-
PDF
- Split View
-
Views
-
Cite
Cite
Helena M. Kok, Lucas L. van den Hoogen, Joel A. G. van Roon, Elisabeth J. M. Adriaansen, Ruth D. E. Fritsch-Stork, Tri Q. Nguyen, Roel Goldschmeding, Timothy R. D. J. Radstake, Niels Bovenschen, Systemic and local granzyme B levels are associated with disease activity, kidney damage and interferon signature in systemic lupus erythematosus, Rheumatology, Volume 56, Issue 12, December 2017, Pages 2129–2134, https://doi.org/10.1093/rheumatology/kex332
Close - Share Icon Share
Abstract
Granzymes (Grs) are serine proteases that eliminate virally infected or tumour cells by inducing apoptosis. GrB has been shown to be associated to the pathophysiology of SLE, whereas the role of the other Grs in SLE remain unknown.
Gr levels were determined in the serum of SLE patients and controls and linked to SLE activity parameters, including the IFN signature. In addition, GrB expression was investigated in LN biopsies and correlated to kidney function parameters and disease severity.
Serum GrK and GrM levels were not elevated in SLE and did not correlate with disease activity. In contrast, GrB was increased in SLE serum, which correlated to both the SLEDAI and IFN signature. GrB expression was detected in LN tissue biopsies. The number of GrB-positive cells in tissue correlated to several kidney function parameters (e.g. serum creatinine, proteinuria) and to the LN chronicity index.
GrB, but not GrK and GrM, is increased in the serum and kidney of patients with SLE and correlates with measures of poor prognosis in LN. These data suggest that GrB may contribute to the pathogenesis of SLE/LN, which indicates the possibility that GrB might be used as a biomarker and/or a therapeutic target.
Rheumatology key messages
Circulating granzyme B but not granzyme K and M levels are elevated in SLE patients.
The circulating granzyme B concentration correlates with the SLEDAI and IFN signature in SLE.
Granzyme B expression in lupus nephritis biopsies correlates with signs of kidney damage.
Introduction
SLE is a chronic and systemic autoimmune disease characterized by the presence of autoantibodies directed against nuclear components. The pathophysiology of SLE is complex and includes genetic and environmental factors, defects in the clearance of apoptotic cells and the activation of immune cells. Overexpression of type I IFN inducible genes due to the activation of plasmacytoid dendritic cells (pDCs) and alterations in B cells as well as helper and cytotoxic T cell subsets drive inflammation in SLE [1]. Several organs can be affected in SLE and up to 50% of patients develop LN. In LN, immune complexes are deposited in the glomeruli, initiate inflammation and induce tissue damage. LN is diagnosed by kidney biopsy and classified into different classes based on histologic findings. Despite improvements in its treatment, LN is still associated with substantial morbidity and mortality in patients with SLE [2].
Granzymes (Grs) are serine proteases mainly produced by cytotoxic lymphocytes such as CD8+ T cells, γδ T cells, natural killer (NK) cells and NK T cells, as well as by pDCs [3]. In humans, five different Grs have been described: GrA, B, H, K and M. All five are cytotoxic proteases that induce apoptosis in abnormal cells, such as virally infected or cancerous cells [4]. Grs also fulfil extracellular functions in inflammation or autoimmune diseases, where they trigger cytokine production and induce tissue damage by cleavage of extracellular matrix proteins [5]. Extracellular GrB is thought be involved in the pathogenesis of SLE, by cleaving autoantigens resulting in the formation of immunogenic neo-epitopes and leading to the formation of pathogenic autoantibodies [6]. Increased levels of GrB expressing CD8+ T cells and NK cells are associated with disease activity in SLE [7]. Similarly, GrB-positive CD8+ T cells infiltrate affected skin of SLE patients [8]. GrB expression in patients with SLE in frequently affected tissues such as the kidney has not been analysed. Likewise, the role of Grs other than GrB has not been studied in SLE, so their role in SLE and/or LN pathogenesis remains unknown. In this study we investigated serum levels of GrB, GrK and GrM according to disease activity and IFN signature in SLE. In addition, renal GrB expression was locally examined in relation to LN class and other signs of kidney damage.
