-
PDF
- Split View
-
Views
-
Cite
Cite
Artur Conde, Paulo Silva, Alice Agasse, Carlos Conde, Hernâni Gerós, Mannitol Transport and Mannitol Dehydrogenase Activities are Coordinated in Olea europaea Under Salt and Osmotic Stresses, Plant and Cell Physiology, Volume 52, Issue 10, October 2011, Pages 1766–1775, https://doi.org/10.1093/pcp/pcr121
Close - Share Icon Share
Abstract
The intracellular accumulation of organic compatible solutes functioning as osmoprotectants, such as polyols, is an important response mechanism of several plants to drought and salinity. In Olea europaea a mannitol transport system (OeMaT1) was previously characterized as a key player in plant response to salinity. In the present study, heterotrophic sink models, such as olive cell suspensions and fruit tissues, and source leaves were used for analytical, biochemical and molecular studies. The kinetic parameters of mannitol dehydrogenase (MTD) determined in cells growing in mannitol, at 25°C and pH 9.0, were as follows: Km, 54.5 mM mannitol; and Vmax, 0.47 μmol h−1 mg−1 protein. The corresponding cDNA was cloned and named OeMTD1. OeMTD1 expression was correlated with MTD activity, OeMaT1 expression and carrier-mediated mannitol transport in mannitol- and sucrose-grown cells. Furthermore, sucrose-grown cells displayed only residual OeMTD activity, even though high levels of OeMTD1 transcription were observed. There is evidence that OeMTD is regulated at both transcriptional and post-transcriptional levels. MTD activity and OeMTD1 expression were repressed after Na+, K+ and polyethylene glycol (PEG) treatments, in both mannitol- and sucrose-grown cells. In contrast, salt and drought significantly increased mannitol transport activity and OeMaT1 expression. Taken together, these studies support that olive trees cope with salinity and drought by coordinating mannitol transport with intracellular metabolism.
The nucleotide sequence reported in this paper has been submitted to GenBank under accession number ABR31791.1.
Introduction
High salinity and drought reduce plant growth and consequently agricultural productivity more than any other environmental stress (reviewed by Hussain et al. 2008). The negative effects of soil salinity are a consequence of ion cytotoxicity (mostly due to Na+, Cl− and SO4−), hyperosmotic stress (reviewed by Zhu 2002), nutritional imbalance (Grattan and Grieve 1999) and oxidative stress (Hernández et al. 2001). Plants living in habitats where soil salinity and drought are significant have developed various morphological, physiological and molecular adaptations to cope with these stresses (Winicov 1998, Munns and Tester 2008). The intracellular accumulation of organic compatible solutes functioning as osmoprotectants, such as polyols, proline and quaternary ammonium compounds, represents an important metabolic adjustment of several stress-tolerant plants (Rathinasabapathi 2000).
Polyols are the reduced form of aldoses and ketoses, and can be found in all living forms (Noiraud et al. 2001a). In some plants these sugar alcohols are, together with sucrose (as in celery) or raffinose saccharides (as in olive), direct products of photosynthesis and serve similar functions such as translocation of carbon skeletons and energy between source and sink organs. Mannitol is the most common polyol in nature and has been observed in >100 vascular plant species of several families including the Apiaceae (celery, parsley and carrot), Rubiaceae (coffee) and Oleaceae (olive and privet) (reviewed by Conde et al. 2008). Mannitol synthesis takes place in mature leaves from mannose-6-phosphate by the joint action of a NADPH-dependent mannose-6-phosphate reductase (M6PR) and a mannose-6-phosphate phosphatase. It is then translocated through the phloem to heterotrophic sink tissues where it can be either stored or oxidized to mannose through an NAD+-dependent mannitol dehydrogenase (MTD) and used as a carbon and energy source (reviewed by Stoop et al. 1996, Noiraud et al. 2001b, Conde et al. 2008).
Olive is an evergreen tree traditionally cultivated in the Mediterranean basin (Loumou and Giourga 2003) where the summer months are characterized by high temperatures, high light levels, high vapor pressure deficit and lack of precipitation. Under these climate conditions, often combined with an increasing salinization of the soils, olive trees have to cope with limited water availability (Angelopoulos et al. 1996). Mannitol is one of the primary photosynthetic products in Olea europaea (Drossopoulos and Niavis 1988, Cataldi et al. 2000) and its content in olive pulp increases during maturation, reaching values of 8 mg g−1 DW in the fully ripe fruit (Marsilio et al. 2001). The elucidation of the role of mannitol as a carbon and energy source for plant growth, as well as a solute protecting against biotic and abiotic assumes critical importance for the enhancement of yield potential of O. europaea and other plants. Indeed, significant knowledge has been achieved in understanding the protective role of mannitol in several plants, including O. europaea (Yancey et al. 1982, Tarczynski et al. 1993, Williamson et al. 1995, Shen et al. 1997b, Jennings et al. 1998, Patonnier et al. 1999, Hu et al. 2005, Voegele et al. 2005, Conde et al. 2007a, Seckin et al. 2009).
