-
PDF
- Split View
-
Views
-
Cite
Cite
Susan B. Sleight, Patricia V. Miranda, Nia-Washington Plaskett, Bernhard Maier, Jeff Lysiak, Heidi Scrable, John C. Herr, Pablo E. Visconti, Isolation and Proteomic Analysis of Mouse Sperm Detergent-Resistant Membrane Fractions: Evidence for Dissociation of Lipid Rafts During Capacitation, Biology of Reproduction, Volume 73, Issue 4, 1 October 2005, Pages 721–729, https://doi.org/10.1095/biolreprod.105.041533
Close - Share Icon Share
Abstract
Mammalian sperm acquire fertilization capacity after residing in the female tract during a process known as capacitation. The present study examined whether cholesterol efflux during capacitation alters the biophysical properties of the sperm plasma membrane by potentially reducing the extent of lipid raft domains as analyzed by the isolation of detergent-resistant membrane fractions using sucrose gradients. In addition, this work investigated whether dissociation of the detergent-resistant membrane fraction during capacitation alters resident sperm raft proteins. Mouse sperm proteins associated with such fractions were studied by silver staining, tandem mass spectrometry, and Western blot analysis. Caveolin 1 was identified in sperm lipid rafts in multimeric states, including a high-molecular-weight oligomer that is sensitive to degradation under reducing conditions at high pH. Capacitation resulted in reduction of the light buoyant-density, detergent-resistant membrane fraction and decreased the array of proteins isolated within this fraction, including loss of the high-molecular-weight caveolin 1 oligomers. Proteomic analysis of sperm proteins isolated in the light buoyant-density fraction identified several proteins, including hexokinase 1, testis serine proteases 1 and 2, TEX101, hyaluronidase (PH20, SPAM1), facilitated glucose transporter 3, lactate dehydrogenase A, carbonic anhydrase IV, IZUMO, pantophysin, basigin, and cysteine-rich inhibitory secretory protein 1. Capacitation also resulted in a significant reduction of sperm labeling by the fluorescent lipid-analog DiIC16, indicating that capacitation alters the liquid-ordered domains in the sperm plasma membrane. The observations that capacitation alters the protein composition of the detergent-resistant membrane fractions is consistent with the hypothesis that cholesterol efflux during capacitation dissociates lipid raft constituents, initiating signaling events that lead to sperm capacitation.
Introduction
Following ejaculation, sperm are motile yet lack fertilizing competence, which they gain in the female reproductive tract during a time-dependent process collectively called capacitation [1, 2]. Capacitation correlates in vitro with a cAMP-dependent rise in tyrosine phosphorylation and is associated with changes in both the head and tail that prepare the sperm to undergo a regulated acrosome reaction (e.g., in response to the zona pellucida of the egg) and to be capable of hyperactivated motility [3]. Capacitation can be accomplished in vitro using caudal epididymal or ejaculated sperm incubated in a defined medium that reflects the electrolytic composition of the oviductal fluid [1].
Capacitation is associated with significant changes in the properties of sperm membranes, including an efflux of cholesterol. An essential component of in vitro capacitation medium, BSA is believed to function as a cholesterol acceptor by removing it from the sperm plasma membrane [4–11]. Cholesterol and/or cholesterol analogs, if added to the capacitation medium, inhibit sperm capacitation [12]. Additionally, other cholesterol-binding agents, such as high-density lipoprotein [12–14] and β-cyclodextrins, may substitute for BSA in capacitation media [15–18].
Previously, we demonstrated that the efflux of cholesterol and other sterols from the plasma membrane precedes the cAMP-regulated tyrosine-phosphorylation cascade leading to capacitation [12, 19, 20]. However, our understanding of how sterol efflux couples to the regulation of signal transduction pathways intrinsic to capacitation remains rudimentary. One possibility is that before capacitation, cholesterol concentrates in specialized plasma membrane microdomains known as lipid rafts.
By definition, rafts are highly enriched in cholesterol, gangliosides, and sphingolipids. This lipid content contributes to the hydrophobic nature of raft domains and leads to two inherent biochemical properties: insolubility at 4°C in Triton X-100 (TX100) detergent, and light buoyant density after centrifugation in a sucrose density gradient. These properties can be used to isolate detergent-resistant membrane (DRM) as a biochemical correlate of lipid rafts [21].
An additional property of lipid rafts, also as a result of their inherent hydrophobic nature, is the ability to recruit specific types of proteins, including transmembrane, membrane-bound, glycophospholipid (GPI)-anchored, and saturated acyl chains lipid-modified proteins [22]. In somatic cells, evidence is accumulating that lipid rafts serve as centers for cholesterol traffic and for signal transduction pathways originating at the plasma membrane [23]. Signaling proteins in these domains often are cell-type and cell-state specific, and they include receptor and nonreceptor tyrosine kinases, G proteins, inositol phospholipids, GPI-anchored proteins, nitric oxide synthase, and others congregate in lipid rafts [24–26].
A subset of lipid raft, called caveolae, is formed by polymerization of caveolins or caveolin-related integral membrane proteins (e.g., flotillin) that tightly bind cholesterol [27]. Caveolin forms high-molecular-weight (HMW) homo- and hetero-oligomers, ranging in size from 200 to 600 kDa [28–30]. Although the general functions of caveolae are still not completely defined, they are believed to be implicated in cholesterol transport [31], membrane trafficking [32], and signal transduction [33] in other systems. In mouse and guinea pig sperm, caveolin 1 (CAV1) is present in the plasma membrane overlying the acrosomal region and the flagellum [34, 35].
Several investigators have used cholesterol depletion as a method to evaluate whether a particular signaling pathway is regulated by changes in the properties of lipid rafts [36, 37]. In some cases, these studies have used β-cyclodextrins, a plant derivative with strong affinity for cholesterol, which drastically depletes cells from their cholesterol content in a nonphysiologic manner. As noted above, not only is cholesterol efflux observed under capacitating conditions [7, 12], cholesterol acceptors (e.g., BSA, β-cyclodextrins, or high-density lipoprotein) in the incubation media are required for capacitation [15, 17]. Taken together, these observations lead to the conclusion that cholesterol efflux is necessary for capacitation and to the primary hypothesis that cholesterol efflux alters the biophysical properties of the sperm plasma membrane by potentially reducing the extent of lipid raft domains. Supporting this hypothesis, a recent study found that two raft components, the GPI-linked protein CD59 and the sphingolipid GM1, showed a partial, sterol loss-dependent shift to the nonraft domain during human sperm capacitation [38]. A secondary hypothesis postulates that dissociation of the raft domains alters the composition or distribution of raft-associated proteins and, in turn, initiates signaling pathways that lead to capacitation.
