-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroyuki Takeda, Takuo Yoshikawa, Xi-Zhen Liu, Naoki Nakagawa, Yi-Qin Li, Naoki Sakurai, Molecular Cloning of Two Exo-β-glucanases and Their in vivo Substrates in the Cell Walls of Lily Pollen Tubes, Plant and Cell Physiology, Volume 45, Issue 4, 15 April 2004, Pages 436–444, https://doi.org/10.1093/pcp/pch049
Close - Share Icon Share
Abstract
Full-length cDNA sequences of two exo-β-glucanases, LP-ExoI and LP-ExoII, secreted into cell walls of lily (Lilium longiflorum) pollen tube, were determined by RT-PCR. LP-ExoI exhibited over 80% similarity to LP-ExoII at both DNA and amino acid levels. RT-PCR showed that LP-ExoI transcripts were abundant in pollen grains and tubes, but could not be detected in leaf, stem, stigma, style, ovary, petal, filament, young root, young bud, and scale leaf of bulb. However, LP-ExoII transcripts ubiquitously existed in all the tissues tested. To determine the potential substrates of exo-β-glucanases, cell wall components of lily tissues were analyzed. Linkage analysis revealed that pollen tubes contained high levels of 3-Glc in hemicellulose (44.3%), while pollen grains had no detectable 3-Glc. The hemicellulose fraction of pollen tubes was treated with lichenase and the product was analyzed by HPLC-PAD to determine the origin of 3-Glc. Specific tetra-saccharide was liberated from hemicellulose of pollen tubes, suggesting the presence of 1,3 : 1,4-β-glucan in lily pollen tube hemicellulose. The structure of this 1,3 : 1,4-β-glucan may be different from cereal plant 1,3 : 1,4-β-glucan, since tri-saccharide was not detected in hemicellulose fraction after lichenase treatment. LP-ExoI and LP-ExoII, expressed in pollen grains and tubes, may be involved in the regulation of pollen tube elongation by hydrolyzing callose and 1,3 : 1,4-β-glucan within pollen tube walls.
(Received September 22, 2003; Accepted January 29, 2004)
Introduction
Pollen germination and pollen tube elongation are crucial processes of sexual reproduction in higher plants. Pollen tube cells display representative polarized growth, which extend only at the apex. The pollen tube wall exhibits a distinctive structure: an inner callose (1,3-β-glucan) lining and an outer fibrillar layer composed largely of pectin, hemicellulose and cellulose (Ferguson et al. 1998). The tip of the growing pollen tube always lacks callose, and contains more methyl esterified pectin compared to the outer wall regions distal to the tip, which permits a more flexible and extensible structure of the tip wall (see reviews: Li et al. 1997, Taylor and Hepler 1997, Franklin-Tong 1999, Hepler et al. 2001, Shivanna 2003).
Pollen germination and pollen tube growth need massive synthesis and reconstruction of the cell wall polysaccharides. Two exo-β-glucanases, LP-ExoI and LP-ExoII, were isolated from cell walls of lily (Lilium longiflorum) pollen tubes (Kotake et al. 2000). Both enzymes exhibited substrate specificity not only for laminarin (1,3-β-glucan), but also for 1,4-β-glucan, 1,3 : 1,4-β-glucan and 1,6-β-glucan. LP-ExoI showed the ability to hydrolyze 1,3- and 1,4-glucosyl linkages in hemicellulosic polysaccharides isolated from the cell wall of lily pollen tubes. Three possible models of exo-β-glucanase function in lily pollen are proposed, i.e., (1) degrading the primary wall in the pollen tube tip, facilitating tip expansion, (2) degrading of callose in the tip to prevent the tip from clogging, and (3) opening the callosic wall for pollen tube–style communication (Kotake et al. 2000, Li et al. 2001). However, further study is required to reveal the details of exo-β-glucanases function in pollen tubes, since potential substrates of exo-β-glucanases are unclear.
1,3 : 1,4-β-Glucan is a major component in Poaceae hemicellulose. Exo-β-glucanase contributes to cell wall loosening in cereals by degrading 1,3 : 1,4-β-glucan (Hrmova et al. 1996, Kotake et al. 1997, Sakurai 2000, Darley et al. 2001, Hrmova and Fincher 2001). Li et al. (1999) analyzed the glycosyl-linkage composition of Nicotiana alata pollen tube walls and reported that elongating pollen tube walls contain both 3-Glc and 4-Glc. These residues are not only components of callose and cellulose, but also those of 1,3 : 1,4-β-glucan. Further study on the existence of 1,3 : 1,4-β-glucan in pollen tubes has as yet not been carried out.
In the present study, we isolated full-length cDNAs of LP-ExoI and LP-ExoII, and analyzed their expression patterns during pollen germination and tube growth in comparison with other lily plant tissues. Furthermore, cell wall components of lily tissues as well as lily pollen tubes were analyzed to determine the potential substrates of LP-ExoI and LP-ExoII.
Results
Cloning of LP-ExoI and LP-ExoII
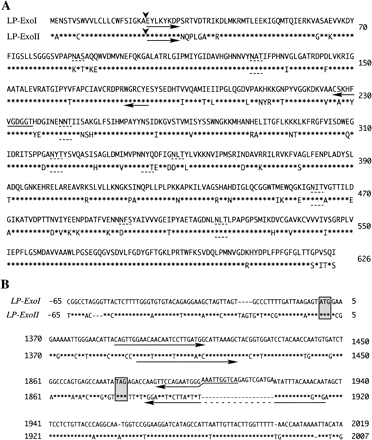
LP-ExoI and LP-ExoII proteins have been isolated from cell walls of lily pollen tube and 10 residues of the N-terminus amino acid sequences were obtained (Kotake et al. 2000). Degenerated sense primers were designed based on the amino acid sequences. Degenerated antisense primers were designed from highly conserved sequences of plant exo-glucanases (Fig. 1A, shown by arrows). Using RT-PCR and RACE, full-length cDNA sequences of two glucanases were determined. The deduced amino acid sequence from cloned LP-ExoII was identical to the N-terminus amino acid sequence of the mature polypeptide. However, the deduced N-terminus amino acid sequence of cloned LP-ExoI (EYLKYKDPSR) was different from the N-terminus sequence of LP-ExoI protein reported before (EYLKYKDPGL, Kotake et al. 2000). Both cDNA sequences of LP-ExoI and LP-ExoII encoded 626 amino acids polypeptides containing 22 amino acids signal peptide sequences at the N-terminus (Fig. 1A, cleavage sites are shown by the arrowhead). The open reading frame of LP-ExoI exhibited 85.2% identity at the cDNA level and 87.2% identity at the deduced amino acid level to those of LP-ExoII. 5′ and 3′ untranslated region of the cDNA sequences showed 76.6% and 66.9% similarity, respectively (Fig. 1B). The 35-base sequences in LP-ExoI and LP-ExoII (from +1,867 to +1,901, see Fig. 1B), around the end codon, showed low similarity (51%), and an additional different 20-base sequence was found in LP-ExoI (from +1,902 to 1,921, Fig. 1B). The deduced amino acid sequence of LP-ExoI exhibited eight potential sites of N-linked glycosylation, while LP-ExoII exhibited six (shown in Fig. 1A, broken lines). Predicted molecular weight and pI value were 65.8 kDa and 8.28 for LP-ExoI, and 66.1 kDa and 8.77 for LP-ExoII.
