-
PDF
- Split View
-
Views
-
Cite
Cite
Marta Palusinska-Szysz, Agnieszka Zdybicka-Barabas, Małgorzata Cytryńska, Sylwia Wdowiak-Wróbel, Elżbieta Chmiel, Wiesław I. Gruszecki, Analysis of cell surface alterations in Legionella pneumophila cells treated with human apolipoprotein E, Pathogens and Disease, Volume 73, Issue 2, March 2015, Pages 1–8, https://doi.org/10.1111/2049-632X.12214
Close - Share Icon Share
Binding of human apolipoprotein E (apoE) to Legionella pneumophila lipopolysaccharide was analysed at the molecular level by Fourier-transform infrared spectroscopy, thereby providing biophysical evidence for apoE-L. pneumophila lipopolysaccharide interaction. Atomic force microscopy imaging of apoE-exposed L. pneumophila cells revealed alterations in the bacterial cell surface topography and nanomechanical properties in comparison with control bacteria. The changes induced by apoE binding to lipopolysaccharide on the surface of L. pneumophila cells may participate in: (1) impeding the penetration of host cells by the bacteria; (2) suppression of pathogen intracellular growth and eventually; and (3) inhibition of the development of infection.
INTRODUCTION
Legionella pneumophila is a Gram-negative pathogenic bacterium residing in both natural and man-made water systems. After inhalation of a water aerosol containing L. pneumophila, the bacteria enter the human lungs to infect alveolar macrophages, which may lead to development of atypical pneumonia known as Legionnaires’ disease. Phagocytosis of L. pneumophila by the host macrophages results in modulation thereof, which enables bacteria to reside within a nascent phagosome by inhibition of phagosome–lysosome fusion (Abu Kwaik et al., 1993). Colonization of human respiratory cells is mediated by specific molecules located on the bacterial cell surface. A number of observations link L. pneumophila virulence to its lipopolysaccharide, a major component of the bacterial outer membrane. Interestingly, c. 80% of L. pneumophila clinical isolates belong to serotype 1 characterized by highly hydrophobic lipopolysaccharide, whose chemical composition and biological activity differs substantially from the endotoxin of other Gram-negative bacteria (Palusinska-Szysz and Russa, 2009).
Human apolipoprotein E (apoE) is a multifunctional 34 kDa glycoprotein that plays a key role as a modulator of lipid metabolism both in the plasma and in the central nervous system. The protein is synthesized and secreted by many cell types including hepatocytes, smooth muscle cells, neuronal cells and macrophages. ApoE exists in three isoforms, designated apoE2, apoE3 and apoE4. Among them, apoE4 is a major genetic risk factor of Alzheimer's disease. Furthermore, disturbances in the apoE level pose a risk of cardiovascular problems, neurodegenerative disorders such as Parkinson's disease and autoimmune disorders, for example multiple sclerosis and psoriasis (Corder et al., 1993; Mahley and Rall 2000; Kockx et al., 2008; Zhang et al., 2011).
Besides its well-established role in lipid transport, apoE exhibits immunomodulatory properties and contributes to host immunity against pathogens. Laskowitz et al. (2000) reported impaired delayed-type hypersensitivity responses and generation of higher levels of antigen-specific IgM after immunization with tetanus toxoid in apoE-deficient mice. Infection of apoE-deficient mice with Listeria monocytogenes or Klebsiella pneumoniae resulted in elevated serum levels of proinflammatory cytokines, such as tumour necrosis factor α (TNF-α), and in a decreased survival rate in comparison with the wild-type animals (Roselaar and Daugherty, 1998; De Bont et al., 1999, 2000). Moreover, apoE-deficient mice were markedly more susceptible to intracellular pathogen Mycobacterium tuberculosis, which was evidenced by 100% mortality within 4 weeks of infection (Martens et al., 2008). In addition, it has been demonstrated that apoE bound Salmonella minnesota lipopolysaccharide and redirected it from macrophages to hepatocytes in vivo, thereby attenuating the host inflammatory response and protecting against lipopolysaccharide-induced mortality (Rensen et al., 1997; Van Oosten et al., 2001).