To address these hypotheses, the present study evaluated the state of DRMs during sperm capacitation. First, CAV1 in the lipid rafts of mouse sperm was shown to form HMW oligomers that are diminished during capacitation. Second, a comparison of capacitated versus noncapacitated sperm showed a decrease of DRM-associated proteins in the capacitated sperm population. Third, more than 25 proteins were identified in these fractions using a combination of SDS-PAGE and tandem mass spectrometry (MS/MS). Many of these proteins are predicted to be associated with membrane domains either directly or via GPI anchors. Fourth, binding of the fluorescent lipid analog-probe DiIC16, which preferentially targets liquid-ordered domains of the plasma membrane [37], was reduced in capacitated compared to noncapacitated sperm.
Materials and Methods
Materials
All chemicals were of reagent grade or better and, unless otherwise specified, were purchased from Sigma or Fisher. Reagents and buffers for SDS-PAGE, including the molecular-weight Precision standards, were from Bio-Rad. The protease-inhibitors aprotinin, pefabloc, and pepstatin were from Roche/Boeringher-Mannheim Biochemicals; rabbit polyclonal immunoglobulin (Ig) G directed against a 20-amino-terminal peptide from human CAV1 was from Santa Cruz. This 20-amino-acid domain is 84% identical to the orthologous mouse CAV1 domain. Horseradish peroxidase (HRP)-conjugated secondary antibodies from Sigma were visualized with either the NEN Lightning or KPL chemiluminescent reagents according to manufacturer’s instructions. TES 101, a monoclonal antibody against the testicular germ cell-specific antigen TEX101 [39] and polyclonal antibody against hexokinase I (HK1), were generously provided by Dr. Yoshihiko Araki (Yamagata University, Yamagata, Japan) and Dr. Wilson (Michigan State University, East Lansing, MI), respectively. Xomat-Blue and MS Kodak films were from NEN Life Sciences. Fluorescent DiIC16 lipid-analog probe that targets liquid-ordered plasma membrane domains [37] was from Molecular Probes. The mounting medium, Vectashield, and the antigen unmasking solution were purchased from Vector Laboratories.
Preparation of Mouse Sperm
Caudal epididymal sperm were collected from retired breeder males (Charles River Laboratories strain) killed in accordance with Institutional Animal Care and Use Committee guidelines. Experimental protocols were approved by the University of Virginia Animal Care Committee. The cauda epididymis from each animal was placed in 1 ml of 37°C modified Krebs-Ringer medium (Whitten-Hepes buffered [WH]) [40] containing 100 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, 1 mM pyruvic acid, 4.8 mM l(+)-lactic acid, and hemicalcium salt in 20 mM Hepesm, overall pH 7.3 (All reagents were Sigma ultra-pure/cell-culture tested). This WH medium, prepared in the absence of BSA and NaHCO3, does not support capacitation. Sperm released into the media during a 10-min period (between 5 and 8 million sperm/epididymis) were counted and collected by centrifugation at 800 × g for 10 min at room temperature (RT; ∼24°C). For immunofluorescence and experiments involving the collection of noncapacitated and capacitated populations, the centrifuged sperm were resuspended to a final concentration of 2 × 107 cells/ml. Aliquots of 500 μl were placed in a round-bottom borosilicate, 4-ml glass tube and overlaid with either 1.5 ml of 37°C WH (noncapacitated conditions) or WH supplemented with 24 mM NaHCO3 and 3 mg/ml of fatty-acid free BSA (catalog no. A-0281; Sigma) (capacitated conditions). The total incubation time for all conditions was 1 h at 37°C in a 5% CO2/95% O2 incubator. Sperm were then prepared either for lysis in sample buffer (see below) and subsequent Western blot analysis or for immunofluorescent localization.
SDS-PAGE and Western Blot Analysis
After incubation, sperm were concentrated by centrifugation at 10 000 × g for 3 min in a Beckman Coulter Microfuge 18 (Beckman, Fullerton, CA); resuspended in buffer containing 50 mM Tris-HCl (pH 7.3), 150 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM NaF, as well as 10 μg/ml each of leupeptin, pepstatin, aprotinin, and benzimidine and 3 μg/ml of Nα-tosyl-l-lysine chloromethyl ketone hydrochloride (TLCK) (TEN); vortexed for 10 sec at maximum setting using a Fisher Vortex Genie 2 (Fisher); and placed on ice for 5 min. To study HMW caveolin complexes, sperm samples were treated for 30 min at 27°C in a standard Laemmli (pH 6.8) reducing buffer, or such HMW complexes were reduced by boiling for 5 min in Laemmli pH 10 buffer as described previously [28, 29]. For SDS-PAGE, a sperm equivalent of 200 000 sperm was extracted in sample buffer as described previously [19] and loaded on a 4– 16% linear gradient gel with a 3% stacking gel to facilitate the entry of possible HMW caveolin oligomers. Gels were silver-stained [41] or electroblotted to a polyvinylidene fluoride (PVDF) membrane (Millipore). Membranes were blocked with 4% BSA in Tris-buffered saline (pH 7.3) with 0.1% Tween-20 (TBST) for 1 h at RT. All primary and secondary antibody incubations were in TBST plus 4% BSA. All membrane washes were at RT for 15 min with TBST. After incubation for 1 h with a 1:1000 dilution of primary antibody, the PVDF membranes were washed four times, then incubated at RT for 1 h with HRP-conjugated secondary antibodies at a 1:10 000 dilution. As control for anti-CAV1, this antibody was preabsorbed with 1.5 μg/ml of the antigenic peptide. The membrane was washed as before and rinsed briefly with water, and then HRP activity was visualized with chemiluminescence. Western blots using antibodies directed against TEX101 were performed on samples electrophoresed under nonreducing conditions (in the absence of β-mercaptoethanol).
