-
PDF
- Split View
-
Views
-
Cite
Cite
C. Duftner, C. Dejaco, A. Klauser, A. Falkenbach, H. J. Lakomek, M. Schirmer, High positive predictive value of specific antibodies cross-reacting with a 28-kDa Drosophila antigen for diagnosis of ankylosing spondylitis, Rheumatology, Volume 45, Issue 1, January 2006, Pages 38–42, https://doi.org/10.1093/rheumatology/kei109
Close - Share Icon Share
Abstract
Objectives. Diagnosis of ankylosing spondylitis (AS) can be difficult, and a specific laboratory test has not yet been introduced as a routine diagnostic tool. Our aim was to evaluate the diagnostic value of antibodies specifically binding to a recombinant 28-kDa antigen for the diagnosis of AS.
Methods. Blinded sera were tested for antibodies binding to the procaryotically expressed 28-kDa protein using an enzyme-linked immunosorbent assay (ELISA). This purified 28-kDa protein is produced by a specific clone from an embryonic Drosophila hydei Xgtl I c-DNA library and is bound by human antibodies cross-reacting with both a 36-kDa protein of chromosomes from Drosophila melanogaster and a 69-Da HeLa S3 protein potentially involved in signal transduction pathways.
Results. Serum concentrations of antibodies cross-reacting with this specific antigen were increased in 371 patients with AS compared with 37 healthy controls (39.5 vs 22.6 U/ml; P = 0.004). The positive predictive values of this ELISA test for AS were between 95.1% (95% confidence interval 90.6–97.9%) for a cut-off level of 50 U/ml and 97.4% (92.7–99.5%) for a cut-off level of 75 U/ml, and the sensitivities were between 42.1% (37.0–47.3%) for a cut-off level of 50 U/ml and 30.7% (26.1–35.7%) for a cut-off level of 75 U/ml.
Conclusions. Serum ELISA tests for antibodies cross-reacting with the 28-kDa antigen show a high positive predictive value for AS of more than 95%.
The prevalence of ankylosing spondylitis (AS) is more than 1% in the general population, but many of those affected are still not diagnosed with the disease [1]. In some AS patients it takes years from onset of symptoms until diagnosis of AS, and this diagnostic delay is even more pronounced in HLA-B27-negative AS patients [2]. This can be explained in several ways. According to the modified New York criteria, the diagnosis requires bony changes detected by X-ray in combination with typical clinical symptoms [3]. Magnetic resonance imaging may help to diagnose early disease, but it is expensive and not always available [4]. Except for HLA-B27, there is no generally available laboratory test for AS so far. HLA-B27 shows a strong association with AS [5], but HLA-B27 is negative in about 10% of AS patients and positive in 5% of healthy controls [2]. Taken together, diagnosis of AS is a challenge for clinical practice, and a specific laboratory test could be helpful to shorten the time until diagnosis of AS.
In the pathogenesis of AS the strong association between HLA-B27 and AS suggests an important role for T cells [6–8], but there are also some reports on B-cell activation, suggesting a role of B cells in AS, too [9]. More than a decade ago, by applying cytoimmunofluorescence and immunoblotting techniques to polytene chromosomes of salivary glands or nuclear proteins from Kc cells of Drosophila melanogaster, Lakomek et al. [10–12] identified five antibodies in serum samples from patients with AS, but these were rarely detectable in sera from healthy controls. By using indirect immunofluorescence staining of polytene chromosomes of Drosophila melanogaster, 39% of the AS patients’ sera were positive for antibodies directed against antigens present at the chromosomal locus 93D (heat shock puff) [11]. The other AS-specific Drosophila antigens detected by antibodies from AS patients had sizes of 36, 45, 52 and 72 kDa [12]. As the 36-kDa antigen was the antigen most frequently recognized by sera from AS patients, these antibodies were further characterized: 34% of sera from 95 patients with definite AS based on typical clinical and radiological changes reacted with this antigen, but none of the 29 sera from patients with rheumatoid arthritis (RA) or the 38 apparently healthy controls showed a reaction [9]. Therefore, this antigen was further purified and isolated, and the antibodies directed against the purified 36-kDa Drosophila antigen were found to cross-react with a 69-kDa HeLa S3 protein. Using an embryonic Drosophila hydei Xgtl I c-DNA library, a clone was isolated producing a 28-kDa protein also recognized by these anti-36-kDa antibodies. This 28-kDa protein has been sequenced and expressed procaryotically in Escherichia coli (unpublished data, Pharmacia Diagnostics, Freiburg, Germany). Until now, this enzyme-linked immunosorbent assay (ELISA) based on the 28-kDa antigen has been successfully tested only in a cohort of 77 AS patients [13] and 197 patients with juvenile arthritis [14].