Apolipophorin III (apoLp-III), an apoE insect homologue, is an abundant haemolymph protein involved in lipid transport and immune response in insects, that is the roles played by apoE in humans. Similar to apoE, apoLp-III can bind molecules classified as pathogen associated molecular patterns, for example lipopolysaccharide (Weers and Ryan 2006; Oztug et al., 2012; Zdybicka-Barabas and Cytryńska 2013). In our previous papers, we demonstrated that Galleria mellonella apoLp-III inhibited growth of Gram-negative bacteria, including Legionella dumoffii and Legionella gormanii (Zdybicka-Barabas and Cytryńska 2011; Zdybicka-Barabas et al., 2011; Palusinska-Szysz et al., 2012; Chmiel et al., 2014). Moreover, our investigations also indicated high susceptibility of L. pneumophila to apoLp-III (Zdybicka-Barabas et al., 2014). These findings and the above mentioned literature data prompted us to conduct research on a possible role of apoE in human immunity against L. pneumophila.
To get deeper insight into molecular mechanisms associated with interaction of apoE with Legionella cells, two modern biophysical techniques were employed: atomic force microscopy (AFM) and Fourier-transform infrared absorption spectroscopy (FTIR). AFM is a surface imaging scanning probe microscopy technique, characterized by a submolecular resolution, based on interaction between a super-sharp tip (radius of curvature in the order of nanometers) and the sample surface. AFM has been increasingly applied to investigate the morphology and ultrastructure of cell surface of Gram-negative bacteria (e.g. Escherichia coli,K. pneumoniae,Pseudomonas aeruginosa and Salmonella typhimurium) and Gram-positive bacteria (e.g. Bacillus cereus,Bacillus circulans,Micrococcus luteus,Staphylococcus aureus and Streptococcus pyogenes) as well as the biological effects of various compounds like antibiotics and antimicrobial peptides on bacterial cells (Li et al., 2010; Liu and Wang 2010; Scheuring and Dufrêne 2010; Zdybicka-Barabas et al., 2011; Cui et al., 2012). Recently, using AFM imaging and analysis, we have demonstrated alterations of L. pneumophila and L. gormanii cell surface after exposure to G. mellonella apoLp-III (Chmiel et al., 2014; Zdybicka-Barabas et al., 2014).
The FTIR technique facilitates recording of infrared absorption spectra of samples containing molecules as well as entire biological organisms, such as bacteria. IR spectra reflect vibrations of chemical bonds and specific groups of molecules, and therefore, they are very sensitive to all kinds of molecular interactions, in particular formation of hydrogen bonds. Such a feature makes the FTIR technique a powerful tool in examination of the molecular mechanisms of the interactions. This technique also provides spectral fingerprints of complex biological structures and can be used to differentiate and identify microorganisms rapidly (Naumann 2000; Barth 2007; Beekes et al., 2007).
In this report, we present an analysis of binding of human apoE to L. pneumophila lipopolysaccharide at the molecular level performed by FTIR spectroscopy, thus providing biophysical evidence for an apoE-lipopolysaccharide interaction. The consequence of this interaction was changes in L. pneumophila cell surface topography and nanomechanical properties which were imaged and analysed using AFM.
MATERIALS AND METHODS
Bacteria and growth conditions
Legionella pneumophila strain ATCC 33152 (serotype 1) was grown on buffered charcoal yeast agar plates (BCYE; Oxoid, Basingstoke, Hampshire, UK) for 3 days at 37°C in humid atmosphere and 5% CO2. For lipopolysaccharide isolation, the cells were harvested and washed twice with saline and once with distilled water. The final cell lyophilizate mass was c. 800 mg.