During leaf maturation in celery, the proportion of mannitol increases dramatically, and mannitol exported to the phloem and sink tissues is directly correlated with increased mannitol biosynthesis, whereas sucrose remains fairly constant (Davis et al. 1988). Due to the similarities between celery and olive regarding the use of mannitol, this is expected also to be the case in O. europaea. In celery, there is a close relationship between the development of photosynthetic capacity, mannitol synthesis and M6PR activity (Everard et al. 1993, Everard et al. 1997). Root zone salinity increased the activity of M6PR up to 6-fold in celery leaves and promoted changes in photosynthetic carbon partitioning from sucrose to mannitol, facilitating its accumulation in leaf tissues, providing improved stress tolerance (Everard et al. 1994). Similarly, in peach (Prunus persica), the in vitro activity of aldose-6-phosphate reductase, the key enzyme in sorbitol synthesis, increased linearly in response to drought stress, as did the partitioning of newly fixed carbon into sorbitol and its extrusion and concentration in the phloem sap (Escobar-Gutiérrez et al. 1998). A significant shift in photosynthetic carbon partitioning to mannitol under salt or drought stress has also been clearly demonstrated in leaves of O. europaea, confirming the role of mannitol as a potential osmoregulator in leaf mesophyll (Tattini et al. 1996, Gucci et al. 1996, Gucci et al. 1997, Gucci et al. 1998, Dichio et al. 2009, Melgar et al. 2009, Remorini et al. 2009, Cimato et al. 2010).
Studies conducted in our laboratory have yielded some information concerning the biochemical and molecular mechanisms involved in sugar and polyol uptake and metabolism in olive (Oliveira et al. 2002, Conde et al. 2007a, Conde et al. 2007b, Conde et al. 2007c). In particular, the relevance of mannitol transport regulation for osmotic adjustments of O. europaea cells in response to NaCl stress was addressed (Conde et al. 2007a). It was shown that the addition of 500 mM NaCl promoted the increase of OeMaT1 (Olea europaea Mannitol Transporter 1) transcription and mannitol transport activity over time when compared with control cells. The parallel between the Vmax of mannitol transport and OeMaT1 message levels supported that the expression of OeMaT1 is responsible for the increase of mannitol transport capacity under salt stress conditions.
In the present study, we performed a detailed biochemical characterization of olive NAD+-dependent MTD, identified its potential encoding cDNA (OeMTD1) and provided a solid body of evidence that indicates that its activity is tightly coordinated with mannitol transport capacity in order to regulate the cellular mannitol pool, which proved to be critical for olive cells to cope with salinity and drought conditions.
Results
Mannitol dehydrogenase activity, OeMTD1 cloning and expression, and coordination with mannitol transport activity and OeMaT1 expression
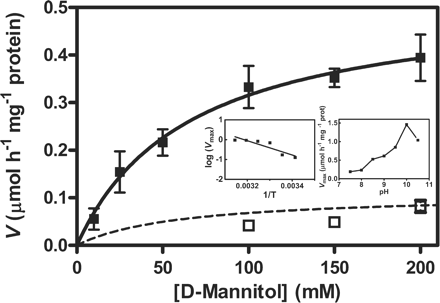
The activity of MTD measured in homogenates from cells of O. europaea growing in 2% mannitol is in line with a previous kinetic characterization (Conde et al. 2007a). In the present study, MTD activity was determined in cells growing in mannitol at different temperatures, ranging from 20 to 50°C, and pH values, ranging from 7.5 to 10.5, and subsequently compared with the activity measured at 25°C and pH 9 in homogenates from cells growing in 2% sucrose (Fig. 1), allowing for the subsequent correlation with gene expression in both growth conditions. The kinetic parameters, at 25°C and pH 9.0, were as follows: Km, 54.5 mM mannitol; and Vmax, 0.47 μmol h−1 mg−1 protein. Temperature and pH dramatically affect enzyme activity, the Vmax being highest at pH 10 and 45°C. An activation energy value of 68.9 kJ mol−1 was estimated (data not shown).
Mannitol dehydrogenase (MTD) activity, measured at 25°C and pH 9.0, in extracts of O. europaea suspension-cultured cells cultivated up to the mid-exponential growth phase with 2% (w/v) mannitol (filled squares) and 2% (w/v) sucrose (open squares). Insets: dependence of Vmax of mannitol oxidation in extracts of cells growing in mannitol on the temperature of the assay medium, at pH 9.0 (left graph) and dependence of Vmax on pH, at 25°C (right graph). Expression of OeMTD1 (Olea europaea mannitol dehydrogenase 1) in both experimental conditions is depicted in Fig. 4. Error bars denote the SD from the mean, n = 3.