Isolation of Light Buoyant-Density DRM Fractions
Caudal epididymal sperm were collected in WH medium and centrifuged at 150 × g for 5 min to clear the sperm suspension. The sperm-containing supernatant was removed and centrifuged at 700 × g for 10 min. The sperm pellet was divided into two aliquots, each containing 80 million sperm in a total of 2 ml of WH medium. One aliquot was incubated in WH medium (noncapacitated) and the other in WH medium containing 3 mg/ml of BSA and 24 mM NaHCO3 (capacitated). After 1 h of incubation, the sperm were collected by centrifugation at 700 × g for 10 min, and the pellet was resuspended in 400 μl of TEN buffer containing 0.5% TX100. The pellet was gently Dounce homogenized and then sonicated with five brief bursts in a Sonifier Cell Disruptor W-350 (Thistlle Scientific) set at output 3 and 50% duty cycle. Samples were kept on ice, and foaming was avoided. The sperm lysates were rotated at 4°C for 45 min to liberate the DRM fractions, which were operationally defined as the lipid raft domains. Lysates were adjusted to 40% sucrose with the addition of 80% sucrose in TEN. To generate a discontinuous sucrose gradient, the sperm lysate was placed in the bottom of a 2-ml Beckman centrifuge tube and gently overlaid with 800 μl of 30% sucrose in TEN, followed by 400 μl of 5% sucrose in TEN. The same procedure was applied for all raft fraction isolations. The samples were then centrifuged at 200 000 × g for 18 h in a TLS55 swinging bucket rotor (Beckman) in a Beckman Optima-TLX ultracentrifuge. The visible opaque light buoyant-density DRM fractions, which float up the sucrose gradient, were apparent as a visible light-scattering band following centrifugation. Nine 200-μl fractions were carefully collected from the top to the bottom of the gradient, and the positions of the visible fractions were noted.
Fractions were prepared for SDS-PAGE with SDS-reducing sample buffer (pH 6.8) and treated at 27°C for 30 min to preserve the HMW caveolin complexes as described previously [28, 29]. Fractions resolved on a 4–16% linear gradient SDS-PAGE gel with a 3% stacking gel were silver-stained or prepared for Western blot analysis as previously described. To obtain peptide sequences from the visible light buoyant-density DRM fraction, proteins that were silver-stained from this fraction were excised from the gel and submitted for MS/MS.
MS/MS Analysis of Raft Proteins
The proteins visualized by silver stain in the visible RAFT fraction were numbered and excised from the gel. Numbered bands were then submitted to the W.M. Keck Biomedical Mass Spectrometry Laboratory facility from the University of Virginia (Charlottesville, VA) for peptide sequencing. Samples were prepared as described previously [42].
Detection of Liquid-Ordered Domains using DiIC16 Probe
Mouse caudal epididymal sperm were treated under noncapactitated and capacitated conditions as described above. DiIC16 was first dissolved in 100% ethanol at 2 mg/ml and vortexed at maximum speed with a Fisher Vortex Genie 2 for 5 min to ensure complete mixing of the lipid. Just before sperm labeling for either fixed or living sperm, the probe was diluted in PBS to a final working concentration of 25 μg/ml, vortexed vigorously, and added to the sperm for 15 min at 37°C. To label fixed sperm, the sperm were fixed in solution with freshly prepared 4% parafomaldehyde in PBS (pH 7.3) for 15 min at 37°C and then spotted directly onto a microscope slide and subsequently labeled with DiIC16. After incubation for 1 h in the appropriate medium, live sperm were labeled for 15 min at 37°C with the probe in WH medium, briefly washed with PBS using gentle centrifugation (at 800 × g for 3 min), and then spotted onto a slide, followed by fixation for 15 min at 37°C in paraformaldehyde/PBS. Labeled sperm were then washed three times with PBS at RT. The slides were mounted with Vectashield. Fluorescence was detected as described above. The Alexa fluor 568-labeled lipid analog-probe DiIC16 emits fluorescence when excited with a wavelength of 568 nM. Photos were taken with a digital camera as described previously [43].
Results
HMW Caveolin Oligomers Are Present in Mouse Sperm
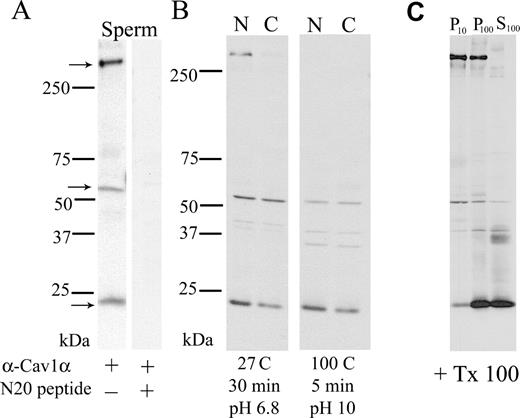
High-molecular-weight caveolin oligomers ranging in size from 200 to 600 kDa have been proposed as the active scaffolding framework for signal transduction proteins [44] and demonstrated to be stabilized by cholesterol and/or long-chain fatty acylation [45]. Although CAV1 has been reported in mammalian sperm [34, 35], those studies have not shown the presence of HMW oligomers. In the present work, HMW caveolin complexes were preserved as described above, and three distinct immunoreactive bands were detected in noncapacitated sperm (Fig. 1A). These bands migrated as a 24-kDa monomer, a 50-kDa dimer (forms noted previously in sperm) [34], and the novel observation of HMW caveolin complexes migrating at molecular weights greater than 250 kDa. Two lines of evidence support the authenticity of these HMW CAV1-containing oligomers. First, they were not detected when the antibody against CAV1 was preincubated with an excess of the N-20 immunogenic CAV1 peptide (1 μg/ml). Second, HMW caveolin oligomers were proposed to be linked by thioester bonds that are reduced only under basic conditions (pH 10) [28–30]. When protein extracts were boiled for 5 min at pH 10 to reduce the thioester bonds, the HMW caveolin complexes were lost (Fig. 1B, right), indicating that the HMW oligomers in sperm behave similarly to previously reported caveolin oligomers [28–30]. More importantly, the pattern of immunoreactive CAV1 proteins from capacitated sperm differed from CAV1 proteins from noncapacitated sperm. In capacitated sperm, the HMW caveolin oligomers were virtually absent, whereas the 24-kDa monomer was reduced (Fig. 1B, left). Little or no change was present in the 50-kDa dimer. To our knowledge, this is the first evidence documenting capacitation-associated reduction in HMW caveolin oligomers. Initially, to evaluate whether caveolin monomers or oligomers associate with sperm membrane fractions, sequential centrifugation of sperm extracts obtained in the presence of 1% TX100 was performed. Western blot analysis of the subcellular fractions was performed using antibodies against CAV1 (Fig. 1C). Although the 24-kDa CAV1 monomer was partially extracted with TX100, the HMW oligomers remained associated with the pellet obtained after ultracentrifugation (P100), indicating that the HMW caveolin oligomers are present in detergent-insoluble domains.