Methods
Serum Gr levels
Serum from 30 patients with SLE fulfilling the 1997 ACR criteria for SLE and 20 age- and sex-matched healthy controls (HCs) was collected and stored at −80 °C until further use. Clinical characteristics were obtained from the patients’ charts and disease activity was assessed by the Safety of Estrogen in Lupus Erythematosus National Assessment (SELENA)-SLEDAI. None of the patients had evidence of an active viral infection at inclusion. Serum levels of GrB, GrK and GrM were determined using ELISA (Uscn Life Science, Wuhan, China) according to the manufacturer’s instructions.
IFN signature
Peripheral blood mononuclear cells from 20 patients with SLE and 20 HCs were isolated from peripheral blood by density gradient isolation using Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA). Monocytes were isolated by CD14 magnetic bead (Miltenyi Biotec, Bergish Gladbach, Germany) isolation using an autoMACSpro according to the manufacturer’s instructions. Purified monocytes (104) were stained for 20 min at 4 °C with CD45-PerCP (BioLegend, San Diego, CA, USA), CD16-PE (Dako, Glostrup, Denmark) and CD14-FITC (Miltenyi) to assess the purity of the isolated monocytes. All samples had a purity of >90% (data not shown). RNA was isolated from purified monocytes using the All prep Universal Kit (Qiagen, Venlo, The Netherlands) by the Qiacube (Qiagen) according to the manufacturer’s instructions. cDNA was generated using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR was conducted in duplicate on the following four type I IFN inducible genes: Ly6E, IFITM1, Serping1 and IFI44L. Gene expression was normalized to the housekeeping gene GUSB. Normalized gene expression values were used to calculate type I IFN scores as previously described [9].
GrB evaluation in LN kidney
Formalin-fixed paraffin-embedded renal biopsies of (a historic cohort of) 34 histologically proven proliferative LN patients (including 3 patients with rebiopsies) were cut into 3 µm slides and used for immunohistochemistry. LN was diagnosed by light microscopy on haematoxylin and eosin–, periodic acid–Schiff– or silver-stained sections in combination with immunofluorescence for IgG, IgM, IgA, C1q, C3 and kappa and lambda light chains using the revised classification criteria for glomerulonephritis in SLE [10] by two experienced renal pathologists (R.G. and T.Q.N.). Activity index and chronicity index were scored based on histology slides as described by Austin et al. [11]. GrB expression was determined by immunohistochemistry using the mouse IgG -7 clone (Thermo Fisher, Waltham, MA, USA) as previously described [12]. The number of GrB-positive cells was corrected for biopsy size and recorded using an Aperio Image scope. In healthy renal tissue sections, GrB cannot be detected as previously described [12]. Serum creatinine levels (μmol/l), 24 h urinary protein excretion (g/l) and urine sediment prior to biopsy were obtained from the patients’ charts.
Ethics
For the serum samples, all patients and healthy donors signed an informed written consent form approved by the Medical Research Ethics Committee University Medical Center (UMC) Utrecht review board prior to participation in the study.
For the renal biopsies, we used archival pathology material, which does not interfere with patient care and does not require physical involvement of the patient. Therefore, no ethical approval was required according to Dutch legislation [13]. The use and storage of pseudo-anonymous or coded leftover material for scientific purposes is part of the standard treatment contract with patients and therefore informed consent was not required for the tissue sections according to our Biobank Research Ethics Committee UMC Utrecht [14].
Statistics
Comparisons between groups were made using Mann–Whitney U test when comparing two groups or Kruskall–Wallis with post hoc Dunn’s test when comparing more than two groups. Correlations between parameters were tested using Spearman’s rank correlation. All tests were conducted two-sided with an alpha level set at 0.05. Statistical analysis was performed using GraphPad Prism (version 6; GraphPad Software, La Jolla, CA, USA).