The cloning of an 816 bp cDNA named OeMTD1 (accession No. ABR31791.1) allowed us to confirm by means of CLUSTAL W alignment its high similarity with other plant MTDs (Fig. 2). Although we cannot exclude that other MTD isoforms may operate in O. europaea that could, at least theoretically, cross-hybridize with the probe in the gene expression studies, the signals observed in Northern experiments were associated with OeMTD1 expression. However, it has been demonstrated that only one isoform of MTD exists in celery, mainly located in the cytoplasm and to a lesser extent in the nucleus, or secreted as in other plants (Williamson et al. 1995, Stoop et al. 1995, Stoop et al. 1996, Yamamoto et al. 1997, Zamski et al. 2001, Blackburn et al. 2010).
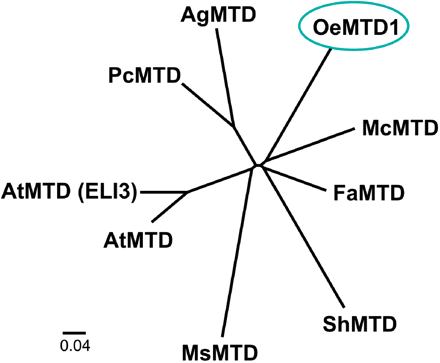
Phylogenetic tree showing the relationship between OeMTD1 and other MTD genes from other plants. Sequence analysis, multiple sequence alignments (using the ClustalW algorithm) and the unrooted phylogenetic tree were carried out using the Phylip 3.68 software package, and the final tree was built using the FigTree 1.1.2. software package. GenBank accession numbers are as follows: Olea europaea mannitol dehydrogenase (OeMTD1), ABR31791.1; Apium graveolens mannitol dehydrogenase (AgMTD), 2117420A; Petroselinum crispum mannitol dehydrogenase (PcMTD), P42754.1; Fragaria ananassa mannitol dehydrogenase (FaMTD), Q9ZRF1; Mesenbryanthemum crystallinum mannitol dehydrogenase (McMTD), P93257; Stylosanthes humilis mannitol dehydrogenase (ShMTD), Q43137.1; Medicago sativa mannitol dehydrogenase (MsMTD), O82515.1; Arabidopsis thaliana putative mannitol dehydrogenase (AtMTD), also known as cinnamyl-alcohol dehydrogenase 8 (AtCAD8), Q02972; and Arabidopsis thaliana putative mannitol dehydrogenase (AtMTD) elicitor-activated 3 (ELI3), NP_001031805.1.
Fig. 3 depicts the correlation between OeMTD1 and OeMaT1 transcript levels and mannitol transport activity throughout cell growth with 0.5% (w/v) mannitol (initial concentration) as the carbon and energy source. In terms of mannitol availability in the extracellular medium, these conditions are equivalent to those from the mid–late exponential growth phase to the stationary phase in cultures with an initial mannitol concentration of 2%. The results indicate that cells keep OeMaT1 transcription at basal levels when mannitol is present in measurable amounts, in line with previous work by Conde et al. (2007a). On the fourth day, when the external concentration of mannitol fell below approximately 0.1% (w/v), the activity of the polyol transport system increased and its maximal activity was reached at day 6, when mannitol was completely exhausted from the culture medium. Although high mannitol levels seem to repress carrier-mediated manitol transport, a different correlation is observed for OeMTD1 transcription because OeMTD1 expression decreases together with the substrate levels in the culture, from day 1 to day 5.
Growth, mannitol/H+ symport activity and OeMaT1 and OeMTD1 expression in O. europaea cell suspensions. (A) Cells were cultivated with 0.5% (w/v) mannitol (initial concentration), and d-[14C]mannitol uptake (filled triangles) was measured in cell aliquots harvested from the culture at the times indicated. Values are represented by the mean of two independent experiments. (B) Northern blot analysis of OeMaT and OeMTD1 expression. In each condition, 50 μg of RNA were used as in Conde et al. (2007a).
Mannitol transport and metabolism under salt and drought stresses in mannitol- and sucrose-grown cells
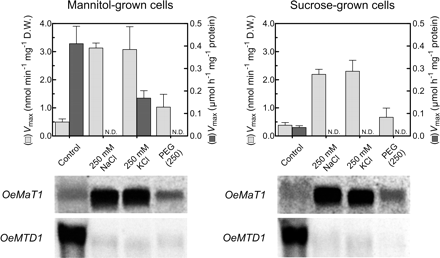
Mannitol transport activity and MTD activity were correlated with OeMaT1 and OeMTD1 expression under salt (NaCl or KCL) and drought [polyethylene glycol (PEG)] stress both in cells growing in 2% mannitol and in cells growing in 2% sucrose, which were collected at the mid–late exponential growth phase. The results showed that non-treated 2% mannitol-grown cells (control mannitol-cells) display basal mannitol transport activity and OeMaT1 transcript levels, together with a high MTD activity and high level of OeMTD1 transcription (Fig. 4), in accordance with the data from Fig. 3 (day 1–4 after subculture). Conversely, in non-treated sucrose-grown cells (control sucrose-cells), both the mannitol transporter and MTD activities are kept at basal levels, but OeMTD expression is high (Fig. 4).