Multiple electrophoretic isoforms of CAV1 are present in mouse sperm. The HMW oligomer and monomer are reduced after capacitation. A) Sperm were prepared as described in Materials and Methods. The extracts (200 000 sperm equivalent/lane) were separated using a 4–16% linear gradient SDS-PAGE and transferred to Immobilon P, and CAV1 was visualized using specific antibodies (left). As a control for antibody specificity, identical blots were developed with the same antibody preabsorbed with the antigenic peptide. B) On the left, noncapacitated (NON) and capacitated (CAP) sperm proteins were prepared to maintain HMW caveolin complexes and analyzed as described in A. On the right, parallel identical samples were boiled for 5 min at high pH to reduce the HMW oligomers and then analyzed as described in A. C) Sperm homogenates were sonicated in buffer containing 1% TX100 and incubated in the same buffer for 30 min on ice. Then, sequential centrifugations were performed. First, a pellet (P10) and supernatant were obtained after centrifugation at 10 000 × g for 10 min. Second, the supernatant was further centrifuged at 100 000 × g for 1 h, and the pellet (P100) and the supernatant (S100) were recovered. Each fraction was then prepared to maintain HMW CAV1 complexes and separated in SDS-PAGE gels as before. The proteins were then transferred to PVDF membranes and the CAV1 visualized with anti-CAV1 by Western blot analysis
Proteins Associated with the DRM Fraction Were Reduced after Capacitation
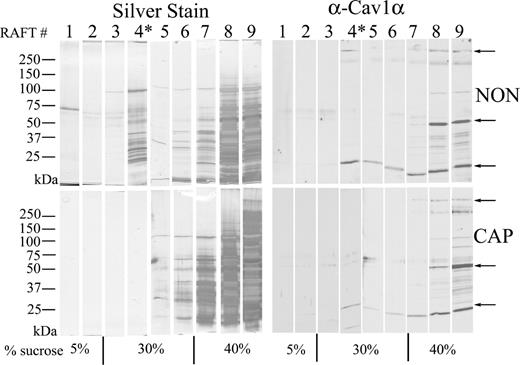
The presence of CAV1 oligomers and monomers in light buoyant-density DRM fractions (Fig. 1C) was consistent with previous reports suggesting the existence of lipid raft microdomains in mouse sperm [46]. Because capacitation is associated with the release of cholesterol from the sperm plasma membrane, the fate of sperm DRM fractions during capacitation was further investigated (Fig. 2). The DRM fractions were prepared from noncapacitated and capacitated mouse sperm as described in Materials and Methods. In noncapacitated sperm, a visible light-scattering band was observed in the sucrose gradient just below the 5%/30% sucrose interface. This light buoyant-density visible band was recovered as fraction 4 (indicated by an asterisk in Fig. 2, lane 4, NON, upper left). The visible light buoyant-density fraction from noncapacitated sperm contained multiple silver-stained proteins ranging from 25 to 125 kDa. In contrast, in capacitated sperm, the fraction just below the 5%/30% interface (fraction 4) did not contain a visible light-scattering band and showed a significant decrease in total protein as determined by silver-stain analysis (Fig. 2, lane 4, CAP, lower left).
Biochemical isolation of DRM lipid rafts by sucrose gradient centrifugation from noncapacitated (NON) and capacitated (CAP) sperm. Both NON TX100 in TEN buffer were adjusted to 40% sucrose. Samples were prepared for DRM isolation as described in Materials and Methods. In each condition, nine 200-μl fractions were then collected from top to bottom, divided in two, and analyzed in a linear gradient SDS-PAGE. One set was analyzed by silver staining of the fractions from the top to bottom of the sucrose gradient. The light-scattering visible light buoyant-density fraction (*) band was observed in the NON population but not in the CAP population. The second set of aliquots was analyzed by linear gradient SDS-PAGE, transferred to Immobilon P, and immunostained with the CAV1 antibody. This experiment was repeated six times with similar results
When the same fractions were analyzed for the presence of CAV1 by Western blot, fraction 4 of noncapacitated sperm contained the HMW complexes as well as the 24-kDa caveolin monomer, but not the 50-kDa dimer (Fig. 2, lane 4, NON, upper right). In contrast, the CAV1 HMW oligomer was not detected in fraction 4 from capacitated sperm, and the 24-kDa caveolin monomer was significantly reduced (Fig. 2, lane 4, CAP, lower right). Thus, the process of in vitro capacitation alters the protein composition of sperm DRM and supports the hypothesis that the protein composition of lipid rafts is altered during capacitation.
Identification of DRM-Associated Proteins from Sperm by MS/MS
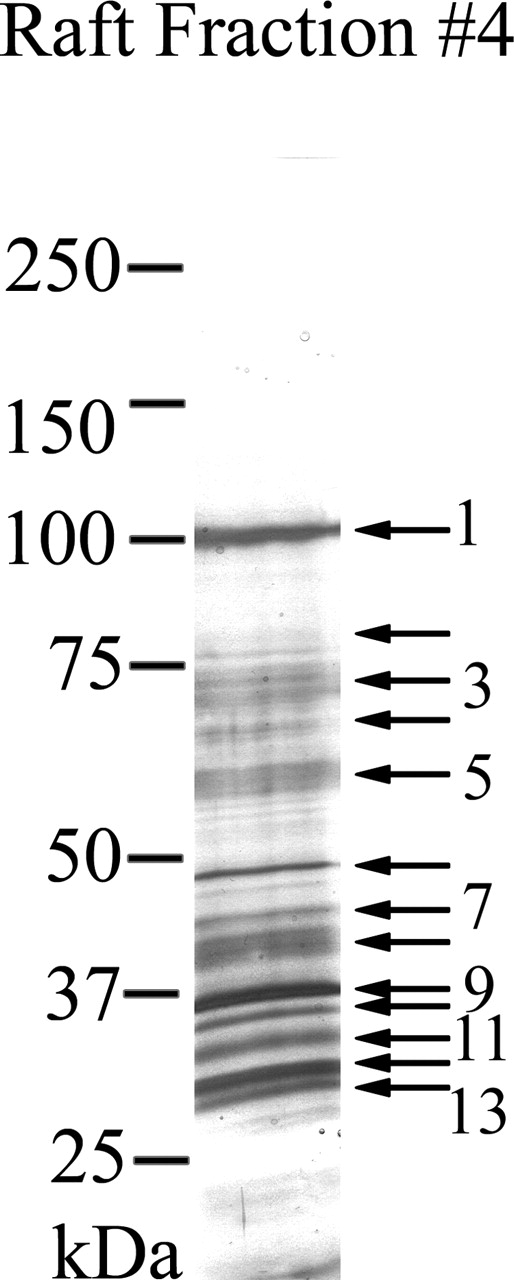
To further analyze the hypothesis that sperm capacitation perturbs lipid rafts, proteins in the light buoyant-density DRM fractions of noncapacitated mouse sperm (Fig. 2, lane 4, NON, upper left) were cut and processed for MS/MS. Using this methodology, it was possible to identify more than 25 DRM-associated proteins (Fig. 3 and Table 1). The proteins include enzymes implicated in glucose transport and metabolism, bicarbonate metabolism, and putative cell receptors. The majority of the identified proteins was membrane-associated and included transmembrane and GPI-linked proteins. A subset of these proteins appears to be testes specific, as determined by a bio-informatic analysis of tissue distribution of expressed sequence tags (ESTs). Interestingly, some of the proteins (e.g., pantophysin, vacuolar ATPase D, and carbonic anhydrase IV [CAR4]) found using this approach were known but never identified in sperm, whereas others (e.g., proteins in bands 5 and 10) are in the database only as hypothetical proteins (e.g., hypothetical polycythemia rubra vera-like 1 and 2) (Table 1).