Expression patterns of LP-ExoI and LP-ExoII
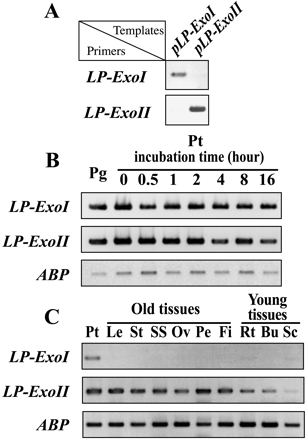
The cDNA sequence of LP-ExoI was so similar to that of LP-ExoII that Northern hybridization with labeled cDNA probes failed to distinguish these two mRNAs. Alternatively, RT-PCR analysis was conducted to detect the respective expression patterns of LP-ExoI and LP-ExoII. Specific primers for LP-ExoI and LP-ExoII were designed, as shown by arrows in Fig. 1B. The primers recognized each gene specifically, and no other products were amplified (Fig. 2A). The amplified products were subsequently confirmed by subcloning and sequencing.
LP-ExoI and LP-ExoII transcripts were abundant in dried pollen grains and pollen tubes (Fig. 2B). Their high expressions in the pollen tube were maintained during the 16-h incubation. LP-ExoI transcripts were almost none in other tissues such as leaf, stem, stigma + style, ovary, petal, filament, young root, young bud, and scale leaf of bulb (Fig. 2C), whereas transcripts of LP-ExoII were present in all these tissues. They were slightly rich in the old tissues, but less in scale leaf (Fig. 2C).
Polysaccharide analysis of cell walls in pollen grains and pollen tubes
The pollen grain wall consists of the inner intine and the outer exine. The exine layer is composed of sporopollenin, a highly resistant organic bio-polymer (Southworth 1985, Shivanna 2003), which can be removed by ethanolamine treatment (Jungfermann et al. 1997). After exine removal, we compared the cell walls of dried pollen grains’ intine (hereafter called pollen grains) with those of germinated and elongated pollen tubes consisting of intine and pollen tube (hereafter called pollen tubes). Cell wall polysaccharides were fractionated into pectin, hemicellulose, and cellulose fractions. Sugar contents and sugar composition of hemicellulose fraction of pollen grains and 5-h incubated pollen tubes are shown in Table 1. After 5-h incubation, total sugar contents of hemicellulose increased remarkably from 1.59 mg to 11.16 mg. The hemicellulose fraction was composed mainly of Glc, which accounted for more than 50% in both pollen grains and tubes. Contents of Ara in hemicellulose increased from 8% to 20% after 5-h incubation.
Hemicellulose fractions of pollen grains and tubes were subjected to methylation analysis to identify the potential substrate of exo-β-glucanase (Table 2). After 5-h incubation, the composition of sugar linkage changed drastically. Pollen grains did not have detectable 3-Glc, but pollen tubes contained high levels of 3-Glc (44.3±1.2%). 4,6-Glc was the most abundant in pollen grains (32.4±2.0%), but low in pollen tubes (6.5±0.1%). 4-Glc content in pollen grains (14.7±0.4%) was higher than in pollen tubes (3.7±0.3%).
Sugar linkage in cellulose fractions of pollen grains and tubes were also analyzed. The result of cellulose fraction using DMSO was essentially similar to that using DMSO containing NMNO (Joseleau et al. 1981). The cellulose fraction of pollen grains consisted mainly of 4-Glc (92.2±4.4%), while that of pollen tubes consisted of not only 4-Glc (80.5±0.9%), but also 3-Glc (15.1±0.8%).
Structure of cell wall polysaccharides of pollen and other tissues
Cell walls of other lily tissues, such as leaf, stigma, style, ovary, petal and filament were extracted and fractionated into pectin, hemicellulose and cellulose. Sugar contents and sugar composition of the hemicellulose fraction are shown in Table 3. The hemicellulose fraction of the tissues was rich in Xyl, Man, Gal, and Glc. Contents of Xyl (from 12 to 24%) and Man (from 9 to 26%) in the other tissues were larger than those of pollen grains and tubes. Exceptionally, stigma and style contained high percentages of Man (24% and 26%, respectively).
Sugar linkage in the hemicellulose fraction of other tissues was analyzed and the results are shown in Table 4. None of these tissues contained 3-Glc. 4-Glc accounted for 16–27% of neutral sugar in the tissues analyzed. 4-Man existed in all the tissues (from 9 to 26%) except pollen grains and tubes. In contrast, Tp-Gal was found in pollen grains and tubes, but not in the other tissues.
Detection of 1,3 : 1,4-β-glucan in pollen tube hemicelluloses
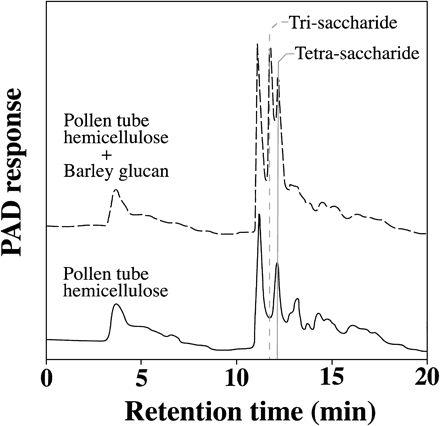
1,3 : 1,4-β-glucan can be detected specifically with a sequence-dependent Bacillus subtilis lichenase (1,3 : 1,4-β-endo-glucanase). To identify 1,3 : 1,4-β-glucan in hemicellulose fraction of pollen tubes and determine the origin of 3-Glc, we partially purified lichenase from B. subtilis α-amylase purchased from Sigma (Huber and Nevins 1977). The hemicellulose fraction of pollen tubes was first applied to an anion-exchange column to remove acidic polysaccharides. The purified neutral hemicellulose was subsequently treated with the lichenase. Oligo-saccharides derived from 1,3 : 1,4-β-glucan were detected by HPLC-PAD. Tetra-saccharides released from the pollen tube hemicellulose after the lichenase treatment are shown in Fig. 3 (shown by a solid line). The mixture of lily pollen hemicellulose and barley 1,3 : 1,4-β-glucan was treated with the lichenase and analyzed by HPLC-PAD. The elution peaks demonstrated that both tri-saccharide and tetra-saccharide were produced (Fig. 3, broken line). The oligo-saccharide peak from digested lily pollen hemicellulose (Rt. 12.2 min) was consistent with the peak of tetra-saccharide derived from barley 1,3 : 1,4-β-glucan, suggesting 1,3 : 1,4-β-glucan exists in lily pollen tubes. The cellulose fraction from pollen tubes was also analyzed as above. However, no oligo-saccharide peak appeared after lichenase treatment. Lichenase was administered to pollen germination medium, but there was no obvious change in morphology or tube length of the pollen tube.
