-induced parkinsonism, Ann. Clin. Exp. Neurol., 7, 34–39.
LeBel, C. P., Ischiropoulos, H., and Bondy, S. C. (1992) Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress, Chem. Res. Toxicol., 5, 227–231.
Vladimirov, Y. A. (1996) Studies of antioxidant activity by measuring chemiluminescence kinetics, Proc. Int. Symp. on Natural Antioxidants, AOCS Publishing, pp. 125–144.
Sariev, A. K., Abaimov, D. A., Tankevich, M. V., Pantyukhova, E. Y., Prokhorov, D. I., Fedorova, T. N., Lopachev, A. V., Stvolinskii, S. L., Konovalova, E. V., and Seifulla, R. D. (2015) Experimental study of the basic pharmacokinetic characteristics of dipeptide carnosine and its efficiency of penetration into brain tissues, Eksp. Klin. Farmakol., 78, 30–35.
Kramer, D., and Minichiello, L. (2010) Cell culture of primary cerebellar granule cells, Methods Mol. Biol., 633, 233–239.
O’Dowd, J. J., Cairns, M. T., Trainor, M., Robins, D. J., and Miller, D. J. (1990) Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain, J. Neurochem., 55, 446–452.
Margolis, F. L. (1974) Carnosine in the primary olfactory pathway, Science, 184, 909–911.
Fedorova, T. N. (2003) Application of chemiluminescent analysis for comparative assessment of antioxidant activity of some pharmacological compounds, Eksp. Klin. Farmakol., 66, 56–58.
Beliaev, M. S. (2008) Carnosine as a Factor for Endoecological Protection of the Body from Damage Caused by Oxidative Stress: PhD thesis [in Russian], RUDN University, Moscow.
Konovalova, E. V., Fedorova, T. N., Makletsova, M. G., and Berezov, T. T. (2013) Protective effect of carnosine under acrolein toxicity in PC-12 cells, Vopr. Biol. Med. Farm. Khim., 6, 43–48.
Fujita, T., Kishida, T., Wada, M., Okada, N., Yamamoto, A., Leibach, F. H., and Ganapathy, V. (2004) Functional characterization of brain peptide transporter in rat cerebral cortex: identification of the high-affinity type H+/peptide transporter PEPT2, Brain Res., 997, 52–61.
Wolf, J. P., Bouhaddi, M., Louisy, F., Mikehiev, A., Mourot, L., Cappelle, S., Vuillier, F., Andre, P., Rumbach, L., and Regnard, J. (2006) Side-effects of L-DOPA on venous tone in Parkinson’s disease: a leg-weighing assessment, Clin. Sci. (London), 110, 369–377.
Sayin, V. I., Ibrahim, M. X., Larsson, E., Nilsson, J. A., Lindahl, P., and Bergo, M. O. (2014) Antioxidants accelerate lung cancer progression in mice, Sci. Transl. Med., 6, 221a15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2016, Vol. 81, No. 5, pp. 678-689.
Rights and permissions
About this article
Cite this article
Lopachev, A.V., Lopacheva, O.M., Abaimov, D.A. et al. Neuroprotective effect of carnosine on primary culture of rat cerebellar cells under oxidative stress. Biochemistry Moscow 81, 511–520 (2016). https://doi.org/10.1134/S0006297916050084
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916050084