List of proteins identified by MS/MS with their respective NCBI accession numbers.
| Band no. . | Proteina . | NCBI accession no. . |
|---|---|---|
| 1 | HEXOKINASE I (HK1) | AAH72628 |
| 2 | MOUSE KERATIN COMPLEX 2 (KRT2) | NP-032499 |
| 5 | IZUMO | BAD91011 |
| 5 | SIMILAR TO PH-20/Hya15 (4933439A12RIK) | BAC55071 |
| 6 | MOUSE SERUM ALBUMIN (ALBI) | CAA09617 |
| 6 | SEMINAL VESICLE SECRETION 5 (SVS5) | NP_333327 |
| 6 | SIMILAR TO CGI-49 (C330023F11RIK) | NP_848768 |
| 7 | PH-20/SPERM ADHESION MOLECULE (SPAM1) | AAP49832 |
| 7, 8 | TESTIS SERINE PROTEASE 1 (TESP1) | NP_033381 |
| 7, 8 | TESTIS SPECIFIC TES101RP (TEX101) | NP_064365 |
| 7, 8 | GLUT3/SOLUTE CARRIER FAMILY 2 (SLC2A3) | NP_035531 |
| 8 | L-LACTATE DEHYDROGENASE A-like (LDHAL6B) | NP_780558 |
| 8 | SCP/TPX-1/CRISP-LIKE #1 (4921508011 RIK) | NP_080499 |
| 8 | TESTIS SERINE PROTEASE 2 (TESSP2) | BAB78735 |
| 8 | VACUOLAR ATPASE D (ATP6V0D1) | NP_038505 |
| 8, 9, 10 | CARBONIC ANHYDRASE IV (CAR4) | NP_031633 |
| 10 | PANTOPHYSIN ISOFORM 1 (SYP1) | NP_038663 |
| 10, 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 1 (4933400F01RIK) | NP_877586 |
| 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 2 (BC049730) | XP_355991 |
| 11, 12, 13 | BASIGIN (BSG) | NP_033898 |
| 11 | SERINE PROTEASE-LIKE# 1 (1700036D21RIK) | BAB63919 |
| 12 | SCP/TPX-1/CRISP-LIKE # 2 (1 70001 1 E04RIK) | BAB24280 |
| 13 | Ig LIGHT CHAIN (IGK-V8) | AAA51141 |
| 13 | Ig GAMMA 2b (IGH-3) | AAB59659 |
| 13 | CYSTEINE-RICH SECRETORY PROTEIN 1 (CRISP1) | NP_033768 |
| 13 | MAJOR URINARY PROTEIN 1 (MUP1) | NP_112465 |
| 13 | CALTRIN/SEMINAL VESICLE SECRETION 7 (SVS7) | NP_064660 |
| Band no. . | Proteina . | NCBI accession no. . |
|---|---|---|
| 1 | HEXOKINASE I (HK1) | AAH72628 |
| 2 | MOUSE KERATIN COMPLEX 2 (KRT2) | NP-032499 |
| 5 | IZUMO | BAD91011 |
| 5 | SIMILAR TO PH-20/Hya15 (4933439A12RIK) | BAC55071 |
| 6 | MOUSE SERUM ALBUMIN (ALBI) | CAA09617 |
| 6 | SEMINAL VESICLE SECRETION 5 (SVS5) | NP_333327 |
| 6 | SIMILAR TO CGI-49 (C330023F11RIK) | NP_848768 |
| 7 | PH-20/SPERM ADHESION MOLECULE (SPAM1) | AAP49832 |
| 7, 8 | TESTIS SERINE PROTEASE 1 (TESP1) | NP_033381 |
| 7, 8 | TESTIS SPECIFIC TES101RP (TEX101) | NP_064365 |
| 7, 8 | GLUT3/SOLUTE CARRIER FAMILY 2 (SLC2A3) | NP_035531 |
| 8 | L-LACTATE DEHYDROGENASE A-like (LDHAL6B) | NP_780558 |
| 8 | SCP/TPX-1/CRISP-LIKE #1 (4921508011 RIK) | NP_080499 |
| 8 | TESTIS SERINE PROTEASE 2 (TESSP2) | BAB78735 |
| 8 | VACUOLAR ATPASE D (ATP6V0D1) | NP_038505 |
| 8, 9, 10 | CARBONIC ANHYDRASE IV (CAR4) | NP_031633 |
| 10 | PANTOPHYSIN ISOFORM 1 (SYP1) | NP_038663 |
| 10, 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 1 (4933400F01RIK) | NP_877586 |
| 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 2 (BC049730) | XP_355991 |
| 11, 12, 13 | BASIGIN (BSG) | NP_033898 |
| 11 | SERINE PROTEASE-LIKE# 1 (1700036D21RIK) | BAB63919 |
| 12 | SCP/TPX-1/CRISP-LIKE # 2 (1 70001 1 E04RIK) | BAB24280 |
| 13 | Ig LIGHT CHAIN (IGK-V8) | AAA51141 |
| 13 | Ig GAMMA 2b (IGH-3) | AAB59659 |
| 13 | CYSTEINE-RICH SECRETORY PROTEIN 1 (CRISP1) | NP_033768 |
| 13 | MAJOR URINARY PROTEIN 1 (MUP1) | NP_112465 |
| 13 | CALTRIN/SEMINAL VESICLE SECRETION 7 (SVS7) | NP_064660 |
Abbreviations in parentheses refer to UniGene nomenclature.