Discussion
Two full-length exo-β-glucanase cDNAs were cloned from lily pollen tube mRNA. The deduced amino acid sequence of LP-ExoII was in agreement with the N-terminus amino acid sequence of isolated LP-ExoII protein. However, the deduced N-terminus 9th and 10th amino acid residues of LP-ExoI were different from those of LP-ExoI reported before (Kotake et al. 2000). We submitted it as LP-ExoI to the database, since only two peaks of 1,3-β-glucanase activities were detected from cell wall-bound proteins of lily pollen tube (Kotake et al. 2000), and both mRNAs of LP-ExoI and LP-ExoII were abundant in pollen tubes. Furthermore, repeated RT-PCR experiments never produced the sequence agreed precisely with the amino acid sequence of LP-ExoI polypeptide reported before (Kotake et al. 2000).
LP-ExoI and LP-ExoII shared extremely high similarity at both cDNA and deduced amino acid level, and the predicted molecular weights were also almost the same. However, the numbers of N-linked glycosylation sites were different between LP-ExoI and LP-ExoII (Fig. 1A). It may result in different molecular weights of mature proteins, which were estimated by SDS-PAGE gel electrophoresis as 83 kDa for LP-ExoI and 71 kDa for LP-ExoII (Kotake et al. 2000).
It has been reported that exo-1,3-β-glucanases are expressed and play an important role in pollen development. Exo- and endo-1,3-β-glucanases are secreted from the tapetum in several species during the early stage of pollen development. It is suggested that the glucanases secreted from tapetum are essential for the normal development of pollen by breaking down the callose surrounding microspore. The peaks of their expressions are all before microspore release. After that, their transcripts decrease immediately, and they are almost absent in mature pollen grain (Stieglitz and Stern 1973, Hird et al. 1993, Futamura et al. 2000). Recently Suen et al. (2003) purified an exo-β-glucanase protein from maize pollen coat and cloned its cDNA. The pollen coat glucanase was not expressed in the microspore, nor in the pollen interior, but in the anther. Its expression was induced later than the anther glucanases that contribute to the microspore development. It is unlikely that LP-ExoI and LP-ExoII are related to the above glucanases, since LP-ExoI and LP-ExoII transcripts were abundant in mature pollen grain and pollen tubes (Fig. 2B).
Pollen-specific genes have been characterized as “early” or “late” gene (Mascarenhas 1990). Transcripts of “early” genes are detectable soon after meiosis and are reduced or undetectable in the mature pollen. In contrast, transcripts of “late” genes are first detected around the time of microspore meiosis and continue to accumulate as pre-synthesized stock mRNAs throughout pollen development. The “late” genes are regarded to be required during pollen maturation or pollen tube growth (Mascarenhas 1993, McCormick 1993, Fernando et al. 2001). LP-ExoI and LP-ExoII, whose mRNAs were synthesized and stored in mature pollen grains, could be regarded as late genes, and this result might reinforce the hypothesis that LP-ExoI and LP-ExoII contribute to pollen tube growth. Furthermore, the transcripts of LP-ExoI and LP-ExoII did not decrease after the pollen germination, but kept high expression during pollen elongation of the whole 16-h incubation (Fig. 2B). It implied that LP-ExoI and LP-ExoII functioned not only in the early stages of pollen germination and tube growth, but also contributed to the sustained growth of pollen tubes. The localization in pollen tube wall using a expression construct of fusion protein with GFP is now under investigation.
LP-ExoI was a pollen-specific expressed gene (Fig. 2C). However, LP-ExoII was expressed not only in pollen, but also in other tissues. It is interesting that LP-ExoI and LP-ExoII were expressed in different manners, even though these two glucanases were similar to each other. It seems that LP-ExoI and LP-ExoII play a similar role in pollen tube elongation, but LP-ExoII may have different function in other tissues. However, it is unlikely that LP-ExoII contributes to the elongation growth in the vegetative tissues, because LP-ExoII transcripts did not abound in young roots and buds, but abounded consistently in the old tissues, and there is neither callose nor 1,3 : 1,4-β-glucan in these tissues (Table 4). It is known that some PR proteins including endo-1,3-β-glucanases are expressed during flowering. The endo-1,3-β-glucanase expression is not only observed in the floral tissues, but also in the tissues outside the flower. It is reported that 1,3-β-glucanases are expressed at high levels in leaves during tobacco flowering (Simmons 1994). Hrmova and Fincher (2001) suggested the possibility that exo-β-glucanase is expressed preemptively to counter potential pathogen attack in young tissues. LP-ExoII protein may attack the cell walls of pathogens, which contain 1,3-β-glucan and 1,3 : 1,6-β-glucan. We propose that LP-ExoII not only contributes to pollen tube growth, but also may act on the defense of young tissues and flowering tissues.
3-linked glucose was the main sugar component in the hemicellulose fractions of pollen tubes (Table 2). 3-Glc generally existed in plant cell walls as a component of 1,3 : 1,4-β-glucan or callose. In Nicotiana alata, linkage analysis (Rae et al. 1985, Schlüpmann et al. 1994, Li et al. 1999) revealed that 3-Glc is not present before pollen germination, whereas it later increases as the pollen tube is elongating. Schlüpmann et al. (1994) reported that 3-Glc accounts for 35% of neutral polysaccharide 4 h after N. alata pollen germination. The amount of 3-Glc in lily pollen tube cell wall was comparable to that of N. alata pollen tubes. Lichenase treatment released tetra-saccharide from the hemicellulose fraction (Fig. 3). It suggests that 1,3 : 1,4-β-glucan is present in the hemicellulose fraction of pollen tubes. 1,3 : 1,4-β-glucan in lily pollen tube cell walls should be considered as a potential substrate of exo-β-glucanases. 1,3 : 1,4-β-Glucan is generally found in Poaceae, and consists of tri (G14G13G) and tetra (G14G14G13G) saccharide units. There are few reports about the existence of 1,3 : 1,4-β-glucan in plants except Poaceae (Sakurai 2000). Vithanage et al. (1980) revealed the existence of mixed glucan in rye pollen tube cell walls using lichenase treatment. However, there is no report to identify 1,3 : 1,4-β-glucan in lily pollen tube cell walls by enzymatic analysis. Recently anti-1,3 : 1,4-β-glucan monoclonal antibody detected 1,3 : 1,4-β-glucan in coniferous tree pollen tubes (Yatomi et al. 2002). Our biochemical and enzymatic analysis not only identified 1,3 : 1,4-β-glucan in hemicellulose of lily pollen tube, but also revealed its unique structure. The fact that lichenase liberated only tetramer from neutral polysaccharides of pollen tube hemicellulose suggests that lily pollen 1,3 : 1,4-β-glucan, if there is any, consists of tetra-saccharide units only. No oligo-saccharide was released from pollen tube cellulose fraction by lichenase, even though 3-Glc was contained in the cellulose fraction. Herth et al. (1974) reported that the alkali-resistant fraction of lily pollen tube walls contains a small amount of crystalline form β-1,3-glucan with β-1,4-glucans. Therefore, the 3-Glc found in the cellulose fraction is not derived from 1,3 : 1,4-β-glucan. 3-Glc was not detected in pollen grains, indicating that there was almost no callose or 1,3 : 1,4-β-glucan in pollen grains.