Effect of NaCl, KCl and PEG on the Vmax of mannitol transport and the Vmax of mannitol oxidation in O. europaea cells growing in mannitol or in sucrose, and expression of OeMaT1 and OeMTD1. Cells were collected at mid-exponential growth phase and subjected to salt and drought treatment during 24 h. Error bars denote the SD from the mean, n = 3. In each lane, 50 μg of RNA were used.
The addition of 250 mM NaCl to cells growing in mannitol caused a severe decrease in MTD activity associated with a repression of OeMTD1 transcription. A similar repression of OeMTD1 transcription was observed after KCl addition, but the enzyme activity was not completely repressed (Vmax = 1.3 nmol min−1 mg−1 DW) (Fig. 4). In contrast, mannitol transport activity paralleled the high increase of OeMaT1 transcript levels in both situations. To mimic drought conditions without the ionic cytotoxic component, 250 mM PEG was added to the culture. PEG caused cells growing in mannitol to repress OeMTD1 transcription, and consequently MTD activity was also reduced to non-detectable levels. However, the up-regulation of OeMaT1 expression and activity was less significant than under salt stress conditions. In agreement with these results, fluorescence microscopy analysis revealed that both NaCl and KCl induced loss of cell viability within 24 h, as assessed by fluorescein diacetate (FDA) and propidium iodide (PI). Cells growing in sucrose, however, were shown to be much more sensitive than cells growing in mannitol to the deleterious effect of both salts (not shown).
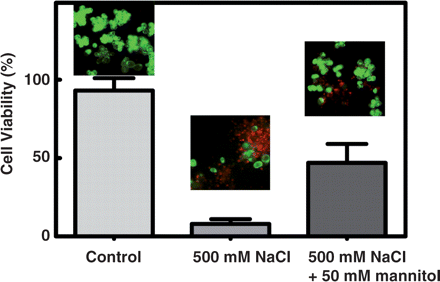
When the effects of NaCl, KCl and PEG were evaluated in cells growing in sucrose, there was also a substantial increase of mannitol transport activity and of OeMaT1 transcript levels, together with a decrease of MTD activity from basal levels to total repression. OeMTD1 transcription was strongly repressed in all experimental stress conditions. Moreover, mannitol addition substantially protected cells growing in sucrose from the deleterious effect of NaCl: the reduction of cell viability in cells incubated in the presence of 500 mM NaCl during 24 h (>90%) diminished in cells incubated with 500 mM NaCl + 50 mM mannitol (~50%), as assessed after incubation of cells with the fluorescent dyes FDA and PI (Fig. 5).
Protective role of mannitol from the deleterious effect of NaCl in O. europaea. Cell viability assays in suspension-cultured cells cultivated with sucrose in the absence of salt (control), 24 h after the addition of 500 mM NaCl and 24 h after the addition of 500 mM NaCl + 50 mM mannitol. Fluorescence was measured after incubation with fluorescein diacetate (FDA, green fluorescence) and propidium iodide (PI, red fluorescence).
Discussion
This study provides a description of how the coordination between the mannitol transport and oxidation steps operating in olive suspension-cultured cells is crucial for salt and drought stress tolerance in O. europaea. A wealth of information is already available on the involvement of mannitol or other solutes in abiotic stress plant tolerance (see the Introduction), whereas much less is known about the regulation of transport activity and metabolism in relation to gene expression and its contribution to the tolerance process. The drawback is that plant organs are often not accessible to such studies. The use of suspension-cultured cells to study mannitol transport and oxidation and its regulatory mechanisms offers a number of distinct advantages over intact plants where bulk diffusion, tissue penetration barriers and cell heterogeneity impair kinetic studies. Moreover, in suspension-cultured cells, the plasma membrane is readily amenable to coping with exogenous substrates such as mannitol or sucrose, which are abundant photoassimilates in O. europaea leaves and fruits. Despite the necessary caution needed to extrapolate the results to a tissue or whole-plant level, cell suspensions provide a useful and convenient experimental system that has already yielded a lot of information that matched observations outside the laboratory in actual field conditions. Extremely useful information on sugar transport or metabolic mechanisms and their regulation (Roitsch and Tanner 1994, Ehness and Roitsch 1997, Oliveira et al. 2002, Cakir et al. 2003, Conde et al. 2006, Conde et al. 2007a), as well as in other key physiological processes, such as hormonal signaling (Cakir et al. 2003), the cell cycle (Riou-Khamlichi et al. 1999) and regulation of gene expression (Graham et al. 1994, Cheng et al. 1999), has been obtained using this model, thus reinforcing that our observations in olive cell suspensions are indeed physiologically relevant. In line with this assumption, both OeMaT and OeMTD expression was observed in olive fruits (not shown) during ripening where they should play an important role in mannitol transport and metabolism.
