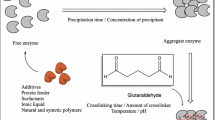
Abstract
A new technique has been developed for the synthesis of cross-linked catalase aggregates by treatment of the enzyme incorporated into the pores of vaterite microspheres with glutaraldehyde and subsequent dissolving of the inorganic matrix. The resulting aggregates have a spherical shape, a narrow particle size distribution, and a high specific activity. The number and enzyme activity of the cross-linked aggregates strongly depends on the rate of the matrix dissolution: mild conditions of dissolution made it possible to increase the number of formed protein particles, whose residual catalase specific activity was only 2 times less than that of the native enzyme. The storage stability of the cross-linked aggregates is comparable to that of the native enzyme of the same concentration.









Similar content being viewed by others
REFERENCES
Brown, G.D., Chemically aggregated enzymes, Methods Enzymol., 1976, vol. 44, pp. 263–280. https://doi.org/10.1016/s0076-6879(76)44022-3
Gupta, M.N., Application of cross-linking techniques to enzyme/protein stabilization and bioconjugate preparation, in Biocatalyst Design for Stability and Specificity, ACS Symp. Ser. Am. Chem. Soc., 1993, pp. 307–324.
Wilson, L., Illanes, A., Soler, L., and Henriquez, M.J., Effect of the degree of cross-linking on the properties of different CLEAs of penicillin acylase, Process Biochem., 2009, vol. 44, pp. 322–326. https://doi.org/10.1016/j.procbio.2008.11.010
Li, X.D., Wu, J., Jia, D.Ch., et al., Preparation of crosslinked glucoamylase aggregates immobilization by using dextrin and xanthan gum as protecting agents, Catalysts, 2016, vol. 6, no. 6, p. 77. https://doi.org/10.3390/catal6060077
Guajardo, N., Ahumada, K., de Domınguez, M.P., and Schrebler, R.A., Remarkable stability of Candida antarctica lipase B immobilized via cross-linking aggregates (CLEA) in deep eutectic solvents, Biocatal. Biotransform., 2019, vol. 37, no. 2, pp. 106–114. https://doi.org/10.1080/10242422.2018.1492567
Wahab, M., Enshasy, H., AbuBakar F., et al., Improvement of cross-linking and stability on cross-linked enzyme aggregate (CLEA)—xylanase by protein surface engineering, Process Biochem., 2019, vol. 86, pp. 40–49. https://www.x-mol.com/paperRedirect/5906292
Illanes, L., Wilson, C., Altamirano, Z., et al., Production of cephalexin in organic medium at high substrateconcentrations with CLEA of penicillin acylase and PGA-450A, Enzyme Microb. Technol., 2007, vol. 40, pp. 195–203. https://doi.org/10.1016/j.enzmictec.2006.03.041
Primozic, M., Kravanja, G., Knez, Z., et al., Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio) degradation, J. Clean. Prod., 2020, vol. 275, article ID 124121. https://doi.org/10.1016/j.jclepro.2020.124121
Guimaraes, J.R., Miranda, L.P., Fernandez-Lafuente, R., and Tardioli, P.W., Immobilization of Eversa® transform via CLEA technology converts it in a suitable biocatalyst for biolubricant production using waste cooking oil, Molecules, 2021, vol. 26, no. 1, p. 193. https://doi.org/10.3390/molecules26010193
Schoevaart, R., Wolbers, M.W., Golubovic, M., et al., Preparation, optimization and structures of cross-linked enzyme aggregates (CLEAs), Biotechnol. Bioeng., 2004, vol. 87, pp. 754–762. https://doi.org/10.1002/bit.2018
Sheldon, R.A., Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts, Biochem. Soc. Trans., 2007, vol. 35, pp. 1583–1587. https://doi.org/10.1042/BST0351583
Sheldon, R.A., Cross-linked enzyme aggregates as industrial biocatalysts, Org. Process Res. Dev., 2011, vol. 15, pp. 213–223. https://doi.org/10.1021/op100289f
Sheldon, R.A., CLEAs, combi-CLEAs and 'smart' magnetic CLEAs: biocatalysis in a bio-based economy, Catalysts, 2019, vol. 9, no. 3, p. 261. https://doi.org/10.3390/catal9030261
Talecar, S., Joshi, A., Joshi, G., et al., Parameters in preparation and characterization of cross-linked enzyme aggregates (CLEAs), RCS Adv., 2013, vol. 3, pp. 12485–12511. https://doi.org/10.1039/C3RA40818C
Roy, I., Mukherjee, J., and Gupta, M.N., Cross-linked enzyme aggregates for applications in aqueous and nonaqueous media, in Enzyme Stabilization and Immobilization, Minteer, S.D., Ed., Humana Press, 2017, pp. 109–123.