The net amounts of 3-Glc and 4-Glc in pollen tube cell walls were calculated from the difference between pollen grains and 5-h incubated pollen tubes in the neutral sugar contents and the results of linkage analysis. After 5-h incubation, 3-Glc increased by 4.86 mg g–1 dried pollen, and 4-Glc by 0.18 mg g–1 dried pollen. Enzymatic analysis revealed that 1,3 : 1,4-β-glucan in the pollen tube consisted of tetra-saccharide units, suggesting that, at most one third of 4-Glc in pollen tube hemicellulose (0.18 mg × 1/3 ≈ 0.06 mg g–1 dried pollen) corresponds to 3-Glc of 1,3 : 1,4-β-glucan. The remaining 3-Glc (ca. 4.80 mg g–1 dried pollen) in the hemicellulose may be derived from callose. Therefore, lily pollen tube hemicellulose contained a large amount of callose (43%) and a slight amount of 1,3 : 1,4-β-glucan (2%). Both 1,3 : 1,4-β-glucan and callose are suitable substrates of LP-ExoI and LP-ExoII (Kotake et al. 2000).
Sugar analysis in the other lily tissues also revealed the distinctive features of cell wall composition. Stigma and style contained large amounts of Man and Glc in the hemicellulose fraction (Table 3). In addition, linkage analysis also showed that 4-Man and 4-Glc were abundant in these tissues (Table 4). These results indicate that glucomannan is present in the hemicellulose of stigma and style. Glucomannan is normally thought to be minor in herbaceous plant tissues, but abundant in secondary walls of coniferous trees (Handford et al. 2003). Our findings might be meaningful for better understanding of cell wall structure of reproductive organs.
d-gluconolactone and nojirimycin, potent inhibitors of glucosidase, severely inhibited the growth of pollen tubes (Kotake et al. 2000). It suggests that hydrolysis of glucans by glucanases is essential for the lily pollen tube growth. Pollen tube cell walls contained a large amount of suitable substrates of exo-β-glucanases–callose and 1,3 : 1,4-glucan. LP-ExoI and LP-ExoII, two exo-β-glucanases, seem to hydrolyze these glucans and play an important role in the regulation of pollen tube elongation.
Materials and Methods
Plant materials and in vitro pollen germination
Fresh anthers of lily (Lilium longiflorum) were dehydrated in a desiccator overnight. Pollen grains were collected and stored at –80°C as described before (Li and Linskens 1983). Stored pollen grains were rehydrated for 1.5 h in a moist chamber at room temperature and cultured in liquid medium which contains 290 mM sucrose, 1.29 mM Ca(NO3)2, 0.16 mM H3BO3 and 0.99 mM KNO3 (Dickinson 1968) on a orbital shaker (120 rpm) at 25°C.
RNA extraction
Lily pollen grains, pollen tubes (0-h to 16-h incubation) and other tissues (leaf, stem + stigma, style, ovary, petal and filament) were ground in liquid nitrogen with mortar and pestle for 5 min. Total RNA was extracted using guanidine HCl method (Logemann et al. 1987) for pollen, and by phenol/SDS-LiCl method (Chirgwin et al. 1979) for the other tissues. Polysaccharides contaminated in the RNA samples were removed as described below. Dried partially purified RNA was dissolved in 600 µl of RNase-free water. One hundred µl of 5 M NaCl and 80 µl of 10% cetyltrimethylammonium bromide (CTAB) containing 0.7 M NaCl were added. The mixture was incubated at 65°C for 10 min. CTAB and polysaccharide was removed by chloroform-isoamylalcohol (24 : 1) extraction. RNA was precipitated with isopropanol at –80°C for 30 min and recovered by centrifugation at 12,000 rpm for 15 min.
Cloning of lily exo-β-glucanases
First-strand cDNA was synthesized from total RNA of pollen tubes using Omniscript Reverse Transcriptase (QIAGEN, Valencia, CA, U.S.A.) with oligo d(T) primer containing an M13 primer M4 sequence (contained in the RNA LA PCR Kit, TAKARA, Otsu, Japan). Degenerated sense primers were designed according to the N-terminus amino acid sequences of lily pollen tube exo-β-glucanases, LP-ExoI and LP-ExoII (Kotake et al. 2000). Degenerated antisense primers were designed from the conserved sequences of plant exo-β-glucanases (Fig. 1A, arrows).
For 3′-RACE, M13 primer M4 was used as antisense primer. First-strand cDNA for 5′ RACE was synthesized using SuperScript III Reverse Transcriptase (Invitrogen Corp, Carlsbad, CA, U.S.A.) and gene specific primers. 5′ RACE was performed with 5′ Full RACE Core Set (Takara). Full-length cDNA of exo-β-glucanases were amplified with KOD-Plus-DNA Polymerase (Toyobo, Osaka, Japan) and gene specific primers. The PCR products were subcloned into pGEM-T vector with TA-cloning kit (Promega, Madison, WI, U.S.A.), and the recombinant plasmids were transformed into E. coli strain DH5α. DNA sequencing was performed by Hokkaido System Science Co., Ltd (Sapporo, Japan).
Sequence analysis
The deduced amino acid sequences of the two full-length cDNAs were subjected to homology analysis with BLAST program (http://www.ncbi.nlm.nih.gov). DNA or amino acid sequences of cloned glucanases were aligned using ClustalW program (http://www.ddbj.nig.ac.jp). Predicted amino acid sequence was subjected to molecular weight (Mw) and isometric point (pI) analysis with Compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html). Signal peptide was predicted with Signal P program (http://www.cbs.dtu.dk/services/SignalP/). Glycosylation sites were predicted using PPsearch program (http://www.ebi.ac.uk/ppsearch/).
Expression of lily exo-β-glucanases
For RT-PCR analysis, total RNA was extracted from dried pollen grain and pollen tubes incubated from 0 to 16 h. Total RNA was also extracted from other lily tissues. Leaf, stem, stigma and style, ovary, petal, and filament were collected from lily plant after flowering. Young root and bud were collected from germinated lily bulb 20 d after plant. Scale leaf was collected from lily bulb after vernalization. First strand cDNA was synthesized with oligo d(T) primer (n = 25) and SuperScript III Reverse Transcriptase. PCR was performed using Takara Ex Taq (Takara) and gene specific primers (shown in Fig. 1B). PCR products were applied to 1.5% agarose gel, and stained by ethidium bromide. The amounts of first-strand cDNAs in the PCR were adjusted by the levels of amplified DNA for lily actin-bundling protein (GenBank AF088901, Vidali et al. 1999). The mock RT reaction without the RT enzyme was performed as negative controls for all reactions.
Cell-wall fractionation
Four grams of pollen grains or 5 h-incubated pollen tubes were heated in boiling 80% ethanol for 10 min to denature endogenous cell wall-bound enzymes. To remove exine, pollen was suspended into 5 ml of ethanolamine and incubated at 80°C for 20 min and centrifuged for 5 min at 1,000×g. Exine was removed three times. After ethanolamine treatment, intine and elongated pollen tubes without exine were microscopically confirmed. The residue of intine and pollen tubes was washed three times with distilled water (DW). Washed residue was homogenized with a glass homogenizer (Asahi Techno Glass, Tokyo, Japan) and subjected to further extraction.
Three g of lily plant tissues were heated in boiling 80% ethanol for 10 min and washed with DW three times. Washed samples were homogenized with a glass homogenizer as described above.