In heterotrophic suspension-cultured cells of O. europaea, carrier-mediated mannitol uptake occurs through a 1 : 1 polyol : H+ symport system (Km of 1.3 mM mannitol). OeMaT1 is down-regulated by high levels of mannitol and up-regulated by high salinity (Conde et al. 2007a). Following transmembrane transport, oxidation to mannose catalyzed by an NAD+-dependent MTD is the first step of the mannitol metabolism in heterotrophic tissues (Stoop et al. 1996). MTD activity measured in homogenates from cultured cells grown with mannitol was substantially higher (up to 10-fold) than in homogenates from cells growing in sucrose, but the transcription of OeMTD1 was high in both conditions (Figs. 1, 5). Moreover, in cells growing in mannitol, the expression of OeMTD1 was completely repressed when mannitol in the culture medium declined to undetectable values, at the end of the exponential growth phase (Fig. 3). These data strongly suggest that MTD activity is up-regulated by its own substrate, at a transcriptional or post-transcriptional level. The significantly higher MTD activity in cells growing in mannitol when compared with cells growing in sucrose is consistent with the observations of Stoop and Pharr (1993), although in celery this increment was only up to 4-fold. The same group observed that the repression of MTD activity by sucrose was linked to a down-regulation of MTD transcription (Prata et al. 1997). The high amount of OeMTD1 transcripts found in cells growing in sucrose suggests that OeMTD1 activity may also be negatively regulated by sucrose at the protein level.
The Km of OeMTD1 is physiologically similar to that of the MTD of celery roots (72 mM mannitol). The specific activity of OeMTD1 is very close to the 0.62 μmol h−1 mg−1 protein determined for the celery MTD in crude extracts of suspension-cultured cells and substantially different from the 0.8 μmol h−1 mg−1 protein displayed in celery roots (Stoop and Pharr 1992, Stoop et al. 1995). Moreover, the Vmax observed for OeMTD1 is very similar to that of the Vicia faba enzyme in normal conditions (Voegele et al. 2005). The negative correlation observed in Fig. 3 between OeMaT1 and OeMTD1 transcription suggested that the availability of the enzyme is higher at lower amounts of intracellular mannitol. However, it must be stressed that in a mannitol-rich medium, while OeMaT1 expression and carrier-mediated mannitol transport activity are maintained at basal levels, energy-independent diffusional uptake is the preferred mode of mannitol absorption, as shown before (Conde et al. 2007a). The nature of this non-saturable mechanism widely reported in the literature is still poorly understood, but the involvement of channel-like proteins has been recently proposed (Conde et al. 2007b, Conde et al. 2010). This low affinity and high capacity mode of mannitol transport ensures high mannitol concentrations that sustain an elevated MTD activity (resulting from high OeMTD1 transcription), which converts mannitol to mannose in order to feed the glycolytic pathway of exponentially growing cells. As external mannitol decreases to residual levels, the linear transport component no longer sustains mannitol uptake at a rate sufficient to allow cell growth/maintenance, and the involvement of a concentrative, energy-dependent transport system becomes critical. In these conditions OeMaT transcript levels and polyol:H+ symport activity increase, but the net amounts of intracellular mannitol are probably lower, which may explain the decrease observed in OeMTD1 expression. However, we may not exclude that at this stage substantial amounts of intracellular mannitol are still present as well as a sustained biochemical activity of OeMTD1, depending on the turnover rate of the protein.
The analysis in Fig. 4 regarding the activity of OeMTD and OeMTD expression in control cells cultivated with mannitol and sucrose as sole carbon and energy sources suggests that OeMTD is regulated at both the transcriptional and post-transcriptional level. Remarkably, gene transcription was completely abolished, and consequently a strong reduction of mannitol oxidation activity was observed after salt, K+ and PEG treatments, in both mannitol- and sucrose-grown cells, suggesting that these stress factors inhibit MTD at the transcriptional level.