Results
Serum GrB but not GrK and GrM levels are elevated in SLE patients
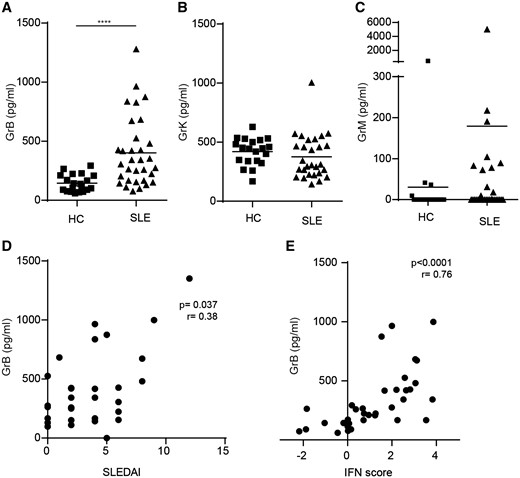
Elevated levels of GrB (P < 0.001) but not GrK (P = 0.12) and GrM (P = 0.40) were found in the sera of the SLE patients (Fig. 1A–C). Only serum GrB levels correlated with disease activity as measured by SELENA-SLEDAI (r = 0.38, P = 0.04; Fig. 1D). Increased levels of GrB were not confined to active SLE patients, as patients with low disease activity [defined as SELENA-SLEDAI ⩽4 (n = 20)] also had elevated serum levels of GrB compared with HCs (P = 0.005, data not shown). Furthermore, GrB levels were strongly associated with the type I IFN score (r = 0.754, P < 0.0001; Fig. 1E). There were no significant differences in serum GrB levels compared with levels of anti-dsDNA antibodies, complement or leucocyte count, nor between patients with or without medication or immunosuppressant use (data not shown). No correlations between disease activity nor the type I IFN score and GrK or GrM levels were observed (data not shown).
Serum GrB, GrK and GrM levels correlate to SLE disease parameters
Serum levels of (A) GrB, (B) GrK and (C) GrM in SLE patients and healthy controls (HCs) were measured using ELISA. (D) Serum GrB levels were correlated to the SLEDAI. (E) GrBs of all available samples (SLE and HCs, n = 35) were correlated with the type I IFN score. Mean Gr levels are depicted and statistical analysis was performed using the Mann–Whitney U test. ****P < 0.0001 compared with HCs.
GrB is expressed in the kidney in LN and associates with signs of kidney damage
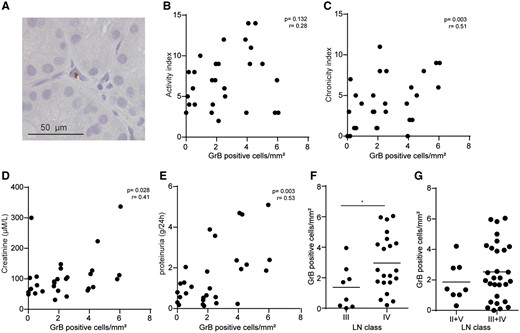
To assess relevant tissue Gr expression beyond the already described GrB presence in SLE skin, we analysed GrB expression in the kidney cortex of proliferative LN from patients with active urinary sediment based on the SELENA-SLEDAI. GrB-positive cells were seen in the majority of biopsies located predominantly in the interstitium between tubules rather than in glomeruli or around blood vessels (Fig. 2A). Staining with an isotype control antibody was negative (data not shown). Whereas no correlation was found between local GrB levels and the LN activity index (r = 0.28, P = 0.13; Fig. 2B), a significant correlation was seen with the LN chronicity index (r = 0.51, P = 0.003; Fig. 2C). Furthermore, GrB expression correlated with signs of kidney damage, such as proteinuria (r = 0.53, P = 0.003; Fig. 2D) and serum creatinine levels (r = 0.41, P = 0.03; Fig. 2E). GrB expression differed between proliferative LN classes III and IV (P = 0.035; Fig. 2F). Finally, there was no significant difference in GrB expression between proliferative classes (III and IV) compared with non-proliferative classes (II and V) (Fig. 2G). Class IV GrB levels were significantly higher than classes II, III and V (P = 0.029) (data not shown).
Local GrB in LN biopsies correlates with kidney damage
(A) A representative picture of a GrB-positive cell in an LN biopsy. GrB-positive cells/mm2 of LN biopsies were determined and correlated with the (B) LN activity index, (C) chronicity index, (D) creatinine and (E) 24 h proteinuria. GrB-positive cells in LN biopsies were observed in (F) different LN classes and (G) non-proliferative classes (II + V) vs proliferative classes (III + IV). *P < 0.05 compared with other LN class.
Discussion
In the present study, the expression levels of several Grs were assessed in serum and kidney samples of patients with SLE. Serum levels of GrK and GrM were not elevated in SLE and did not correlate with disease activity. In contrast, serum GrB levels were increased and correlated with disease activity and the IFN signature. Additionally, GrB levels were associated with kidney damage in LN patients.