The homogenate was centrifuged at 1,000×g for 10 min. Precipitate was washed three times each with acetone and DW, and finally suspended in 50 mM MOPS buffer (pH 7.0). The suspension was heated at 100°C for 1 min and cooled, then treated with 100 units of an α-amylase (from porcine pancreas, Boehringer, Ingelheim, Germany) for 2 h at 37°C. The reaction mixture was centrifuged for 10 min at 1,000×g. The precipitate was washed three times each with hot-water treatment for 15 min. Then the residue was treated three times with EDTA (50 mM, pH 6.8) for 15 min at 90°C to extract pectic substances. The residue was treated three times with 17.5% NaOH containing 0.02% NaBH4 for a total of 24 h. The alkaline extract was neutralized with glacial acetic acid, dialyzed and centrifuged. The supernatant was lyophilized and designated as hemicellulose fraction. The residue after alkali extraction was washed with 0.03 M acetic acid, then dehydrated with ethanol and diethyl ether. The residue was dried at room temperature and designated as cellulose fraction.
Sugar analysis
Total sugar contents were estimated by phenol-sulfuric acid method (Dubois et al. 1956) and UA by m-hydroxydiphenyl method (Blumenkrantz and Asboe-Hansen 1973).
Sugar composition of pectin and hemicellulose fraction was analyzed by acethylation analysis (Albersheim et al. 1968). Lyophilized powder (1 mg) was dissolved in 1 ml of 2 M trifluoroacetic acid containing 300 µg of myo-inositol as internal standard. The polysaccharides were hydrolyzed, reduced and acetylated by acetic anhydride with 1-methylimidazole as a catalyst (Blackeny et al. 1983). The acetylated sugars were extracted by chloroform, dried and dissolved in 100 µl of acetone.
Sugar linkage was analyzed by methylation analysis (Conrad 1972). Sugar samples of hemicellulose or cellulose were dissolved in 1 ml of DMSO and methylated with methyliodine. In some experiment, cellulose fraction was dissolved in DMSO containing NMNO to facilitate the solubility of cellulose (Joseleau et al. 1981). Permethylated polysaccharides were extracted with methanol/chloroform (1 : 1), dried, dissolved in 1 ml of 2 M trifluoroacetic acid containing 300 µg of myo-inositol, hydrolized at 121°C for 1 h, and acetylated.
One µl of acetylated or methylated samples was introduced into a GC-MS system (GCMS-QP5000, Shimadzu, Kyoto, Japan) with a capillary column SP-2340 (15 m × 0.25 mm, Supelco, Bellefonte, PA, U.S.A.). The column was maintained at 160°C for 1 min and then the temperature was raised to 245°C at 2°C min–1.
Purification of lichenase from commercial B. subtilis α-amylase product
Lichenase activity was purified from commercially available B. subtilis α-amylase product (type II-A, lot number 33F-5162, Sigma, St. Louis, MO, U.S.A.) with two different chromatographic protocols. α-Amylase sample was dissolved in 20 mM sodium-acetate buffer (pH 5.4), applied to a G3000SW HPLC column (0.8×30 cm, Tosoh, Tokyo, Japan) and eluted with 20 mM sodium-acetate buffer (pH 5.4) containing 200 mM NaCl. Fractions containing lichenase activity were collected and desalted with PD-10 column (Amersham, Buckinghamshire, U.K.) equilibrated by 50 mM MOPS buffer (pH 7.0). The desalted fraction was applied to a Q-Sepharose FF column (0.7×3 cm, Amersham). The column was eluted with 50 mM MOPS containing 100 mM NaCl.
Lichenase activity was measured by a neocuproin method (Dygert et al. 1965). During purification of the enzyme, barley 1,3 : 1,4-β-glucan (Sigma) and soluble starch (Katayama Chemical, Osaka, Japan) were used as substrates.
Identification of 1,3 : 1,4-β-glucan
Glucans of lily pollen tube wall were partially purified using anion exchange chromatography (DEAE sephadex A-25, Amersham). Barley 1,3 : 1,4-β-glucan or purified lily pollen glucan was dissolved in MOPS buffer (50 mM, pH 7.0) at a concentration of 1 mg ml–1. Eighty µl of substrate were mixed with 20 µl of partially purified lichenase. The reaction mixes were incubated for 30 min at 37°C. The reaction products were applied to HPLC-PAD (Dionex, Sunnyvale, CA, U.S.A.) system as described before (Timotiwu and Sakurai 2002).
Acknowledgments
This work was supported in part by cooperative programs between Japan and China (Japan Society for the Promotion of Science), and partly supported by National Natural Science Foundation of China (project No. 30170090). We thank Prof. Dr. Donald J. Nevins for a generous gift of B. subtilis α-amylase. We also thank Dr. Toshihisa Kotake for advising and providing degenerated primers used in this study. Finally, we are grateful to the Miyasako Flower Shop in Higashi Hiroshima for the supply of lily anthers.
Corresponding author: E-mail, nsakura@hiroshima-u.ac.jp; Fax, +81-824-24-0758.
Fig. 1 Sequences of LP-ExoI and LP-ExoII. (A) Amino acid sequences of LP-ExoI and LP-ExoII. Amino acid residues of LP-ExoII identical to those of LP-ExoI are indicated by asterisks. Arrowheads show the cleavage sites of signal peptide. Broken under lines show the possible N-glycosylation sites. The positions of degenerated primers used for cloning of exo-β-glucanases are shown by arrows. (B) cDNA sequence alignment of LP-ExoI and LP-ExoII. Nucleotides of LP-ExoII identical to those of LP-ExoI are indicated by asterisks, and gaps are indicated by hyphen. The shadow boxes show the position of start codon and stop codon. The positions of gene specific primers used in RT-PCR analysis are shown by arrows.
Fig. 2 RT-PCR analysis. (A) Specificity of gene specific primers. The plasmids containing cDNA of LP-ExoI (shown as pLP-ExoI) or LP-ExoII (pLP-ExoII) were used as PCR template with gene specific primers to check their specificity by 28 PCR cycles. (B) Expressions of LP-ExoI and LP-ExoII in lily pollen. Total RNAs were converted to cDNA by reverse transcriptase. Then 523-bp LP-ExoI or LP-ExoII fragment was amplified. Lily actin-bundling protein (ABP) cDNA was amplified for normalization. The mock RT reaction without the reverse transcriptase enzyme was performed as a negative control for all reactions. Pg, pollen grains; Pt, pollen tubes. (C) Expressions of LP-ExoI and LP-ExoII in lily tissues. Leaf (Le), stem (St), stigma and style (SS), ovary (Ov), petal (Pe) and filament (Fi) were collected from lilies after flowering. Root (Rt) and bud (Bu) were collected from germinated lily bulbs 20 d after planting. Scale leaves (Sc) were collected from lily bulbs after vernalization.
Fig. 3 Identification of 1,3 : 1,4-β-glucan in the hemicellulose fraction of lily pollen tubes. Hemicellulose fraction (indicated by a solid line) and hemicellulose fraction mixed with barley 1,3 : 1,4-β-glucan (indicated by a broken line) were treated with partially purified lichenase from B. subtilis. The reaction products were applied to an HPLC-PAD system. The eluting positions of tri-saccharide and tetra-saccharide are shown.