The aim of this study was to test the value of this new ELISA test in detecting antibodies cross-reacting with a procaryotically expressed 28-kDa antigen in a larger cohort of adult AS patients, and to compare the results with those of unselected healthy controls and patients with rheumatic diseases other than AS.
Patients and methods
Patients’ characteristics
Stored sera from 371 patients with AS according to the modified New York criteria [3] and 37 healthy controls were used to evaluate this laboratory test. Residual sera from routinely drawn peripheral venous blood were used, in a manner approved by the local ethics committee. To evaluate the clinical relevance of antibodies cross-reacting with the 28-kDa antigen, we also included sera from patients with immune-mediated diagnoses other than AS, including Behçet's disease (BD, n = 17), RA (n = 68) and primary Sjögren's syndrome (SS, n = 13) according to current diagnostic criteria [15–17].
The patients’ characteristics are summarized in Table 1. All probands were treated either in the Heilstollen Hospital Badgastein-Böckstein, Salzburg (n = 371) or the rheumatic out-patient unit of the Innsbruck Medical University, Austria. The results of the Bath Ankylosing Spondylitis Metrology Index (BASMI) were available for 75 AS patients, and of the Bath Ankylosing Spondylitis Functional Index (BASFI) and the Health Assessment Questionnaire specific for AS (S-HAQ) for 55 AS patients [18–20]. HLA-B27 status was available for 252 AS patients. Of these AS patients, 89.3% were known to be HLA-B27-positive. Control sera were obtained from healthy persons without history of any malignancy or a history of immune-mediated chronic diseases.
Patients’ and controls’ characteristics
| . | Number . | Sex (% female) . | Age (yr) . | ESR (mm/h) . | CRP (mg/dl) . |
|---|---|---|---|---|---|
| Controls | 37 | 54.1 | 44.9±13.6 | <15 (normal) | <0.7(normal) |
| Ankylosing spondylitis | 371 | 21.0 | 51.3±11.2 | 21.9±20.0 | 6.5±14.7 |
| Behçet's disease | 17 | 52.9 | 38.9±10.5 | 16.6±7.2 | 0.8±0.1 |
| Rheumatoid arthritis | 68 | 82.4 | 54.3±15.4 | 44.4±17.1 | 2.4±1.0 |
| Sjögren's syndrome | 13 | 92.3 | 47.9±14.2 | 13.6±9.8 | 0.6±0.3 |
| . | Number . | Sex (% female) . | Age (yr) . | ESR (mm/h) . | CRP (mg/dl) . |
|---|---|---|---|---|---|
| Controls | 37 | 54.1 | 44.9±13.6 | <15 (normal) | <0.7(normal) |
| Ankylosing spondylitis | 371 | 21.0 | 51.3±11.2 | 21.9±20.0 | 6.5±14.7 |
| Behçet's disease | 17 | 52.9 | 38.9±10.5 | 16.6±7.2 | 0.8±0.1 |
| Rheumatoid arthritis | 68 | 82.4 | 54.3±15.4 | 44.4±17.1 | 2.4±1.0 |
| Sjögren's syndrome | 13 | 92.3 | 47.9±14.2 | 13.6±9.8 | 0.6±0.3 |
Patients’ and controls’ characteristics
| . | Number . | Sex (% female) . | Age (yr) . | ESR (mm/h) . | CRP (mg/dl) . |
|---|---|---|---|---|---|
| Controls | 37 | 54.1 | 44.9±13.6 | <15 (normal) | <0.7(normal) |