Assay of the L. pneumophila survival rate
The effect of apoE on L. pneumophila survival was tested using a colony-counting assay. The bacteria (10 μL of suspension of OD620 nm = 0.1 diluted 2 × 10−5-fold in pyrogen-free water) were incubated for 1 h at 37°C without (control) or in the presence of human apoE4 (Cat. No A3234; Sigma-Aldrich) at the final concentrations 0.1–0.8 mg mL−1. Then, the bacteria were transferred onto BCYE plates, and the number of colony-forming units was determined after 3 days of incubation at 37°C and 5% CO2. The controls defined the total (100%) survival of L. pneumophila cells in the experimental conditions. The results represent the mean of three independent experiments, each performed in triplicate.
Isolation and purification of L. pneumophila lipopolysaccharide
The smooth form of L. pneumophila lipopolysaccharide was isolated employing the phenol/water extraction procedure (Westphal and Jann 1965; Johnson and Perry 1976) as described in detail in our recent paper (Zdybicka-Barabas et al., 2014). First, free lipids and membrane phospholipids were removed according to the Bligh and Dyer method (Bligh and Dyer 1959). After incubation with intensive stirring for 2 h at room temperature, the suspension was centrifuged at 8 000 g for 20 min. The delipidated bacterial mass was washed twice with a freshly prepared single-phase Bligh-Dyer mixture and lyophilized. Then, the lyophilizate was suspended in 50 mM sodium phosphate buffer (pH 7.0) supplemented with 5 mM EDTA and digested with lysozyme (6 mg 1 g−1 dry wt) for 18 h at 4°C. Nucleic acids were degraded with DNase and RNase (1 mg mL−1, 24 h, 37°C) with addition of 5 mM MgCl2, and proteins with proteinase K (0.3 mg 1 mg−1 dry wt, 18 h, 37°C). Next, the suspension was dialysed against distilled water, and the lipopolysaccharide was recovered using the hot 45% phenol/water procedure. The phenol layer containing lipopolysaccharide was dialyzed against tap and distilled water. The crude lipopolysaccharide was purified by repeated ultracentrifugation at 105 000 g, 4 h, 4°C (Beckman Coulter). To remove cations, the lipopolysaccharide (c. 16 mg) was then electrodialysed for 24 h (500 mA, 200 V, 40 W) using Bio-Rad electrophoresis apparatus and lyophilized (Galanos and Lüderitz, 1975). The lipopolysaccharide purity was checked spectrophotometrically at 260 and 280 nm (SmartSpec™ 3000; BioRad). Before further analyses, the lipopolysaccharide suspended in water was sonificated in a water bath at a room temperature for 10 min (Elmasonic S100H, Elma, Germany).
Atomic force microscopy
Forty microliters of L. pneumophila suspension (OD620 nm = 0.2) was incubated without (control) or in the presence of apoE (final concentration 0.4 mg mL−1) at 37°C for 1 h. After centrifugation (8000 g, 10 min, 4°C), the bacteria were prepared on mica discs for imaging as described previously (Zdybicka-Barabas et al., 2011).
The bacterial cell surface was imaged using a NanoScope V AFM (Veeco) (Analytical Laboratory, Faculty of Chemistry, UMCS, Lublin, Poland) in the ‘Peak Force QNM’ operation mode using a silicon tip with a spring constant of 20 N m−1 (NSG30, NT-MDT, Russia). Three fields were imaged on each mica disc. The topography of the examined samples was presented as the height and peak force error images. The DMT (Derjaguin, Muller and Toporov) modulus, adhesion and deformation maps reflected bacterial cell surface stiffness, adhesion forces between the cell surface and a tip and penetration of the tip into the cell surface, respectively. The values of average root mean square (RMS) roughness, DMT modulus, adhesion forces and the deformation rate of the cell surface were calculated from measurements of 60 fields (120 × 120 nm) in 1 × 1 μm images of the bacterial cell surface. The data were analysed with nanoscope analysis ver. 1.40 software (Veeco). The section profiles of the cells were generated using wsxm 5.0 software (Nanotec, Spain; Horcas et al., 2007).
Interaction of human apoE with L. pneumophila lipopolysaccharide
The L. pneumophila lipopolysaccharide (4 and 50 μg for SDS-PAGE and FTIR analysis, respectively) and apoE4 (Cat. No A3234; Sigma-Aldrich) were incubated for 1 h at 37°C alone (controls) and in combination in the ratios 1 : 1 and 5 : 1, respectively, for SDS-PAGE and FTIR analysis. The temperature conditions used were close to the lipopolysaccharide phase transition temperature (37–40°C) according to Oztug et al., 2012.