Sugar contents and monosaccharide composition in hemicellulose fractions of lily pollen grains and tubes
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | Total | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Pollen grains | 1.51 | 0.08 | 1.59 | 1 | 1 | 8 | 7 | 4 | 6 | 68 | 5 | |
| Pollen tubes | 10.95 | 0.22 | 11.17 | 3 | 2 | 20 | 8 | 1 | 9 | 55 | 2 | |
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | Total | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Pollen grains | 1.51 | 0.08 | 1.59 | 1 | 1 | 8 | 7 | 4 | 6 | 68 | 5 | |
| Pollen tubes | 10.95 | 0.22 | 11.17 | 3 | 2 | 20 | 8 | 1 | 9 | 55 | 2 | |
Pollen grains and 5-h incubated pollen tubes were treated with ethanolamine and α-amylase to remove exine and starch, respectively. Cell wall polysaccharides were fractionated into pectin, hemicellulose and cellulose fractions. NS, neutral sugar; UA, uronic acid.
Sugar contents and monosaccharide composition in hemicellulose fractions of lily pollen grains and tubes
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | Total | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Pollen grains | 1.51 | 0.08 | 1.59 | 1 | 1 | 8 | 7 | 4 | 6 | 68 | 5 | |
| Pollen tubes | 10.95 | 0.22 | 11.17 | 3 | 2 | 20 | 8 | 1 | 9 | 55 | 2 | |
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | Total | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Pollen grains | 1.51 | 0.08 | 1.59 | 1 | 1 | 8 | 7 | 4 | 6 | 68 | 5 | |
| Pollen tubes | 10.95 | 0.22 | 11.17 | 3 | 2 | 20 | 8 | 1 | 9 | 55 | 2 | |
Pollen grains and 5-h incubated pollen tubes were treated with ethanolamine and α-amylase to remove exine and starch, respectively. Cell wall polysaccharides were fractionated into pectin, hemicellulose and cellulose fractions. NS, neutral sugar; UA, uronic acid.
Methylation analysis in hemicellulose fractions of pollen grains and tubes. SEs (n = 3) are shown
| Fraction | Pollen grains (mol %) | Pollen tubes (mol %) | |
| Rha | T- | 0 | 0.7±0.1 |
| 3- | 1.0±0.2 | 0 | |
| 2,4- | 4.6±0.3 | 0 | |
| Ara | Tp- | 0 | 0.3±0.1 |
| 5f- | 18.3±0.7 | 16.3±0.6 | |
| 2,5- | 4.6±0.3 | 7.9±0.5 | |
| Xyl | 4p- | 3.6±0.6 | 0.9±0.0 |
| Man | 4- | 1.4±0.1 | 0.4±0.0 |
| 6- | 5.8±0.2 | 3.2±0.1 | |
| 3,4- | 0.7±0.1 | 0 | |
| Gal | Tp- | 3.8±0.1 | 3.4±0.1 |
| 3- | 0 | 0.6±0.1 | |
| 4- | 2.3±0.1 | 0 | |
| 3,6- | 0.9±0.2 | 0 | |
| 4,6- | 0 | 0.2±0.0 | |
| Glc | T- | 0 | 1.6±0.0 |
| 2- | 2.2±0.4 | 0 | |
| 3- | 0 | 44.3±1.2 | |
| 4- | 14.7±0.4 | 3.7±0.1 | |
| 6- | 1.4±0.1 | 2.9±0.1 | |
| 2,3- | 0 | 0.7±0.1 | |
| 3,6- | 1.1±0.1 | 3.7±0.3 | |
| 4,6- | 32.4±2.0 | 6.5±0.1 | |
| 3,4,6- | 0.7±0.1 | 1.1±0.3 | |
| 2,3,4,6- | 0.6±0.1 | 0 | |
| Fraction | Pollen grains (mol %) | Pollen tubes (mol %) | |
| Rha | T- | 0 | 0.7±0.1 |
| 3- | 1.0±0.2 | 0 | |
| 2,4- | 4.6±0.3 | 0 | |
| Ara | Tp- | 0 | 0.3±0.1 |
| 5f- | 18.3±0.7 | 16.3±0.6 | |
| 2,5- | 4.6±0.3 | 7.9±0.5 | |
| Xyl | 4p- | 3.6±0.6 | 0.9±0.0 |
| Man | 4- | 1.4±0.1 | 0.4±0.0 |
| 6- | 5.8±0.2 | 3.2±0.1 | |
| 3,4- | 0.7±0.1 | 0 | |
| Gal | Tp- | 3.8±0.1 | 3.4±0.1 |
| 3- | 0 | 0.6±0.1 | |
| 4- | 2.3±0.1 | 0 | |
| 3,6- | 0.9±0.2 | 0 | |
| 4,6- | 0 | 0.2±0.0 | |
| Glc | T- | 0 | 1.6±0.0 |
| 2- | 2.2±0.4 | 0 | |
| 3- | 0 | 44.3±1.2 | |
| 4- | 14.7±0.4 | 3.7±0.1 | |
| 6- | 1.4±0.1 | 2.9±0.1 | |
| 2,3- | 0 | 0.7±0.1 | |
| 3,6- | 1.1±0.1 | 3.7±0.3 | |
| 4,6- | 32.4±2.0 | 6.5±0.1 | |
| 3,4,6- | 0.7±0.1 | 1.1±0.3 | |
| 2,3,4,6- | 0.6±0.1 | 0 | |
Methylation analysis in hemicellulose fractions of pollen grains and tubes. SEs (n = 3) are shown
| Fraction | Pollen grains (mol %) | Pollen tubes (mol %) | |
| Rha | T- | 0 | 0.7±0.1 |
| 3- | 1.0±0.2 | 0 | |
| 2,4- | 4.6±0.3 | 0 | |
| Ara | Tp- | 0 | 0.3±0.1 |
| 5f- | 18.3±0.7 | 16.3±0.6 | |
| 2,5- | 4.6±0.3 | 7.9±0.5 | |
| Xyl | 4p- | 3.6±0.6 | 0.9±0.0 |
| Man | 4- | 1.4±0.1 | 0.4±0.0 |
| 6- | 5.8±0.2 | 3.2±0.1 | |
| 3,4- | 0.7±0.1 | 0 | |
| Gal | Tp- | 3.8±0.1 | 3.4±0.1 |
| 3- | 0 | 0.6±0.1 | |
| 4- | 2.3±0.1 | 0 | |
| 3,6- | 0.9±0.2 | 0 | |
| 4,6- | 0 | 0.2±0.0 | |
| Glc | T- | 0 | 1.6±0.0 |
| 2- | 2.2±0.4 | 0 | |
| 3- | 0 | 44.3±1.2 | |
| 4- | 14.7±0.4 | 3.7±0.1 | |
| 6- | 1.4±0.1 | 2.9±0.1 | |
| 2,3- | 0 | 0.7±0.1 | |
| 3,6- | 1.1±0.1 | 3.7±0.3 | |
| 4,6- | 32.4±2.0 | 6.5±0.1 | |
| 3,4,6- | 0.7±0.1 | 1.1±0.3 | |
| 2,3,4,6- | 0.6±0.1 | 0 | |