List of proteins identified by MS/MS with their respective NCBI accession numbers.
| Band no. . | Proteina . | NCBI accession no. . |
|---|---|---|
| 1 | HEXOKINASE I (HK1) | AAH72628 |
| 2 | MOUSE KERATIN COMPLEX 2 (KRT2) | NP-032499 |
| 5 | IZUMO | BAD91011 |
| 5 | SIMILAR TO PH-20/Hya15 (4933439A12RIK) | BAC55071 |
| 6 | MOUSE SERUM ALBUMIN (ALBI) | CAA09617 |
| 6 | SEMINAL VESICLE SECRETION 5 (SVS5) | NP_333327 |
| 6 | SIMILAR TO CGI-49 (C330023F11RIK) | NP_848768 |
| 7 | PH-20/SPERM ADHESION MOLECULE (SPAM1) | AAP49832 |
| 7, 8 | TESTIS SERINE PROTEASE 1 (TESP1) | NP_033381 |
| 7, 8 | TESTIS SPECIFIC TES101RP (TEX101) | NP_064365 |
| 7, 8 | GLUT3/SOLUTE CARRIER FAMILY 2 (SLC2A3) | NP_035531 |
| 8 | L-LACTATE DEHYDROGENASE A-like (LDHAL6B) | NP_780558 |
| 8 | SCP/TPX-1/CRISP-LIKE #1 (4921508011 RIK) | NP_080499 |
| 8 | TESTIS SERINE PROTEASE 2 (TESSP2) | BAB78735 |
| 8 | VACUOLAR ATPASE D (ATP6V0D1) | NP_038505 |
| 8, 9, 10 | CARBONIC ANHYDRASE IV (CAR4) | NP_031633 |
| 10 | PANTOPHYSIN ISOFORM 1 (SYP1) | NP_038663 |
| 10, 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 1 (4933400F01RIK) | NP_877586 |
| 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 2 (BC049730) | XP_355991 |
| 11, 12, 13 | BASIGIN (BSG) | NP_033898 |
| 11 | SERINE PROTEASE-LIKE# 1 (1700036D21RIK) | BAB63919 |
| 12 | SCP/TPX-1/CRISP-LIKE # 2 (1 70001 1 E04RIK) | BAB24280 |
| 13 | Ig LIGHT CHAIN (IGK-V8) | AAA51141 |
| 13 | Ig GAMMA 2b (IGH-3) | AAB59659 |
| 13 | CYSTEINE-RICH SECRETORY PROTEIN 1 (CRISP1) | NP_033768 |
| 13 | MAJOR URINARY PROTEIN 1 (MUP1) | NP_112465 |
| 13 | CALTRIN/SEMINAL VESICLE SECRETION 7 (SVS7) | NP_064660 |
| Band no. . | Proteina . | NCBI accession no. . |
|---|---|---|
| 1 | HEXOKINASE I (HK1) | AAH72628 |
| 2 | MOUSE KERATIN COMPLEX 2 (KRT2) | NP-032499 |
| 5 | IZUMO | BAD91011 |
| 5 | SIMILAR TO PH-20/Hya15 (4933439A12RIK) | BAC55071 |
| 6 | MOUSE SERUM ALBUMIN (ALBI) | CAA09617 |
| 6 | SEMINAL VESICLE SECRETION 5 (SVS5) | NP_333327 |
| 6 | SIMILAR TO CGI-49 (C330023F11RIK) | NP_848768 |
| 7 | PH-20/SPERM ADHESION MOLECULE (SPAM1) | AAP49832 |
| 7, 8 | TESTIS SERINE PROTEASE 1 (TESP1) | NP_033381 |
| 7, 8 | TESTIS SPECIFIC TES101RP (TEX101) | NP_064365 |
| 7, 8 | GLUT3/SOLUTE CARRIER FAMILY 2 (SLC2A3) | NP_035531 |
| 8 | L-LACTATE DEHYDROGENASE A-like (LDHAL6B) | NP_780558 |
| 8 | SCP/TPX-1/CRISP-LIKE #1 (4921508011 RIK) | NP_080499 |
| 8 | TESTIS SERINE PROTEASE 2 (TESSP2) | BAB78735 |
| 8 | VACUOLAR ATPASE D (ATP6V0D1) | NP_038505 |
| 8, 9, 10 | CARBONIC ANHYDRASE IV (CAR4) | NP_031633 |
| 10 | PANTOPHYSIN ISOFORM 1 (SYP1) | NP_038663 |
| 10, 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 1 (4933400F01RIK) | NP_877586 |
| 11 | POLYCYTHEMIA RUBRA VERA-LIKE # 2 (BC049730) | XP_355991 |
| 11, 12, 13 | BASIGIN (BSG) | NP_033898 |
| 11 | SERINE PROTEASE-LIKE# 1 (1700036D21RIK) | BAB63919 |
| 12 | SCP/TPX-1/CRISP-LIKE # 2 (1 70001 1 E04RIK) | BAB24280 |
| 13 | Ig LIGHT CHAIN (IGK-V8) | AAA51141 |
| 13 | Ig GAMMA 2b (IGH-3) | AAB59659 |
| 13 | CYSTEINE-RICH SECRETORY PROTEIN 1 (CRISP1) | NP_033768 |
| 13 | MAJOR URINARY PROTEIN 1 (MUP1) | NP_112465 |
| 13 | CALTRIN/SEMINAL VESICLE SECRETION 7 (SVS7) | NP_064660 |
Abbreviations in parentheses refer to UniGene nomenclature.