SDS-polyacrylamide gel electrophoresis
After incubation of L. pneumophila lipopolysaccharide with apoE in the ratio 1 : 1, the samples were prepared in a sample buffer (175 mM Tris-HCl pH 6.8, 0.25% SDS, 2% glycerol, 0.04% bromophenol blue) and analysed by Tris-tricine SDS-PAGE initially at 15 mA and then at 35 mA (Lesse et al., 1990). Then, lipopolysaccharide was visualised by silver staining (Tsai and Frasch 1982).
FTIR spectroscopy
Infrared absorption spectra were recorded with a Vector 33 FTIR absorption spectrometer (Bruker, Germany) equipped with an attenuated total reflection set-up (ATR). The internal reflection element was a ZnSe crystal (45° cut) yielding 10 internal reflections. Typically, 20 scans were collected, Fourier-transformed and averaged for each measurement. Absorption spectra were collected in the region between 4000 and 600 cm−1 at a resolution of one data point every 2 cm−1. The spectrum of a clean crystal was used as the background. The samples were deposited to the ZnSe crystal via partial evaporation from water solutions. The instrument was purged with dry air for 40 min, before and continuously during the measurements. The ATR crystals were cleaned with organic solvents. All the measurements were taken at 21°C. Spectral analysis was performed with OPUS (Bruker, Germany) and grams ai software from Thermo Galactic. The spectra were corrected by subtraction of the water vapour contributions and smoothing procedure (Golay-Savitzky on 21 points). More details of the measurements were presented previously (Gagos et al., 2005).
Statistical analysis
The Student's t-test was used to establish the differences between two mean values. P values of < 0.05 were considered significant.
RESULTS AND DISCUSSION
FTIR analysis of the interaction between human apoE and L. pneumophila lipopolysaccharide
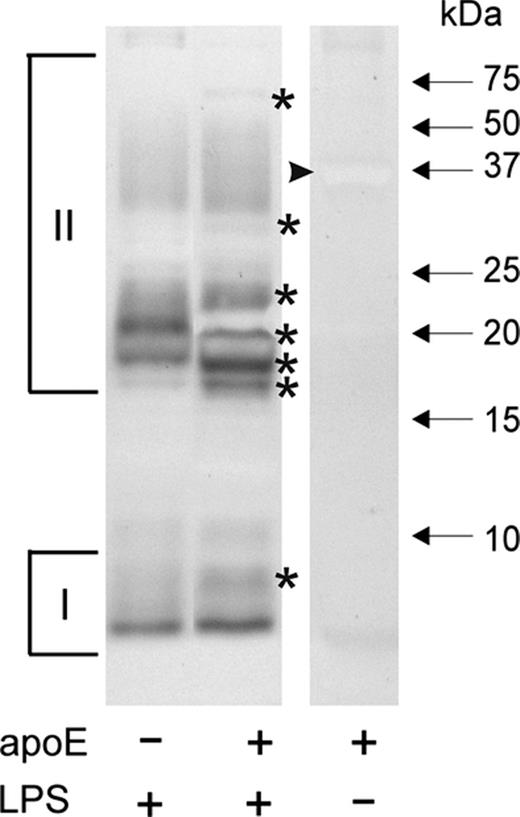
SDS-PAGE analysis of L. pneumophila lipopolysaccharide revealed that incubation with human apoE resulted in changes in its electrophoretic mobility, suggesting strong interaction of apoE with this component of L. pneumophila outer membrane (Fig. 1). A detailed FTIR analysis was performed to provide more insight into the apoE-L. pneumophila lipopolysaccharide interaction.