| Ankylosing spondylitis | 371 | 21.0 | 51.3±11.2 | 21.9±20.0 | 6.5±14.7 |
| Behçet's disease | 17 | 52.9 | 38.9±10.5 | 16.6±7.2 | 0.8±0.1 |
| Rheumatoid arthritis | 68 | 82.4 | 54.3±15.4 | 44.4±17.1 | 2.4±1.0 |
| Sjögren's syndrome | 13 | 92.3 | 47.9±14.2 | 13.6±9.8 | 0.6±0.3 |
| . | Number . | Sex (% female) . | Age (yr) . | ESR (mm/h) . | CRP (mg/dl) . |
|---|---|---|---|---|---|
| Controls | 37 | 54.1 | 44.9±13.6 | <15 (normal) | <0.7(normal) |
| Ankylosing spondylitis | 371 | 21.0 | 51.3±11.2 | 21.9±20.0 | 6.5±14.7 |
| Behçet's disease | 17 | 52.9 | 38.9±10.5 | 16.6±7.2 | 0.8±0.1 |
| Rheumatoid arthritis | 68 | 82.4 | 54.3±15.4 | 44.4±17.1 | 2.4±1.0 |
| Sjögren's syndrome | 13 | 92.3 | 47.9±14.2 | 13.6±9.8 | 0.6±0.3 |
Protein and Western blotting
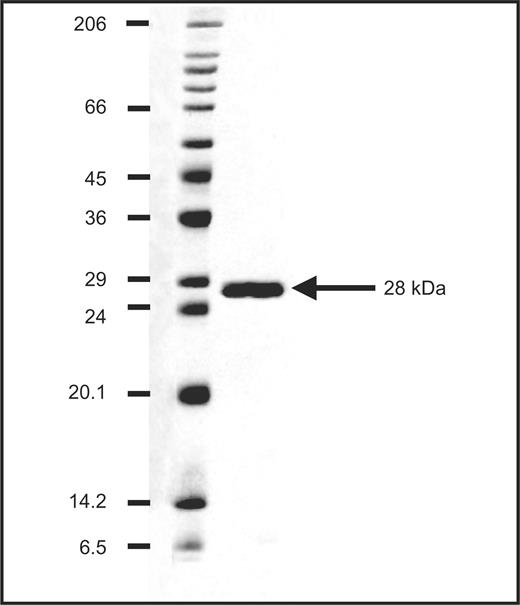
The 28-kDa Drosophila antigen used for the ELISA was procaryotically expressed in E. coli (Pharmacia Diagnostics). As shown in Fig. 1, protein blotting of the recombinant 28-kDa antigen lot 14080 was performed on sodium dodecyl sulphate (SDS) gels with 4% stacking and 13.5% separation gel using a Hoefer Mighty Small II vertical electrophoresis chamber (Hoefer Science Instruments, San Francisco, CA, USA). Ten microlitres of the protein sample (concentration 0.5 mg/ml) and molecular weight standards (Protein Standard: Sigma M-4038, Wide Range; Sigma, Munich, Germany) were run under reducing conditions and stained with Coomassie Brilliant blue.
Protein blotting of the antigen lot 14080 of the 28-kDa Drosophila antigen used for the ELISA testing. Staining of purified Drosophila antigen and wide-range molecular weight markers (protein standard: Sigma M-4038, Wide Range, Sigma, Munich, Germany) with Coomassie Brilliant blue after SDS–PAGE under reducing conditions.
Western blotting of E. coli extract (strain BL21) was performed on SDS gels with 4% stacking and 13.5% separation gel under reducing conditions using a Hoefer Mighty Small II vertical electrophoresis chamber. After transfer to polyvinylidene fluoride (PVDF) membranes (Semi-Phor™; Hoefer Science Instruments), the membranes were sliced, incubated with rabbit anti-E. coli polyclonal antibodies (DakoCytometation, Glostrup, Denmark; B-0357, 1:5000), patients’ sera and control sera, and counterstained with alkaline phosphatase (AP)-labelled anti-rabbit monoclonal antibodies (Sigma A-2306, 1:3000) or anti-human IgG-AP labelled antibodies (Sigma A-3187, 1:30 000), as appropriate. Accordingly, the recombinant 28-kDa antigen lot 14080 protein sample (concentration 0.124 mg/ml) was loaded and, after transfer to the PVDF membrane, incubated with rabbit anti-E. coli polyclonal antibodies, patients’ sera at concentrations of 1:5000 and 1:2000 and sera from controls at a concentration of 1:2000 and counterstained again with AP-labelled anti-rabbit monoclonal antibodies or anti-human IgG antibodies.