MS/MS analysis of lipid rafts. Proteins detected by silver stain in the light buoyant-density DRM fraction (fraction 4) of noncapacitated sperm were numbered, excised, and submitted for peptide analysis and protein identification as described in Materials and Methods. For simplicity, every other protein band is numbered, whereas every arrow represents an excised silver-stained band. The Sequest algorithmic program or manual EST database searches were used to match the peptides to known proteins. Proteins identified by this method are shown in Table 1
Proteins Associated with Light Buoyant-Density DRM Fractions Redistribute in Sucrose Gradients During Capacitation
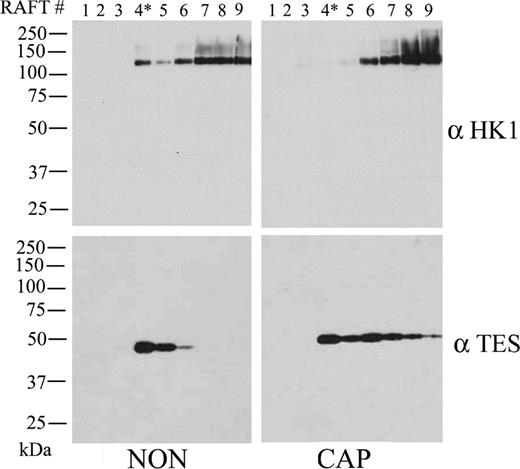
As noted above, silver staining of sucrose gradient fractions indicated that during mouse sperm capacitation in vitro, the protein content decreases in the light buoyant-density DRM fraction. To investigate further changes in specific proteins with capacitation two proteins, TEX101 and HK1, identified by MS/MS were analyzed along the sucrose gradient by Western blot (Fig. 4). Both proteins were confirmed to be in the light buoyant-density DRM fractions isolated from noncapacitated sperm. However, whereas TEX101 was located exclusively in the light buoyant-density fractions (Fig. 4, lanes 4 and 5, NON, left), HK1 was found in the light-density as well as in the heavier fractions (Fig. 4, NON, upper left). After capacitation, TEX101 appeared in heavier fractions of the sucrose gradient, whereas HK1 was diminished in the light buoyant-density fraction and appeared in the heavier fractions (Fig. 4, right), confirming results presented in Figure 2. Thus, in vitro capacitation changes the distribution of selected proteins contained in light buoyant-density DRM fractions, suggesting redistribution of raft proteins may occur as a result of cholesterol removal.
Detection of TEX101 and HK1 after sucrose gradient fractionation in noncapacitated and capacitated mouse sperm. Both noncapacitated (NON) and capacitated (CAP) sperm (80–100 million) treated with 0.5% TX100 in TEN buffer were adjusted to 40% sucrose and prepared for DRM isolation as described in Materials and Methods. In each condition, nine 200-μl fractions were then collected from top to bottom, divided in two, and analyzed by linear gradient SDS-PAGE. The gels were then transferred to Immobilon P, and Western blot analysis was conducted with antibodies against HK1 and TEX101, respectively
Sperm Liquid-Ordered Domains Are Reduced During Capacitation
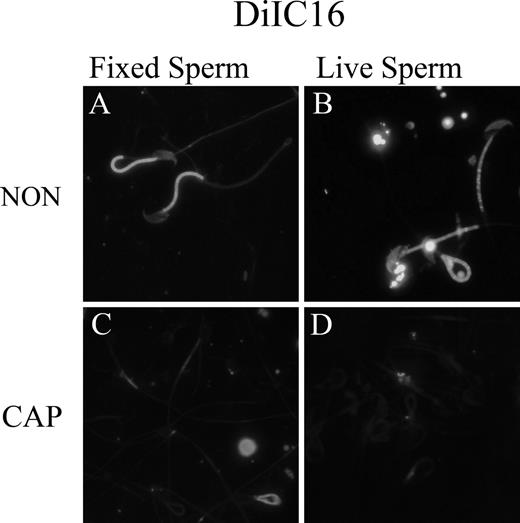
Studies of model membranes containing cholesterol and other lipid components have indicated that the lipid properties in DRMs purified by sucrose gradients are similar to those of liquid-ordered domains, which are characterized by tightly packed hydrocarbon tails. Cholesterol is thought to contribute to the tight packing of lipids in liquid-ordered domains by filling interstitial spaces between lipid molecules, and the formation of liquid-ordered domains is seen only within certain ranges of cholesterol concentration. DiIC16 is a lipid-analog probe that has been shown to partition into relatively ordered regions of membrane in living cells with properties similar to those of DRMs [37]. Because a significant reduction of the proteins in DRMs purified by discontinuous sucrose gradient had been noted, DiIC16 binding to sperm incubated under noncapacitating or capacitating conditions was undertaken to monitor changes in liquid-ordered domains. Noncapacitated sperm exhibited a strong DiIC16 labeling in the flagellar midpiece and lighter staining of the head as well as of other tail structures (Fig. 5, NON). When the sperm were incubated under capacitating conditions, a significant reduction in DiIC16 staining was observed in both live and fixed sperm (Fig. 5, CAP). These observations suggest that during capacitation, the sperm membrane undergoes reorganization, with reduction of liquid-ordered domains. These data are consistent with the capacitation-associated increase in membrane fluidity as measured by fluorescence recovery after photobleaching [47].
Detection of liquid-ordered plasma membrane domains in noncapacitated (NON) and capacitated (CAP) sperm with the lipid analog-probe DiIC16. Both NON (top; A and B) and CAP (bottom; C and D) sperm were labeled with the lipid analog-probe DiIC16 to detect liquid-ordered domains as described in Materials and Methods. Labeling with DiIC16 of fixed (left; A and C) and live (right; B and D) sperm was performed as described in Materials and Methods to compare probe binding of liquid-ordered domains in both conditions. Original magnification ×40
Discussion
One current representation using model membranes envisions lipid rafts as transient phase separations conferring ordered microdomains on an otherwise fluid lipid bilayer. From this biophysical model, it has been proposed that biological membranes contain a minimum of two lipid phases: a typically, but not always, more abundant liquid-disordered phase; and a usually less abundant liquid-ordered phase [48, 49]. The distribution of liquid-ordered versus liquid-disordered is dependent on the type and the state of each cell. Also proposed is that lateral diffusion of proteins in liquid-ordered domains is restricted and, hence, function with localized and discrete effects within the field of the plasma membrane. The present work examined the hypotheses that 1) capacitation-associated cholesterol efflux alters the biophysical properties of the sperm plasma membrane by reducing the extent of lipid raft domains and 2) dissociation of lipid raft domains during capacitation alters resident sperm raft proteins. These steps may represent early events of capacitation in which cholesterol removal from the sperm plasma membrane initiates signaling pathways.