SDS-PAGE analysis of Legionella pneumophila lipopolysaccharide after incubation with human apoE. Lipopolysaccharide (4 μg) was incubated without (control) and in the presence of apoE in the ratio 1 : 1, separated by Tris-tricine SDS-PAGE, and visualized by silver staining. I and II indicate the fast (low molecular mass) and slow (high molecular mass) migrating lipopolysaccharide fractions, respectively. The bands with changed electrophoretic mobility are marked by asterisks. The arrowhead indicates negatively stained apoE band.
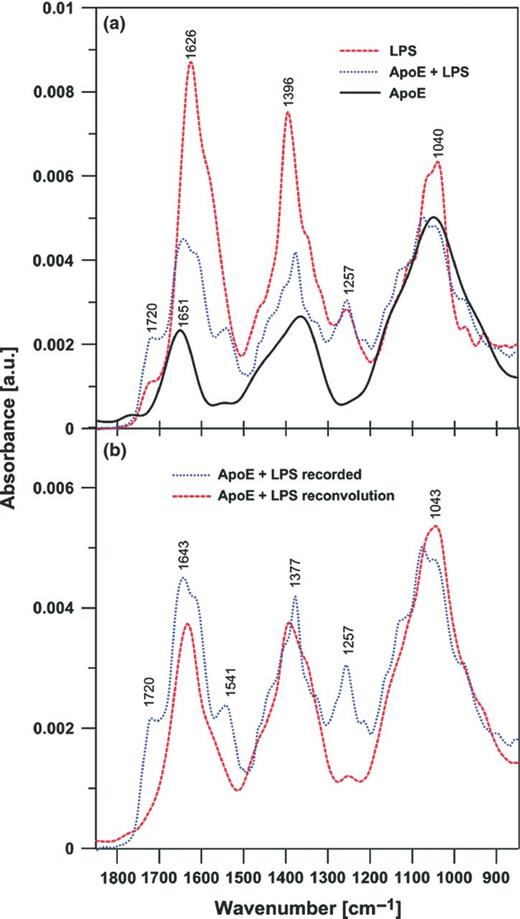
Figure 2a presents the IR absorption spectra of apoE, lipopolysaccharide and the two-component system of apoE + lipopolysaccharide. In the case of apoE, the principal band between 1600 and 1700 cm−1 is referred to as the amide I band. The maximum of this band at 1651 cm−1 corresponds to the α-helical secondary structure dominant in the protein (Tamm and Tatulian 1997). Several relatively intensive IR absorption bands can also be visible in the lipopolysaccharide spectra. The major band centred at 1626 cm−1 can be assigned to the C=O stretching vibrations. Three other spectral bands can be assigned to the deformation vibrations of CH3 groups (1396 cm−1), |${PO^{-}_{2}}$| antisymmetric stretching (1257 cm−1) and stretching of the C–O–P–O fragments of lipopolysaccharide (1040 cm−1). Figure 2b presents comparison of the IR absorption spectra of the apoE + lipopolysaccharide two-component system, recorded and reconvoluted on the basis of the spectra of the pure components (presented in Fig. 2a). The experimental and calculated spectra are in relatively good agreement, except the spectral regions representing the C=O stretching and the |${PO^{-}_{2}}$| stretching vibrations. In the latter case, the band is distinctly more intensive in the mixture, as could be predicted based on the component spectra. The increased oscillator strength reflects involvement of this particular group in the formation of hydrogen bonds between lipopolysaccharide and the protein. The additional band that appears in the experimental spectrum of apoE + lipopolysaccharide at 1541 cm−1 can be assigned to the stretching vibrations of the carbonyl groups of lipopolysaccharide, more intensive than the pure component spectrum and shifted towards lower frequencies from the band visible as a shoulder at 1577 cm−1. Both effects are consistent with the involvement of the C=O groups in hydrogen bonding in the two-component system. An additional pronounced difference between the calculated and experimental spectra of apoE + lipopolysaccharide is the increased intensity of the band at 1720 cm−1, which can be assigned to the ester carbonyl group vibrations in lipopolysaccharide. Such an effect indicates that, upon interaction with the protein environment, those groups are involved in binding via hydrogen bonding.