Determination of antibodies cross-reacting with the 28-kDa Drosophila antigen
All sera were blinded before further testing. Concentrations of antibodies cross-reacting with the 28-kDa Drosophila antigen were determined by the laboratory of Pharmacia Diagnostics (Freiburg, Germany) using an ELISA test system. Ninety-six-well plates were coated with the antigen overnight. After saturation of the plates with a 1% solution of bovine serum albumin, calibrators (ready to use), control sera and patients’ sera in dilutions from 1:100 to 1:6400 were incubated for 30 min at room temperature. After washing, peroxidase-conjugated anti-IgG was incubated for 30 min at room temperature followed by another washing step. Then 3,3′,5,5′-tetramethylbenzidine was added as a chromogenic substrate. The substrate reaction was stopped after 10 min by 0.5 M sulphuric acid and analysis was performed by photometry at 492 nm with a reference filter at 620 nm. The antibody concentrations of the patient samples were calculated from the calibration curve in arbitrary units. The cut-off levels of 50 and 60 U/ml antibody concentrations were the same as those used previously [13]. The cut-off level of 60 U/ml has been described as reliable, and levels between 50 and 60 U/ml were considered as suspicious for AS. The cut-off level of 75 U/ml was chosen arbitrarily to test for a possible further increment in the ELISA's specificity.
Statistical evaluation
Data are expressed as median and range as the antibody titres showed a non-parametric distribution (Kolmogorov–Smirnov test). The Mann–Whitney test was used to compare serum antibody concentrations between groups and Spearman's ρ to assess possible correlations between serum antibody titres and clinical measurements. All statistical tests were performed using the SPSS program, version 11.0 (SPSS, Chicago, IL, USA). The χ2 test was performed using Java-supported biometry from the University of Münster. In the case of multiple testing, Bonferroni adjustment was performed as indicated. P<0.05 was considered significant and P<0.01 highly significant. The likelihood ratio was calculated as sensitivity/(1 − specificity).
Results
Specific reaction of patients’ sera to the recombinant 28-kDa antigen but not to E. coli antigens
The purity and molecular size of the 28-kDa Drosophila antigen used for the ELISA to detect antibodies specifically directed against this antigen are shown in Fig. 1.
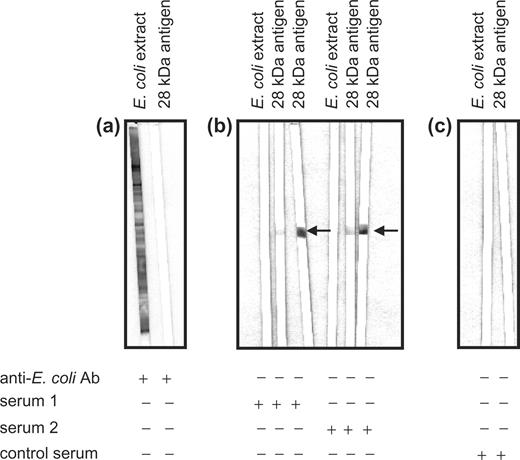
As the 28-kDa Drosophila antigen is procaryotically expressed in E. coli, the Western blot technique was used to detect impurities of E. coli in the protein preparation. Using polyclonal, polyspecific antibodies against E. coli antigens, no reaction to the purified 28-kDa antigen was found (Fig. 2a).
Specific reaction of the patient's sera to the recombinant 28-kDa Drosophila antigen. Western blot analysis to show (a) lack of cross-reaction of polyclonal, polyspecific antibodies against E. coli antigens (anti-E. coli Ab) to the purified 28-kDa Drosophila antigen, and (b) lack of detection of E. coli antigens by two independent patients’ sera (sera 1 and 2) at two different concentrations. (c) Sera from healthy controls (control serum) showed a reaction neither to the E. coli antigens nor to the 28-kDa Drosophila antigen.