Previously, CAV1 has been reported in lipid rafts [50]; in the present study, an important observation was that CAV1 in mouse sperm forms HMW oligomers. In somatic cells, the presence of similar caveolin oligomers has been linked to the distribution of caveolin in cholesterol-rich domains in the plasma membrane [28, 29]. In sperm, these HMW CAV1-containing complexes are significantly decreased after 1 h of in vitro capacitation, an observation that is consistent with previous reports of cholesterol release during the capacitation process [11, 12]. Accompanying the reduction in CAV1 oligomers, the overall content of proteins from the lipid raft light buoyant-density DRM fraction from sucrose gradients was reduced in capacitated sperm. In addition, binding of DiIC16, a lipid probe shown to partition into relatively liquid-ordered regions of membrane [37], was significantly decreased in both head and tail regions of capacitated sperm compared with a noncapacitated population. These results are consistent with recent reports in boar [51] and human [38] sperm, showing evidence that cholesterol efflux alters lipid raft stability and distribution during capacitation. Together, these results indicate that in vitro capacitation disrupts lipid raft domains and causes a shift in the overall membrane fluidity of the sperm plasma membrane. Because raft membrane domains typically are considered to be of small diameter [52], individual rafts will contain a limited array of signaling components, an organization that may be important for maintaining the off state of certain signaling pathways [53]. Disruption of lipid rafts in sperm may induce the interaction of several raft resident proteins to initiate signaling pathways associated with the capacitation process. It is believed that not every sperm within a population capacitates in vitro, as determined from chlortetracycline fluorescence and the zona pellucida-induced acrosome reaction [19]. Following these criteria, approximately 50% of sperm are capacitated after 1.5 h of incubation in a complete media. This number is similar to the one obtained by single-cell analysis of the capacitation-associated hyperpolarization [54]. In this respect, whether the whole sperm population or only a fraction undergoes disruption of lipid rafts is still an open question.
Proteomic analysis identified more than 25 resident raft proteins from noncapacitated mouse sperm. The majority are membrane-associated, either as transmembrane, integral membrane, GPI-linked, or acyl chain-recruited proteins. Because of the methodology used, proteins in the light buoyant-density DRM could, in theory, belong to any sperm membrane fraction, not only to the plasma membrane. However, analysis of Table 1 does not show mitochondria or nuclear protein markers, suggesting that the presence of mitochondrial or nuclear membranes in the DRM preparation is unlikely. At present, the possibility that some of the identified proteins localized to the inner or outer acrosomal membranes cannot be discarded. In terms of enzymatic activity and function, these proteins fall into several categories. The first group includes proteins that function in metabolic processes. Several proteins associated with glucose metabolism were found in lipid raft fractions, including HK1, a testis-specific lactate dehydrogenase, the facilitated glucose transporter Glut3, and pantophysin. This last protein is believed to form an active energy-transport complex for glucose with Glut3 [55]. The present finding of HK1 in the raft fraction agrees with previous results demonstrating that HK1 in sperm is tightly associated with membrane fractions [56]. The presence of metabolic enzymes in sperm lipid rafts suggests that cholesterol removal mediates some of the changes in energy metabolism observed during capacitation and warrants further investigation.
A GPI-anchored carbonic anhydrase, CAR4 has been linked in other systems to |${\rm{HCO}}_3^ - $| transport [57]. Recent work in human embryonic kidney (HEK293) cells showed a direct interaction between CAR4 and the |${\rm{N}}{{\rm{a}}^ + }{\rm{/HCO}}_3^ - $| cotransporter (NBC). This interaction appears to be necessary for full NBC activity [58]. In context, our recent finding of an electrogenic NBC in mouse sperm [59] and the present identification of CAR4 in mouse sperm lipid raft suggest that similar mechanisms might play a role in the transmembrane movement of |${\rm{HCO}}_3^ - $| anions into sperm.
Three other groups of proteins identified are novel members of the urokinase plasminogen activator-receptor PLAU/polycythemia rubra vera 1 family, proteins belonging to the cysteine-rich inhibitory secretory protein (CRISP) family, and members of the Ig superfamily. Members of the PLAU family are receptors that fulfill a diverse set of functions, ranging from proteolytic inhibition to interaction with signaling complexes. Members of the CRISP protein family include Tpx 1 and a series of snake venoms recently described [60]. Although very little is known about the function of this protein family in sperm, one of its members, CRISP1 (also known as DE), has been proposed to mediate sperm-egg fusion [61]. Finally, recent attention has been given to the Ig superfamily in sperm, particularly IZUMO, a protein that recently was shown to be required for sperm to fuse with eggs [62]. It might be speculated that the capacitation-associated changes in sperm lipid rafts plays a role in positioning IZUMO for fusion events during fertilization.
To our knowledge, several of the proteins identified in the present work have not been described before, and in many cases, their sequences have been theoretically composed by analysis of genomic sequences. Thus, little is known about their tissue distribution. To initiate the investigation of tissue expression of the sperm lipid raft proteins, their sequences were blasted to EST databases, and both mouse and human ESTs were evaluated. The EST analysis indicates that several of the sperm raft proteins have a testis-specific expression in both the mouse and the human. Among these proteins are TEX101, IZUMO, the hypothetical polycythemia rubra vera-like proteins, serine proteases, and some members of the CRISP protein family. Identification of proteins that have a restricted testicular expression suggests functions related exclusively to this tissue. In some cases, this unique distribution might make them suitable targets for novel contraceptive strategies.
In summary, we have identified more than 25 lipid raft-associated proteins in sperm and have shown that these proteins change their migration properties in sucrose gradients when sperm are incubated under capacitating conditions. Although the function of these proteins in sperm is not known, it is tempting, because of the established function of cholesterol efflux in sperm capacitation, to speculate that proteins present in raft microdomains might play a role in the regulation of sperm signaling. Understanding how cholesterol efflux affects the activity of lipid raft-resident proteins will improve our knowledge of capacitation in mammalian sperm.
Acknowledgments
We are grateful to Dr. John E. Wilson (Michigan State University) and Dr. Yoshihiko Araki (Yamagata University) for providing us with anti-HK1 and anti-TEX101 antibodies. The one-dimensional gel bands were sequenced by Dr. Nick Sherman (W.M. Keck Biomedical Mass Spectrometry, University of Virginia).
References
Author notes
Supported by the Andrew W. Mellon Foundation, NIH HD38082, and HD44044 (to P.E.V.), U54 29099 (to J.C.H.), and grants from Schering AG (to J.C.H.).