| Fraction | Pollen grains (mol %) | Pollen tubes (mol %) | |
| Rha | T- | 0 | 0.7±0.1 |
| 3- | 1.0±0.2 | 0 | |
| 2,4- | 4.6±0.3 | 0 | |
| Ara | Tp- | 0 | 0.3±0.1 |
| 5f- | 18.3±0.7 | 16.3±0.6 | |
| 2,5- | 4.6±0.3 | 7.9±0.5 | |
| Xyl | 4p- | 3.6±0.6 | 0.9±0.0 |
| Man | 4- | 1.4±0.1 | 0.4±0.0 |
| 6- | 5.8±0.2 | 3.2±0.1 | |
| 3,4- | 0.7±0.1 | 0 | |
| Gal | Tp- | 3.8±0.1 | 3.4±0.1 |
| 3- | 0 | 0.6±0.1 | |
| 4- | 2.3±0.1 | 0 | |
| 3,6- | 0.9±0.2 | 0 | |
| 4,6- | 0 | 0.2±0.0 | |
| Glc | T- | 0 | 1.6±0.0 |
| 2- | 2.2±0.4 | 0 | |
| 3- | 0 | 44.3±1.2 | |
| 4- | 14.7±0.4 | 3.7±0.1 | |
| 6- | 1.4±0.1 | 2.9±0.1 | |
| 2,3- | 0 | 0.7±0.1 | |
| 3,6- | 1.1±0.1 | 3.7±0.3 | |
| 4,6- | 32.4±2.0 | 6.5±0.1 | |
| 3,4,6- | 0.7±0.1 | 1.1±0.3 | |
| 2,3,4,6- | 0.6±0.1 | 0 | |
Sugar contents and monosaccharide composition in hemicellulose fraction of lily tissues except pollen
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | TS | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Leaf | 4.1 | 0.3 | 4.4 | 2 | 2 | 7 | 24 | 9 | 12 | 37 | 7 | |
| Stigma | 3.5 | 0.5 | 4.0 | 1 | 1 | 5 | 12 | 24 | 9 | 35 | 13 | |
| Style | 12.8 | 0.3 | 13.1 | 1 | 1 | 4 | 16 | 26 | 11 | 39 | 2 | |
| Ovary | 1.9 | 0.2 | 2.1 | 2 | 2 | 9 | 19 | 14 | 12 | 37 | 4 | |
| Petal | 5.8 | 0.1 | 5.9 | 1 | 2 | 2 | 19 | 15 | 17 | 42 | 2 | |
| Filament | 6.3 | 0.2 | 6.5 | 2 | 2 | 3 | 21 | 13 | 15 | 42 | 3 | |
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | TS | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Leaf | 4.1 | 0.3 | 4.4 | 2 | 2 | 7 | 24 | 9 | 12 | 37 | 7 | |
| Stigma | 3.5 | 0.5 | 4.0 | 1 | 1 | 5 | 12 | 24 | 9 | 35 | 13 | |
| Style | 12.8 | 0.3 | 13.1 | 1 | 1 | 4 | 16 | 26 | 11 | 39 | 2 | |
| Ovary | 1.9 | 0.2 | 2.1 | 2 | 2 | 9 | 19 | 14 | 12 | 37 | 4 | |
| Petal | 5.8 | 0.1 | 5.9 | 1 | 2 | 2 | 19 | 15 | 17 | 42 | 2 | |
| Filament | 6.3 | 0.2 | 6.5 | 2 | 2 | 3 | 21 | 13 | 15 | 42 | 3 | |
Sugar contents and monosaccharide composition in hemicellulose fraction of lily tissues except pollen
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | TS | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Leaf | 4.1 | 0.3 | 4.4 | 2 | 2 | 7 | 24 | 9 | 12 | 37 | 7 | |
| Stigma | 3.5 | 0.5 | 4.0 | 1 | 1 | 5 | 12 | 24 | 9 | 35 | 13 | |
| Style | 12.8 | 0.3 | 13.1 | 1 | 1 | 4 | 16 | 26 | 11 | 39 | 2 | |
| Ovary | 1.9 | 0.2 | 2.1 | 2 | 2 | 9 | 19 | 14 | 12 | 37 | 4 | |
| Petal | 5.8 | 0.1 | 5.9 | 1 | 2 | 2 | 19 | 15 | 17 | 42 | 2 | |
| Filament | 6.3 | 0.2 | 6.5 | 2 | 2 | 3 | 21 | 13 | 15 | 42 | 3 | |
| Sugar contents [mg (g pollen grain)–1] | Sugar composition (%) | |||||||||||
| NS | UA | TS | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| Leaf | 4.1 | 0.3 | 4.4 | 2 | 2 | 7 | 24 | 9 | 12 | 37 | 7 | |
| Stigma | 3.5 | 0.5 | 4.0 | 1 | 1 | 5 | 12 | 24 | 9 | 35 | 13 | |
| Style | 12.8 | 0.3 | 13.1 | 1 | 1 | 4 | 16 | 26 | 11 | 39 | 2 | |
| Ovary | 1.9 | 0.2 | 2.1 | 2 | 2 | 9 | 19 | 14 | 12 | 37 | 4 | |
| Petal | 5.8 | 0.1 | 5.9 | 1 | 2 | 2 | 19 | 15 | 17 | 42 | 2 | |
| Filament | 6.3 | 0.2 | 6.5 | 2 | 2 | 3 | 21 | 13 | 15 | 42 | 3 | |
Methylation analysis in hemicellulose fractions of lily tissues except pollen
| Leaf (mol %) | Stigma (mol %) | Style (mol %) | Ovary (mol %) | Petal (mol %) | Filament (mol %) | ||
| Rha | T- | 1 | 0 | 0 | 0 | 1 | 0 |
| 2- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,4- | 1 | 1 | 2 | 3 | 1 | 3 | |
| 2,3,4- | 0 | 0 | 0 | 0 | 0 | 1 | |
| Ara | Tf- | 1 | 0 | 0 | 1 | 0 | 0 |
| Tp- | 6 | 1 | 2 | 3 | 4 | 2 | |
| 5f- | 4 | 0 | 0 | 0 | 1 | 2 | |
| 5p- | 0 | 1 | 1 | 7 | 0 | 0 | |
| Xyl | 4p- | 16 | 4 | 9 | 7 | 11 | 6 |
| 2,3f- | 0 | 2 | 1 | 2 | 0 | 1 | |
| Man | Tp- | 5 | 1 | 1 | 3 | 4 | 2 |
| 4- | 10 | 18 | 26 | 12 | 20 | 9 | |
| 6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 2 | 1 | 1 | 1 | 2 | 1 | |
| 2,3,4,6- | 0 | 11 | 4 | 2 | 0 | 5 | |
| Gal | Tp- | 0 | 0 | 0 | 0 | 0 | 0 |
| 4- | 3 | 2 | 1 | 4 | 7 | 3 | |
| 3,6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,3,4,6- | 0 | 2 | 4 | 2 | 0 | 6 | |
| Glc | Tp- | 0 | 0 | 0 | 0 | 1 | 0 |
| 2- | 4 | 2 | 2 | 4 | 4 | 3 | |
| 3- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4- | 21 | 21 | 23 | 22 | 27 | 16 | |
| 2,6- | 1 | 2 | 0 | 0 | 1 | 0 | |
| 3,4- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 22 | 10 | 13 | 22 | 15 | 17 | |