Western blotting was also performed to exclude detection of E. coli antigens by patients’ sera. In these experiments no reaction of patients’ sera with E. coli extract was detected, but patients’ sera reacted with the 28-kDa Drosophila antigen at two different concentrations (Fig. 2b). Sera from healthy control subjects were used as negative controls and showed neither a reaction to the 28-kDa Drosophila antigen nor to the E. coli antigens (Fig. 2c).
Increased concentrations of antibodies cross-reacting with a purified 28-kDa Drosophila antigen in ankylosing spondylitis
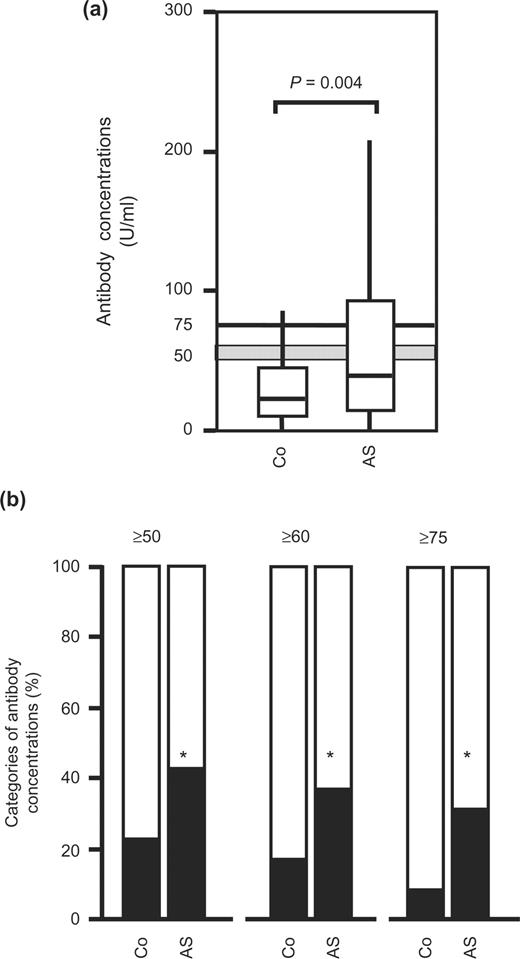
In this blinded design of serum testing, concentrations of antibodies cross-reacting with this 28-kDa Drosophila antigen were increased in sera from AS patients [39.5 U/ml (0.8–330)] compared with healthy controls [22.6 U/ml (1.3–330)] (Fig. 3a). As shown in Fig. 3b, the percentages of antibody-positive patients were significantly higher in the AS group than in the healthy controls, irrespective of the cut-off levels of 50, 60 and 75 U/ml (P = 0.048, P = 0.045 and P = 0.016 after Bonferroni adjustment, respectively).
Antibodies in healthy controls and patients with ankylosing spondylitis. (a) Concentrations of antibodies cross-reacting with a 28-kDa Drosophila antigen of D. melanogaster are shown from unselected controls (Co, n = 37) and patients with ankylosing spondylitis (AS, n = 371). Box and whisker plots show 50% of cases within the boxes and all data excluding outliers (values ≥1.5 of the length of the boxes) between the end-points of the whiskers (lines). P-values <0.05 are considered significant (Mann–Whitney test). (b) Classification of controls (Co) and patients with ankylosing spondylitis (AS) in categories according to the concentrations of antibodies cross-reacting with the 28-kDa Drosophila antigen (≥50, ≥60 and ≥75 U/ml). *Significant differences (P<0.05, χ2 test with Bonferroni adjustment for multiple testing).
Accordingly, the diagnostic value of the test also depended on the cut-off levels (Table 2). Most notably, the positive predictive value was higher than 95.1% even with the lowest cut-off level of 50 U/ml. The odds ratios were calculated to be 2.6, 2.9 and 5.0 and the likelihood ratios were 1.9, 2.2 and 3.8 for the cut-off levels of 50, 60 and 75 U/ml, respectively.