FTIR spectra of apoE, lipopolysaccharide and the apoE − lipopolysaccharide two-component mixture (a) and a FTIR spectrum of the apoE and lipopolysaccharide mixture recorded and the effect of reconvolution based on the experimental spectra of apoE and lipopolysaccharide (b). In (a), the spectra were normalized by dividing by the area beneath each spectrum in the region between 900 and 1200 cm−1. In (b), the normalized spectra of apoE and lipopolysaccharide (presented in a) have been added after multiplication by the fraction factors (0.7 and 0.3, respectively) to reproduce the band between 900 and 1200 cm−1. Note that such a procedure reproduces the spectral region between 1300 and 1500 cm−1 equally well.
AFM imaging and analysis of L. pneumophila cell surface after treatment with apoE
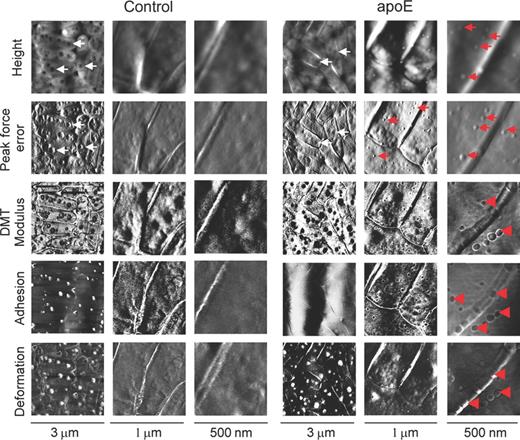
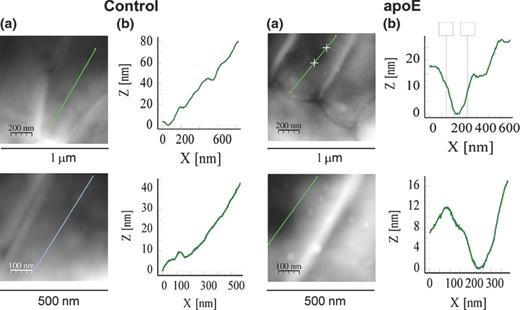
As apoE bound to L. pneumophila lipopolysaccharide, a component forming the outermost layer of the bacterial cell envelope, the effects of the exposure of the bacteria to apoE on their cell surface were investigated. AFM imaging revealed alterations in the cell surface topography and properties caused by the apoE treatment (Figs 3 and 4; Table 1). On the surface of the rod-shaped control cells, regular round areas, probably reflecting intracellular vacuoles, were well visible in all types of images (white arrows in Fig. 3). In contrast, this feature was much less evident in the height and peak force error images of the cells treated with apoE, indicating changes in cell surface characteristics. Moreover, small, clearly demarcated granule-like protuberances appeared on the surface of the apoE-exposed bacteria (red arrows in Fig. 3), although their surface seemed to be smoother in comparison with the control ones (Fig. 3). As presented in Fig. 4, 150–200 nm in diameter and 12–20 nm in depth recesses appeared in the surface of the bacteria exposed to apoE. In addition, the treatment of L. pneumophila with apoE influenced the nanomechanical properties of the bacteria, which was reflected by changes evident in the peak force error, DMT modulus, adhesion and deformation images (Fig. 3) as well as in the calculated values corresponding to cell surface roughness, elasticity and stiffness (Table 1). All the analysed parameters of cell surface of the apoE-treated bacteria were significantly different from the control ones. Their cell surface became considerably less rough and much softer, which was reflected by c. 2.5-fold reduction of the DMT modulus value. Accordingly, these alterations were accompanied by a c. 2.5-fold decrease in adhesion forces and a c. 2.7-fold increase in the deformation value, indicating that the surface was more easily deformed upon the tip action (Table 1).