Tai, C.Y. and Chen, F.B., Polymorphism of CaCO3 precipitated in a constant-composition environment, AIChE J., 1998, vol. 44, pp. 1790–1798. https://doi.org/10.1002/aic.690440810
Volodkin, D.V., Petrov, A.I., Prevot, M., and Sukhorukov, G.B., Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation, Langmuir, 2004, vol. 20, pp. 3398–3406.
Feoktistova, N., Rose, J., Prokopovich, V.Z., et al., Controlling the vaterite CaCO3 crystal pores. Design of tailor-made polymer based microcapsules by hard templating, Langmuir, 2016, vol. 32, no. 17, pp. 4229–4238. https://doi.org/10.1021/acs.langmuir.6b00717
Balabushevich, N.G., Lopez de Guerenu A., Feoktistova N.A., et al. Protein-containing multilayer capsules by templating on mesoporous CaCO3 particles: POST and PRE-loading approaches, Macromol. Biosci., 2015, pp. 2523–2530. https://doi.org/10.1002/mabi.201500243
Binevski, P.V., Balabushevich, N.G., Uvarova, V.I., et al., Biofriendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology, Colloids Surf., 2019, vol. 181, pp. 437–449. https://doi.org/10.1016/j.colsurfb.2019.05.077
Feoktistova, N.A., Vikulina, A.S., Balabushevich, N.G., et al., Bioactivity of catalase loaded into vaterite CaCO3 crystals via adsorption and co-synthesis, Mater. Des., 2020, vol. 185, article ID 108223. https://doi.org/10.1016/j.matdes.2019.108223
Lee, Ch.H., Lee, H.S., Lee, J.W., et al., Evaluating enzyme stabilizations in calcium carbonate: comparing in situ and crosslinking mediated immobilization, Int. J. Biol. Macromol., 2021, vol. 175, pp. 341–350. https://doi.org/10.1016/j.ijbiomac.2021.02.028
Feoktistova, N.G., Stoychev, N., Puretskiy, N., et al., Porous thermo-responsive pNIPAM microgels, Eur. Polym. J., 2015, vol. 68, pp. 650–656. https://doi.org/10.1016/j.eurpolymj.2015.03.040
Schmidt, S., Behra, M., Uhlig, K., et al., Mesoporous protein particles through colloidal CaCO3 templates, Adv. Funct. Mater., 2013, vol. 23, pp. 116–123. https://doi.org/10.1002/adfm.201201321
Beers, R.F. and Sizer, I.W., A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase, J. Biol. Chem., 1954, vol. 195, no. 1, pp. 133–140. https://doi.org/10.1016/0009-8981(96)06374-7
Tukel, S.S., Hurrem, F., Yildirim, D., and Alptekin, O., Preparation of crosslinked enzyme aggregates (CLEA) of catalase and its characterization, J. Mol. Cat. B: Enzym., 2013, vol. 97, pp. 252–257. https://doi.org/10.5151/chemeng-cobeq2014-1205-20483-160850
ACKNOWLEDGMENTS
The authors are grateful to the staff of Moscow State University A.N. Prusov for help in obtaining TEM images and A.A. Tatarintseva for assistance in obtaining SEM images.
Funding
This work was financially supported by the State Registration Topic AAAA-A21-121011290089-4. The equipment was purchased within the framework of the Moscow State University Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors outside the scope of people’s normal professional activities.
Additional information
Translated by I. Gordon
Abbreviations: Cat, catalase co-precipitated with vaterite after dissolution of the carbonate matrix; Cat–CLEAs, cross-linked catalase aggregates; CC, vaterite microspheres; Cat–GA, catalase co-precipitated with vaterite microspheres and treated with glutaraldehyde after dissolution of the carbonate matrix; CC–Cat, vaterite microspheres co-precipitated with catalase; CC–Cat–GA, vaterite microspheres co-precipitated with catalase and treated with glutaraldehyde; EDTA, ethylenediaminetetraacetic acid; GA, glutaraldehyde; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Rights and permissions
About this article
Cite this article
Tagirova, M.A., Eremeev, N.L., Balabushevich, N.G. et al. Preparation of Catalase Cross-Linked Aggregates Based on Vaterite Matrix. Appl Biochem Microbiol 58, 923–931 (2022). https://doi.org/10.1134/S0003683822080075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822080075