Diagnostic values of antibody concentrations cross-reacting with the 28-kDa Drosophila antigen compared with healthy controls
| . | Cut-off levels of antibodies cross-reacting with the 28-kDa Drosophila antigen (U/ml) . | . | . | ||
|---|---|---|---|---|---|
| Diagnostic value . | ≥50 (95% CI) . | ≥60 (95% CI) . | ≥75 (95% CI) . | ||
| Sensitivity | 42.1 (37.0–47.3) | 36.1 (31.2–41.2) | 30.7 (26.1–35.7) | ||
| Negative predictive value | 11.9 (8.1–16.6) | 11.6 (8.0–16.0) | 11.7 (8.2–15.9) | ||
| Specificity | 78.4 (61.8–90.2) | 83.8 (68.0–93.8) | 91.9 (78.1–98.3) | ||
| Positive predictive value | 95.1 (90.6–97.9) | 95.7 (90.9–98.4) | 97.4 (92.7–99.5) | ||
| Odds ratio | 2.6 (1.1–6.4) | 2.9 (1.1–8.0) | 5.0 (1.4–21.0) | ||
| Likelihood ratio | 1.9 (1.2–3.8) | 2.2 (1.3–5.0) | 3.8 (1.6–15.4) | ||
| . | Cut-off levels of antibodies cross-reacting with the 28-kDa Drosophila antigen (U/ml) . | . | . | ||
|---|---|---|---|---|---|
| Diagnostic value . | ≥50 (95% CI) . | ≥60 (95% CI) . | ≥75 (95% CI) . | ||
| Sensitivity | 42.1 (37.0–47.3) | 36.1 (31.2–41.2) | 30.7 (26.1–35.7) | ||
| Negative predictive value | 11.9 (8.1–16.6) | 11.6 (8.0–16.0) | 11.7 (8.2–15.9) | ||
| Specificity | 78.4 (61.8–90.2) | 83.8 (68.0–93.8) | 91.9 (78.1–98.3) | ||
| Positive predictive value | 95.1 (90.6–97.9) | 95.7 (90.9–98.4) | 97.4 (92.7–99.5) | ||
| Odds ratio | 2.6 (1.1–6.4) | 2.9 (1.1–8.0) | 5.0 (1.4–21.0) | ||
| Likelihood ratio | 1.9 (1.2–3.8) | 2.2 (1.3–5.0) | 3.8 (1.6–15.4) | ||
The likelihood ratio was calculated as sensitivity/(100 − specificity) to provide the post-test probability used for the diagnostic algorithm by Rudwaleit et al. [21].
Diagnostic values of antibody concentrations cross-reacting with the 28-kDa Drosophila antigen compared with healthy controls
| . | Cut-off levels of antibodies cross-reacting with the 28-kDa Drosophila antigen (U/ml) . | . | . | ||
|---|---|---|---|---|---|
| Diagnostic value . | ≥50 (95% CI) . | ≥60 (95% CI) . | ≥75 (95% CI) . | ||
| Sensitivity | 42.1 (37.0–47.3) | 36.1 (31.2–41.2) | 30.7 (26.1–35.7) | ||
| Negative predictive value | 11.9 (8.1–16.6) | 11.6 (8.0–16.0) | 11.7 (8.2–15.9) | ||
| Specificity | 78.4 (61.8–90.2) | 83.8 (68.0–93.8) | 91.9 (78.1–98.3) | ||
| Positive predictive value | 95.1 (90.6–97.9) | 95.7 (90.9–98.4) | 97.4 (92.7–99.5) | ||
| Odds ratio | 2.6 (1.1–6.4) | 2.9 (1.1–8.0) | 5.0 (1.4–21.0) | ||
| Likelihood ratio | 1.9 (1.2–3.8) | 2.2 (1.3–5.0) | 3.8 (1.6–15.4) | ||
| . | Cut-off levels of antibodies cross-reacting with the 28-kDa Drosophila antigen (U/ml) . | . | . | ||
|---|---|---|---|---|---|
| Diagnostic value . | ≥50 (95% CI) . | ≥60 (95% CI) . | ≥75 (95% CI) . | ||
| Sensitivity | 42.1 (37.0–47.3) | 36.1 (31.2–41.2) | 30.7 (26.1–35.7) | ||
| Negative predictive value | 11.9 (8.1–16.6) | 11.6 (8.0–16.0) | 11.7 (8.2–15.9) | ||
| Specificity | 78.4 (61.8–90.2) | 83.8 (68.0–93.8) | 91.9 (78.1–98.3) | ||
| Positive predictive value | 95.1 (90.6–97.9) | 95.7 (90.9–98.4) | 97.4 (92.7–99.5) | ||
| Odds ratio | 2.6 (1.1–6.4) | 2.9 (1.1–8.0) | 5.0 (1.4–21.0) | ||
| Likelihood ratio | 1.9 (1.2–3.8) | 2.2 (1.3–5.0) | 3.8 (1.6–15.4) | ||
The likelihood ratio was calculated as sensitivity/(100 − specificity) to provide the post-test probability used for the diagnostic algorithm by Rudwaleit et al. [21].