The effect of human apoE on the nanomechanical properties of the Legionella pneumophila cell surface.
| . | Control cells . | ApoE-treated cells . |
|---|---|---|
| RMS roughness (nm) | 3.126 (± 1.429) | 1.944 (± 0.979)** |
| DMT modulus (GPa) | 16.909 (± 8.1) | 6.675 (± 0.903)*** |
| Adhesion (nN) | 12.673 (± 4.989) | 5.075 (± 1.995)*** |
| Deformation (nm) | 0.228 (± 0.094) | 0.616 (± 0.654)*** |
| . | Control cells . | ApoE-treated cells . |
|---|---|---|
| RMS roughness (nm) | 3.126 (± 1.429) | 1.944 (± 0.979)** |
| DMT modulus (GPa) | 16.909 (± 8.1) | 6.675 (± 0.903)*** |
| Adhesion (nN) | 12.673 (± 4.989) | 5.075 (± 1.995)*** |
| Deformation (nm) | 0.228 (± 0.094) | 0.616 (± 0.654)*** |
The bacteria were incubated without (control) or with apoE (0.4 mg mL−1) and analysed by AFM. The results are presented as ± SD. Statistical significance: **P ≤ 0.01; ***P ≤ 0.001.
The effect of human apoE on the nanomechanical properties of the Legionella pneumophila cell surface.
| . | Control cells . | ApoE-treated cells . |
|---|---|---|
| RMS roughness (nm) | 3.126 (± 1.429) | 1.944 (± 0.979)** |
| DMT modulus (GPa) | 16.909 (± 8.1) | 6.675 (± 0.903)*** |
| Adhesion (nN) | 12.673 (± 4.989) | 5.075 (± 1.995)*** |
| Deformation (nm) | 0.228 (± 0.094) | 0.616 (± 0.654)*** |
| . | Control cells . | ApoE-treated cells . |
|---|---|---|
| RMS roughness (nm) | 3.126 (± 1.429) | 1.944 (± 0.979)** |
| DMT modulus (GPa) | 16.909 (± 8.1) | 6.675 (± 0.903)*** |
| Adhesion (nN) | 12.673 (± 4.989) | 5.075 (± 1.995)*** |
| Deformation (nm) | 0.228 (± 0.094) | 0.616 (± 0.654)*** |
The bacteria were incubated without (control) or with apoE (0.4 mg mL−1) and analysed by AFM. The results are presented as ± SD. Statistical significance: **P ≤ 0.01; ***P ≤ 0.001.
AFM imaging of Legionella pneumophila cells treated with human apoE. The bacteria were incubated without (control) or in the presence of apoE (0.4 mg mL−1) and imaged by AFM. The height, peak force error, adhesion, elasticity (DMT modulus) and deformation images are presented. The brighter and darker areas of the images correspond to the higher and lower values of the parameters, respectively. The round structures reflecting the vacuoles and granule-like protuberances are marked by white and red arrows, respectively. In the DMT modulus, adhesion and deformation maps of the apoE-treated bacteria the red arrowheads indicate separate areas of distinct properties in comparison with the rest of the surface.
Analysis of alterations in the Legionella pneumophila cell surface after treatment with human apoE. The bacteria were incubated without (control) or in the presence of apoE (0.4 mg mL−1) and examined by AFM. The height images (a) and section profiles corresponding to the lines in the height images (b) are presented.