Carrier-mediated mannitol transport was up-regulated at a transcriptional level by NaCl, KCl and PEG in both mannitol- and sucrose-grown cells. In all the experimental conditions there was an increase of the transcript levels of OeMat1 and an increase of the Vmax of carrier-mediated mannitol transport over the control that paralleled the repression of OeMTD. Taken together, these observations suggest that olive cells display an integrated response that leads to a nearly universal reaction to salt and osmotic stresses: intracellular osmolyte accumulation. This is thought to allow osmotic adjustment in order to compensate for the decrease of external water potential and allow for oxidative detoxification. As this highly integrated response occurred even in the absence of extracellular mannitol, the stress signaling pathways involved are epistatic over sugar-mediated regulation of gene expression. Thus, mannitol addition to cells growing in sucrose substantially alleviated the damage caused by salt, as shown by fluorescence microscopy with vital fluorescent stains.
An important question that needs further investigation regards the protective role of mannitol relative to its intracellular compartmentation. That is, how could mannitol protect the cells if a significant part of it is compartmented in the vacuole under normal conditions? In common ivy (Hedera helix), the branched chain sugar alcohol hamamelitol is predominantly present in the vacuole of mesophyll cells and at lower levels in the cytosol, but it undergoes a significant redistribution from the vacuole to the cytosol under drought stress (Moore et al. 1997). Moreover, in other plants such as celery, peach and Plantago major (common plantain), mannitol and other sugar alcohols such as sorbitol are present in significant quantities in the cytosol, vacuole and even the stroma of mesophyll cells (Keller and Matile 1989, Nadwodnik and Lohaus 2008). Despite the shared cytosolic and vacuolar presence of mannitol in a mannitol-synthesizing plant such as celery, which may also be the case in O. europaea, immunolocalization of MTD in both celery suspension-cultured cells and plants showed that MTD is primarily a cytoplasmic enzyme, with a less significant presence observed in the nucleus (Zamski et al. 1996, Yamamoto et al. 1997). This fact may suggest and reinforce that mannitol is clearly present in high levels in the cytosol of cells from mannitol-producing plant species, so that it can be readily oxidized in its metabolic pathway for energy gain. This seems also to be inferred from several other studies on celery (Williamson et al. 1995, Stoop et al. 1995, Stoop et al. 1996, Yamamoto et al. 1997, Zamski et al. 2001, Cheng et al. 2009). Nonetheless, if a vacuolar localization of mannitol occurs significantly in olive cells under normal conditions, there is no doubt that rapid and efficient mechanisms providing its translocation to the cytosol are present at the tonoplast, so that mannitol can be accumulated here to act as an osmoregulator and osmoprotectant under abiotic stress. In future experiments, it would undoubtedly be interesting to study in more detail the compartmentalization of mannitol in olive cells, and, in particular, an eventual redistribution in its subcellular localization in response to osmotic stress.
In conclusion, in the present study conducted with heterotrophic cells as sink models, we have studied how cells coordinate the activity of mannitol/H+ symport with the activity of NAD+-dependent MTD at the gene expression and protein activity level under salinity and drought stress. By adjusting mannitol transport and intracellular metabolism, the olive tree should be able to cope with increased salinity and drought stress, typical of the Mediterranean basin (Table 1). In agreement with this, the large increase in mannitol concentration in salt-treated leaves of O. europaea indicates that it may function primarily as a compatible solute, as in salinity adaptation of Phillyrea latifolia (Tattini et al. 2002). However, in O. europaea mannitol may also function as an antioxidant osmoprotectant against oxidative stress resulting from salt/drought stress and even solar irradiance (Melgar et al. 2009, Remorini et al. 2009, Cimato et al. 2010). Mannitol accumulation has recently been suggested to protect salt-treated leaves in full sunshine from heat stress-induced oxidative damage to a greater extent than leaves growing under partial shading (Cimato et al. 2010). Concordantly, mannitol has been described as a potent scavenger of hydroxyl radicals that may result from salt or drought stress (Shen et al. 1997a, Shen et al. 1997b). In plants, it is suggested that the antioxidant function of mannitol may be to shield susceptible thiol-regulated enzymes such as phosphoribulokinase, thioredoxin, ferredoxin and glutathione from inactivation by hydroxyl radicals (Shen et al. 1997b). Thus, accumulation of mannitol may have dual functions: providing osmotic adjustment and supporting redox control (Shen et al. 1999). In the case of O. europaea, and in the light of our results, the coordination of mannitol transport via OeMaT1 with the significantly lowered mannitol oxidation via OeMTD1 suggests that the function of mannitol in salt or drought stress tolerance (whether resulting from excessive concentrations of Na+ or K+ ions, or from actual low water availability) is mainly as an osmoregulator, but a function as an osmoprotectant against oxidative damage is also perfectly plausible. However, it is not yet completely clear if the abiotic stress-induced accumulation of such osmoprotectant compounds may by itself directly allow an increased tolerance, or if it is just a participant in a much more complex and intricate mechanism of stress tolerance by acting synergistically with other key intervenients. Given the huge importance of mannitol accumulation in abiotic stress tolerance, a coordination of mannitol transport and oxidation allowing that osmoregulator/antioxidant pool is extremely logical in sink cells and its tight regulation is critical, as demonstrated in the present work. The continued study of the signaling pathways responsible for responses to salt, drought and other abiotic stresses may allow for the treatment of plants with exogenous compounds, such as mannitol and other osmoprotectants and antioxidants, without resorting to genetic manipulation, thus avoiding the introduction of genetically manipulated plants in nature. The protective role of mannitol when added to salt-stressed sucrose-grown cells of O. europaea (present study) or its enhancing effect on several antioxidant enzymes of wheat, which does not produce mannitol, under high salinity conditions (Seckin et al. 2009), confirms that this research topic requires continuous investment from the scientific community.