HLA-B27 predisposition, age, sex, clinical scores and the concentrations of antibodies cross-reacting with the 28-kDa Drosophila antigen
Antibody concentrations were independent of HLA-B27 status. No difference in the concentrations of antibodies cross-reacting with 28-kDa antigen was seen between HLA-B27-negative patients [33.8 U/ml (0.8–242.5)] and HLA-B27 positive patients [37.5 U/ml (1.4–330)]. Also, the antibody concentrations did not depend on the age of the patients, and did not correlate with the BASMI, BASFI and S-HAQ scores using Spearman's ρ.
Although the difference was not significant, women tended to have a higher antibody concentration [49.2 U/ml (2.3–330.0 U/ml)] than men [38.2 U/ml (0.8–330.0 U/ml)] (P = 0.091).
Antibodies cross-reacting with a purified 28-kDa Drosophila antigen in immune-mediated rheumatic diseases other than AS
No difference in the concentration of antibodies was seen between healthy controls [22.6 U/ml (1.3–330.0 U/ml)] and patients with BD, RA and SS [15.3 U/ml (1.2–83.60 U/ml), 20.1 U/ml (1.1–320.0 U/ml) and 24.1 U/ml (1.2–67.4 U/ml), respectively]. Also, when categorized according to the different cut-off value (50, 60 and 75 U/ml), there were no differences in the percentages of antibody-positive BD, RA and SS patients and healthy controls.
Discussion
This study shows for a large cohort of AS patients that serum testing for antibodies cross-reacting with a specific recombinant 28-kDa antigen has a high positive predictive value (>95%) for AS compared with healthy controls (Table 2). Given the high prevalence of low back pain in the general population and the difficulty of early diagnosis of AS in clinical practice, this test may be helpful despite its limited sensitivity (between 30.7% and 42.1%). This test, for example, may be helpful in increasing the post-test-probability, which has been recently introduced by Rudwaleit et al. [21] as an important tool in a new diagnostic algorithm for the early diagnosis of axial spondyloarthritis (SpA) and AS. Compared with the likelihood ratio of 9.0 for HLA-B27, for example, our new laboratory test shows a likelihood ratio of 1.9, 2.2 and 3.8 for cut-off levels of ≥50, 60 and 75 U/ml, respectively. Thus, especially in HLA-B27-negative patients, in whom the diagnosis of AS is delayed up to 11.4 yr from the onset of the first symptoms [2], a positive ELISA test result can contribute to the diagnostic evaluation.
The major limitation of this test is its relatively low sensitivity, especially when using the higher cut-off level. In our hands the sensitivity of the test ranged between 30.7% and 42.1% for cut-off levels between 50 and 75 U/ml (Table 2). This corresponds to an earlier study, which showed a sensitivity of 20.8% in AS patients, whereas in undifferentiated SpA the sensitivity was as high as 42.3% [13]. Therefore, a negative test result certainly does not exclude patients from further examination. As we had recruited not only patients with early active AS but also patients with long-standing, inactive disease, we may have underestimated the sensitivity of this test for early active disease. In these elderly patients, B-cell activation with subsequent production of autoantibodies is certainly reduced, and may not have been detected in our study. Contamination of the 28-kDa antigen lot with E. coli could be excluded as E. coli antigen preparations were not detected by patients’ sera using the Western blot technique (Fig. 2). Vice versa, the 28-kDa antigen was not detected by polyclonal anti E. coli antibodies.