Although the treatment of L. pneumophila cells with human apoE led to remarkable alterations in the bacterial cell surface topography and properties, the bacteria remained viable. ApoE applied at the concentrations 0.1–0.8 mg mL−1 did not reduce the L. pneumophila survival rate in vitro (data not shown). The fact that apoE was unable to kill the bacteria in our in vitro experiments does not exclude a possibility of apoE involvement in human anti-Legionella immunity. It is known that lipopolysaccharide released from the outer membrane of most Gram-negative bacteria into the bloodstream during infection acts as a potent activator of human mononuclear cells and macrophages, leading to local inflammation, which may be beneficial to the host. However, when the amount of the lipopolysaccharide released exceeds a certain threshold, the strong induction of inflammatory cytokines, such as interleukins and TNF-α, results in severe sepsis and septic shock with organ dysfunction, which is responsible for the 30–40% death rate among patients with sepsis, as no specific effective treatment is available (Schletter et al., 1995). Therapeutic strategies that may be used to protect against lipopolysaccharide-induced endotoxemia include cytokines, lipopolysaccharide receptors and antibodies against lipopolysaccharide-binding protein (LBP), as well as recombinant apoE (Van Oosten et al., 2001). The investigated L. pneumophila lipopolysaccharide is a weak inducer of pro-inflammatory cytokines, which is due to a failure of the interaction with the lipopolysaccharide receptor CD14 and with its soluble form (Neumeister et al., 1998). Probably, long-chain fatty acids and high hydrophobicity of L. pneumophila lipid A can abolish the interaction of the lipopolysaccharide with receptor CD14 (Girard et al., 2003). This constitutes one of the mechanisms with lipopolysaccharide involvement through which bacteria evade the host immune system. Although a severe type of pneumonia can result from L. pneumophila replication in host macrophages, most infections are presumably constrained and cleared by the immune system without causing a disease. The presented investigations have shown that L. pneumophila lipopolysaccharide with an unusual structure has an ability to bind human apoE. A consequence of the apoE interaction with lipopolysaccharide on the L. pneumophila cell surface could be alteration to its properties impeding the penetration of the host cells by the bacteria and establishment of infection. It has been reported that pulmonary collectins, hydrophilic surfactant proteins A and D implicated in the regulation of host defence in the lungs, specifically interact with L. pneumophila lipopolysaccharide (Sawada et al., 2010). They promote localization of the bacteria to an acid compartment of lysosomes, thus suppressing intracellular growth of phagocytosed pathogens. Therefore, involvement of other lipopolysaccharide-binding proteins, for example apoE, in suppression of L. pneumophila intracellular growth could be speculated.
In addition to its important role in the interaction with host cells, lipopolysaccharide of L. pneumophila is engaged in modulation of intracellular trafficking independently of the type IV Dot/Icm secretion system, which is also essential for intracellular multiplication of Legionella spp. During the E-phase, replicative noninfective phase growth, and during the PE transmissive growth phase characterized by preferential expression of genes required for virulence, L. pneumophila shed a high-molecular-weight lipopolysaccharide and lipopolysaccharide-rich outer membrane vesicles (OMVs; Seeger et al., 2010). In the E-phase, OMVs are attached to the bacterial cell wall but expel lipopolysaccharide structures, whereas in the PE-phase, the vesicles are profusely released (Helbig et al., 2007). Recently, Jäger et al. (2014) have demonstrated OMV-induced histological damage to living human lung tissue resembling the destructive effects caused by L. pneumophila. It has been proposed that OMVs contribute to L. pneumophila extracellular pathogenicity. Both OMV-bound and unbound lipopolysaccharides can intercalate into the phagosomal membrane leading to inhibition of fusion of phagosomes with lysosomes. Thus, not only phagosome maturation is temporarily arrested but also remodelling of the phagosome surface important for L. pneumophila intracellular replication is promoted (Fernandez-Moreira et al., 2006; Seeger et al., 2010). Interestingly, during the E-phase, L. pneumophila lipopolysaccharide is much more hydrophobic than in the PE-phase (Seeger et al., 2010), which facilitates the interaction with apoE. In the light of the above, binding of apoE with lipopolysaccharide may hinder intracellular growth of L. pneumophila.
Given the involvement of apoE in immune response against extracellular and intracellular pathogenic bacteria (Roselaar and Daugherty 1998; De Bont et al., 1999, 2000; Martens et al., 2008) and the results presented in this work, participation of apoE in the immune response towards Legionella should be considered. Further in vivo studies using, for example apoE-deficient mice are necessary to shed more light on this issue.
The authors are grateful to Prof. Teresa Jakubowicz (Department of Immunobiology, Maria Curie-Sklodowska University, Lublin, Poland) for critical reading of the manuscript. This work was supported by the grant from the Ministry of Science and Higher Education No N N303 822640 and in part from the Foundation for Polish Science within the project ‘Molecular Spectroscopy for BioMedical Study’ (the TEAM program). The research was partially carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-06-024/09 Centre of Functional Nanomaterials). The authors declare no conflict of interest.
Conflict of interest statement. None declared.
REFERENCES