Olea europaea copes with salt and osmotic stresses by adjusting mannitol transport and metabolism as pinpointed in the present study and several previous publications (see the references in the text)
| . | No salt/drought . | Salt/drought . |
|---|---|---|
| Source tissues | Important photoassimilate | Increased mannitol synthesis |
| Sink tissues | Stored sugar; carbon and energy source | Osmolyte/antioxidant (increased transport; decreased oxidation) |
| . | No salt/drought . | Salt/drought . |
|---|---|---|
| Source tissues | Important photoassimilate | Increased mannitol synthesis |
| Sink tissues | Stored sugar; carbon and energy source | Osmolyte/antioxidant (increased transport; decreased oxidation) |
Olea europaea copes with salt and osmotic stresses by adjusting mannitol transport and metabolism as pinpointed in the present study and several previous publications (see the references in the text)
| . | No salt/drought . | Salt/drought . |
|---|---|---|
| Source tissues | Important photoassimilate | Increased mannitol synthesis |
| Sink tissues | Stored sugar; carbon and energy source | Osmolyte/antioxidant (increased transport; decreased oxidation) |
| . | No salt/drought . | Salt/drought . |
|---|---|---|
| Source tissues | Important photoassimilate | Increased mannitol synthesis |
| Sink tissues | Stored sugar; carbon and energy source | Osmolyte/antioxidant (increased transport; decreased oxidation) |
Materials and Methods
Cell suspensions and growth conditions
Cell suspensions of O. europaea L. var. Galega Vulgar were grown in 250 ml flasks on a shaker at 100 r.p.m., in the dark, at 25°C on modified Murashige and Skoog (MS) medium (Murashige and Skoog 1962), supplemented with 0.5 or 2% (w/v) mannitol or 2% (w/v) sucrose. Cells were subcultured weekly by transferring 10 ml aliquots to 70 ml of fresh medium. Cell growth was assayed as previously described (Conde et al. 2006). Salt (and consequently osmotic) stress was induced by addition of NaCl or KCl at final concentrations of 250 mM. To mimic drought conditions without the ionic cytotoxic component, PEG was added to provide the osmotic potential in MPa reached with 250 mM NaCl. The stress treatments were carried out for a 24 h period at the mid-exponential growth phase of the cells.
Determination of the mannitol dehydrogenase (OeMTD1) activity
Protein extraction and OeMTD1 activity determination was carried out as described by Stoop and Pharr (1993). Olea europaea suspension-cultured cells were harvested as described above and ground using a chilled mortar and pestle in an approximately 1 : 1 (v/v) powder : buffer ratio. The protein extraction buffer contained 50 mM MOPS (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM dithiothreitol (DTT) and 1% (v/v) Triton X-100. The homogenates were then centrifuged at 20,000×g for 20 min and the supernatants were maintained on ice and used in the enzymatic assays. MTD activity assays, except for the temperature effect evaluation, were performed at room temperature (25°C), in a total volume of 1 ml. The reaction mixture contained enzyme extract, 100 mM Bis-Tris propane (pH 9.0, or the desired pH in the case of the pH effect evaluation), 2 mM NAD+, and d-mannitol at the desired final concentration. The reduction of NAD+ was evaluated spectrophotometrically at 340 nm. All reactions were initiated by the addition of mannitol. For the assessment of the effects of temperature and pH on OeMTD1 activity, 200 mM d-mannitol was used to ensure the Vmax of the enzyme. Total protein concentrations of the extracts were determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Cloning of the O. europaea mannitol dehydrogenase gene OeMTD1
In order to identify and clone putative cDNA sequences encoding MTDs in O. europaea, conserved regions of several plant MTDs were identified and subsequently used to design degenerated primers. Reverse transcription–PCR (RT–PCR) was performed on mRNA extracted from suspension-cultured cells grown with 2% (w/v) mannitol as carbon and energy source. An 816 bp cDNA sequence was amplified and cloned into pGEM T-Easy vector (Promega) according to the manufacturer's instructions and subsequently sequenced and submitted to GenBank (accession No. ABR31791.1) and named OeMTD1.