Given the high prevalence of SpA in the general population, we cannot exclude the possibility that some of our controls will develop SpA during follow-up. In view of the goal of diagnosing AS early, the patients with higher titres of antibodies and clinical signs of low back pain will be examined irrespective of their underlying diagnosis during follow-up. Indeed, sacroiliitis has been described even in RA [22, 23]. BD has also been associated with SpA in a number of studies, and some authors still consider BD a distinct entity in the group of SpA. Data by Brandt et al. [24] suggested an increased prevalence of sicca symptoms and SS in patients with SpA. Patients with SS may therefore include those with previously undiagnosed AS.
In conclusion, this ELISA test to detect antibodies cross-reacting with a specific procaryotically expressed 28-kDa antigen is easy and useful. A positive test result has a high positive predictive value, thus offering an additional tool for further diagnostic decision-making. The positive predictive values of the antibodies for AS compared with healthy controls were higher than 95% and sensitivities were between 30.7% (26.1–35.7) and 42.1% (37.0–47.3), depending on the cut-off values used.

*These authors contributed equally to the design and performance of the study, and thus both have to be considered as last authors.
We thank Dr Höpfl and Dr Schach of Pharmacia Diagnostics, Freiburg, for the protein and Western blots, the blinded performance of the ELISAs and providing the investigators with the blinded results.
The authors have declared no conflicts of interest.
References
Olivieri I, van Tubergen A, Salvarani C, van der Linden S. Seronegative spondyloarthritides.
Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs positive patients with ankylosing spondylitis.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria.
Klauser A, Bollow M, Calin A et al. Workshop report: clinical diagnosis and imaging of sacroiliitis, Innsbruck, Austria, October 9, 2003.
Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27.
Hermann E, Fleischer B, Meyer zum Buschenfelde KH. Bacteria-specific cytotoxic CD8+ T cells: a missing link in the pathogenesis of the HLA-B27-associated spondylarthropathies.
Boyle LH, Hill Gaston JS. Breaking the rules: the unconventional recognition of HLA-B27 by CD4+ T lymphocytes as an insight into the pathogenesis of the spondyloarthropathies.
Duftner C, Goldberger C, Falkenbach A et al. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28–T cells in ankylosing spondylitis.
Lakomek HJ, Plomann M, Specker C, Schwochau M. Ankylosing spondylitis:an autoimmune disease?
Will H, Lakomek HJ, Bautz EK. Reaction of human auto-antibodies with antigens of polytene chromosomes of Drosophila.
Lakomek HJ, Will H, Zech M, Kruskemper HL. A new serologic marker in ankylosing spondylitis.
Lakomek HJ, Schwochau M, Decken K, Juli E, Will H, Kruskemper HL. Attempts towards a serological diagnosis of ankylosing spondylitis.
Lakomek HJ, Hagemann O, Schochau M, Northemann W. Ein neuer Antikörper für die Frühdiagnose der Spondarthritiden.
Wachter U, Lakomek HJ, Pentrop S, Ganser S. Spondylarthropathy-antibody—a predictive parameter to assess courses of disease in juvenile arthritis?
Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease.
Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis.
Vitali C, Bombardieri S, Moutsopoulos HM et al. Preliminary criteria for the classification of Sjoegren's syndrome.
Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index.
Calin A, Garrett S, Whitelock H et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index.
Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloarthropathies.
Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early.
Luthra HS, Ferguson RH, Conn DL. Coexistence of ankylosing spondylitis and rheumatoid arthritis.
Toussirot E, Acquaviva PC. Coexisting rheumatoid arthritis and ankylosing spondylitis discussion of 3 cases with review of the literature.
Author notes
Department of Internal Medicine and 1Department of Radiology, Innsbruck Medical University, Innsbruck and 2Rehabilitationszentrum-Sonderkrankenanstalt Bad-Ischl der Pensionsversicherungsanstalt, Bad Ischl, and Gastein Research Institute, Bad Gastein, Austria and 3Clinic of Rheumatology and Physical Medicine, Minden, Germany.




Comments