Similar to the results of our cohort of patients with SLE, GrK levels were shown not to be altered in the sera of patients with RA [15]. In contrast, GrK has previously been found to be elevated in the circulation of patients with sepsis [16] and during viral infections [15]. This discrepancy suggests that serum levels of specific Grs are differentially regulated in infection-driven as compared with autoimmune-induced inflammation.
Serum GrB levels correlated with the type I IFN signature in SLE patients. The type I IFN signature, a typical feature of SLE immune pathology, is thought to be caused by the activation of pDC endosomal toll-like receptor (TLR) ligands [1]. pDCs are able to express and release GrB [3]. However, stimulation of pDCs with endosomal TLR agonists inhibits GrB release [17]. Hence it is unlikely that TLR triggering leads to the release of both type I IFN and GrB by pDCs in patients with SLE. However, elevated levels of type I IFN in the sera of patients with SLE drive DCs to induce GrB expression in CD8+ cytotoxic T cells in patients with SLE [7]. Therefore pDCs, by producing type I IFN, might have a more indirect effect on GrB release via DCs and CD8+ T cells.
In this study we found positive correlations between SLE disease severity and serum GrB or GrB-positive cell influx in the kidney. Consistent with this, increased levels of GrB-positive CD8+ T cells in the peripheral blood of patients with SLE have been shown to be associated with disease activity [7]. In contrast to our findings, a decrease of GrB-positive SLAMF4+ CD8+ T cells and GrB-positive NK cells in the circulation of patients with SLE has also been demonstrated [18, 19]. Multiple reasons could explain this discrepancy. First, GrB can be expressed by multiple cell types other than SLAMF4+ CD8+ T cells or NK cells, including CD4+ T cells, Treg cells, γδ T cells, NK T cells, neutrophils, basophils, dendritic cells, macrophages and mast cells [20]. Second, GrB might be released from these specific cell types, leading to a selective loss of these T cells and NK cells in SLE [18]. Alternatively, we cannot rule out the possibility that NK cells or GrB-positive SLAMF4+ CD8+ T cells migrate from the circulation into the kidney. A correlation between circulating GrB levels and the SLEDAI in patients with active SLE [mean SLEDAI 31 (range 12–48)] was reported [21]. In our cohort, the median SLEDAI was 4 (range 0–12), indicating a more quiescent and representative SLE cohort, which nevertheless expressed an elevated GrB compared with HCs.
Besides elevated systemic levels, an increased expression of GrB in the kidney was detected. In contrast to immune complexes, which are deposited in the glomeruli, we observed GrB-expressing cells mostly in tubulo-interstitial areas. This pattern of GrB expression is in line with a previous report on GrB expression in renal allografts during acute rejection where the recipient’s T cells are thought to primarily target tubular epithelial cells [12]. Although GrB expression was not observed in the glomeruli, its expression correlated with proteinuria and serum creatinine levels.
Non-proliferative classes II and V showed comparable GrB expression as compared with proliferative classes III and IV. In this context it should be mentioned that 30% of class II biopsies are usually sample errors from class III [22]. Therefore we cannot fully exclude the possibility that we overestimated GrB expression in non-proliferative classes. This is compatible with the notion that the three non-proliferative cases with the highest GrB expression are class II cases. Furthermore, GrB expression correlated to the chronicity index. Since the degree of proteinuria, higher serum creatinine levels and higher chronicity index are associated with poor clinical outcome in LN [23, 24], local GrB expression might influence the prognosis of LN.
Whether or not GrB exerts more impact on lupus-related damage or on SLE disease activity remains an open question. Besides the role of GrB in immune cell–mediated cytotoxicity, GrB may also contribute to autoantigen processing, cytokine responses or matrix remodelling in (renal) fibrosis by cleaving extracellular matrix proteins [6, 7]. This is compatible with potential roles of GrB in the interstitial facet of LN as well as systemically in SLE, influencing the overall prognosis [25]. Further research is required to elucidate the importance of GrB in SLE and LN pathogenesis, to determine how the biomarker value of GrB might be further increased and whether inhibition of GrB might be a valid target for novel therapies.
Funding: This work was supported by the University Medical Center, Utrecht.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
Central Committee on Research Involving Human Subjects (Centrale Commissie Mensgebonden Onderzoek). http://www.ccmo.nl/en/ (7 June 2017, date last accessed).



Comments