Transport studies with radiolabeled mannitol
Harvested cells were centrifuged, washed twice with ice-cold culture medium without sugar at pH 4.5, and re-suspended in the same medium at a final concentration of 5 mg DW ml−1. To estimate the initial uptake rates of d-[14C]mannitol, 1 ml of cell suspension was added to 10 ml flasks, with shaking at 100 r.p.m. After 2 min of incubation at 25°C, the reaction was started by the addition of 40 μl of an aqueous solution of radiolabeled mannitol at the desired specific activity and concentration. The specific activities were defined according to the final concentration of the polyol in the reaction mixture, as follows: 500 d.p.m. nmol−1 (0.1–2 mM), 100 d.p.m. nmol−1 (5–20 mM). Washing, radioactivity measurements and calculations were performed as described by Conde et al. (2006).
RNA gel blot analysis
Total RNAs from olive suspension-cultured cells were obtained by phenol extraction combined with a 2 M LiCl precipitation step [adapted from Howell and Hull (1978)]. RNA blot analysis was performed for both OeMaT1 and OeMTD1 as described by Conde et al. (2006), using partial [32P]OeMaT1 and [32P]OeMTD1 probes, respectively.
Mannitol quantification by HPLC analysis
The quantification of mannitol in the culture medium was performed in a HPLC system from Gibson (132 RI Detector) using a Hyperrez H+ column (Hypersil), at a flow rate of 0.5 ml min−1, with 2.5 mM H2SO4 as the mobile phase. Before each experiment, the column was equilibrated for 30 min at 30°C. The standard solution contained glucose, sucrose, fructose, raffinose and acetate (internal standard), all at 0.5% (w/v). The samples were diluted 1 : 1 in an acetate solution of 1% (w/v), and 25 μl of the sample and standard solutions were injected in the column.
Determination of cell viability
FDA and PI double staining was used to evaluate cell viability, as described in Jones and Senft (1985). Stock solutions of FDA (500 mg ml−1, Sigma) and PI (500 mg ml−1, Sigma) were prepared in dimethylsulfoxide and water, respectively. For the double staining protocol, 1 ml of cell suspension was incubated with 10 μl of FDA stock solution and 1 μl of PI stock solution for 10 min at room temperature in the dark. Stained cells were observed under a Leitz Laborlux S epifluorescence microscope with a 50 W mercury lamp and appropriate filter settings. Images were acquired with a 3CCD color video camera (Sony, DXC-9100P), a frame grabber (IMAGRAPH, IMASCAN/Chroma P) and software for image management and archiving (AxioVision Version 3.0, Carl Zeiss Vision, GmbH).
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT) [research project ref. PTDC/AGR-ALI/100636/2008; grant ref. SFRH/BD/47699/2008 to A.C., grant ref. SFRH/BPD/34998/2007 to C.C. and grant ref. SFRH/BD/13460/2003 to P.S.].
Acknowledgments
The authors would like to thank the work of BabeliUM, the Language Centre of the University of Minho, specifically Ana Teresa Correia, for revising the English version of the manuscript. The authors also thank Prof. Pedro Fevereiro (ITQB/UNL) for kindly providing O. europaea cell suspensions.
Abbreviations
- FDA
fluorescein diacetate
- M6PR
mannose-6-phosphate reductase
- MTD
mannitol dehydrogenase
- PEG
polyethylene glycol
- PI
propidium iodide


![Growth, mannitol/H+ symport activity and OeMaT1 and OeMTD1 expression in O. europaea cell suspensions. (A) Cells were cultivated with 0.5% (w/v) mannitol (initial concentration), and d-[14C]mannitol uptake (filled triangles) was measured in cell aliquots harvested from the culture at the times indicated. Values are represented by the mean of two independent experiments. (B) Northern blot analysis of OeMaT and OeMTD1 expression. In each condition, 50 μg of RNA were used as in Conde et al. (2007a).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/52/10/10.1093/pcp/pcr121/2/m_pcr121f3.gif?Expires=1716401529&Signature=4-Upx91kx-nLZNdwkEnzJCepIiqZT92cC8XzcvWNHW9OnfckQ51NZL685QJGSll8XsC6iuqvdqMf6yV3b1MSFeBfUcz77oNF1U~s-dIeaS3ziZD6QDXTrrj3n8majTnBxLiQ55MqUudlRmBfSFg95ZXJqFolJFnvUx8Bta-JVnEqoZf52LhQm52W~Mo5tS~sV9RI2xCsuXv45JrwbbyrW2UGOyWzD0HF0yAC~HspJXYjAFnEbzBAa7U8VH-VOj6LDeMA442dqGjJ90S4eq63-BWBHFhkEDmUDjioxbCBfva~7gdlvcl1mcUzPL5D3l36UHgTei-GJP84MwMewBUSlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)