| 2,3,4,6- | 0 | 15 | 6 | 5 | 0 | 15 |
| Leaf (mol %) | Stigma (mol %) | Style (mol %) | Ovary (mol %) | Petal (mol %) | Filament (mol %) | ||
| Rha | T- | 1 | 0 | 0 | 0 | 1 | 0 |
| 2- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,4- | 1 | 1 | 2 | 3 | 1 | 3 | |
| 2,3,4- | 0 | 0 | 0 | 0 | 0 | 1 | |
| Ara | Tf- | 1 | 0 | 0 | 1 | 0 | 0 |
| Tp- | 6 | 1 | 2 | 3 | 4 | 2 | |
| 5f- | 4 | 0 | 0 | 0 | 1 | 2 | |
| 5p- | 0 | 1 | 1 | 7 | 0 | 0 | |
| Xyl | 4p- | 16 | 4 | 9 | 7 | 11 | 6 |
| 2,3f- | 0 | 2 | 1 | 2 | 0 | 1 | |
| Man | Tp- | 5 | 1 | 1 | 3 | 4 | 2 |
| 4- | 10 | 18 | 26 | 12 | 20 | 9 | |
| 6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 2 | 1 | 1 | 1 | 2 | 1 | |
| 2,3,4,6- | 0 | 11 | 4 | 2 | 0 | 5 | |
| Gal | Tp- | 0 | 0 | 0 | 0 | 0 | 0 |
| 4- | 3 | 2 | 1 | 4 | 7 | 3 | |
| 3,6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,3,4,6- | 0 | 2 | 4 | 2 | 0 | 6 | |
| Glc | Tp- | 0 | 0 | 0 | 0 | 1 | 0 |
| 2- | 4 | 2 | 2 | 4 | 4 | 3 | |
| 3- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4- | 21 | 21 | 23 | 22 | 27 | 16 | |
| 2,6- | 1 | 2 | 0 | 0 | 1 | 0 | |
| 3,4- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 22 | 10 | 13 | 22 | 15 | 17 | |
| 2,3,4,6- | 0 | 15 | 6 | 5 | 0 | 15 |
Methylation analysis in hemicellulose fractions of lily tissues except pollen
| Leaf (mol %) | Stigma (mol %) | Style (mol %) | Ovary (mol %) | Petal (mol %) | Filament (mol %) | ||
| Rha | T- | 1 | 0 | 0 | 0 | 1 | 0 |
| 2- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,4- | 1 | 1 | 2 | 3 | 1 | 3 | |
| 2,3,4- | 0 | 0 | 0 | 0 | 0 | 1 | |
| Ara | Tf- | 1 | 0 | 0 | 1 | 0 | 0 |
| Tp- | 6 | 1 | 2 | 3 | 4 | 2 | |
| 5f- | 4 | 0 | 0 | 0 | 1 | 2 | |
| 5p- | 0 | 1 | 1 | 7 | 0 | 0 | |
| Xyl | 4p- | 16 | 4 | 9 | 7 | 11 | 6 |
| 2,3f- | 0 | 2 | 1 | 2 | 0 | 1 | |
| Man | Tp- | 5 | 1 | 1 | 3 | 4 | 2 |
| 4- | 10 | 18 | 26 | 12 | 20 | 9 | |
| 6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 2 | 1 | 1 | 1 | 2 | 1 | |
| 2,3,4,6- | 0 | 11 | 4 | 2 | 0 | 5 | |
| Gal | Tp- | 0 | 0 | 0 | 0 | 0 | 0 |
| 4- | 3 | 2 | 1 | 4 | 7 | 3 | |
| 3,6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,3,4,6- | 0 | 2 | 4 | 2 | 0 | 6 | |
| Glc | Tp- | 0 | 0 | 0 | 0 | 1 | 0 |
| 2- | 4 | 2 | 2 | 4 | 4 | 3 | |
| 3- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4- | 21 | 21 | 23 | 22 | 27 | 16 | |
| 2,6- | 1 | 2 | 0 | 0 | 1 | 0 | |
| 3,4- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 22 | 10 | 13 | 22 | 15 | 17 | |
| 2,3,4,6- | 0 | 15 | 6 | 5 | 0 | 15 |
| Leaf (mol %) | Stigma (mol %) | Style (mol %) | Ovary (mol %) | Petal (mol %) | Filament (mol %) | ||
| Rha | T- | 1 | 0 | 0 | 0 | 1 | 0 |
| 2- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,4- | 1 | 1 | 2 | 3 | 1 | 3 | |
| 2,3,4- | 0 | 0 | 0 | 0 | 0 | 1 | |
| Ara | Tf- | 1 | 0 | 0 | 1 | 0 | 0 |
| Tp- | 6 | 1 | 2 | 3 | 4 | 2 | |
| 5f- | 4 | 0 | 0 | 0 | 1 | 2 | |
| 5p- | 0 | 1 | 1 | 7 | 0 | 0 | |
| Xyl | 4p- | 16 | 4 | 9 | 7 | 11 | 6 |
| 2,3f- | 0 | 2 | 1 | 2 | 0 | 1 | |
| Man | Tp- | 5 | 1 | 1 | 3 | 4 | 2 |
| 4- | 10 | 18 | 26 | 12 | 20 | 9 | |
| 6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 2 | 1 | 1 | 1 | 2 | 1 | |
| 2,3,4,6- | 0 | 11 | 4 | 2 | 0 | 5 | |
| Gal | Tp- | 0 | 0 | 0 | 0 | 0 | 0 |
| 4- | 3 | 2 | 1 | 4 | 7 | 3 | |
| 3,6- | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2,3,4,6- | 0 | 2 | 4 | 2 | 0 | 6 | |
| Glc | Tp- | 0 | 0 | 0 | 0 | 1 | 0 |
| 2- | 4 | 2 | 2 | 4 | 4 | 3 | |
| 3- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4- | 21 | 21 | 23 | 22 | 27 | 16 | |
| 2,6- | 1 | 2 | 0 | 0 | 1 | 0 | |
| 3,4- | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4,6- | 22 | 10 | 13 | 22 | 15 | 17 | |
| 2,3,4,6- | 0 | 15 | 6 | 5 | 0 | 15 |
Abbreviations
- Ara
arabinose
- DMSO
dimethyl sulfoxide
- DW
deionized water
- Fuc
fucose
- Gal
galactose
- Glc
glucose
- HPLC-PAD
high performance liquid chromatography with pulsed amperometric detector
- NMNO
N-morpholino N-oxide
- Man
mannose
- MOPS
3-(N-morpholino) propanesulfonic acid
- NS
neutral sugar
- RACE
rapid amplification of cDNA ends
- Rha
rhamnose
- RT
reverse transcript
- Xyl
xylose.
The nucleotide sequences, reported in this paper have been submitted to GenBank/ DDBJ under accession numbers AY357946 (LP-ExoI) and AB119653 (LP-ExoII).
References
Albersheim, P., Nevins, D.J., English, P.D. and Karr, A. (
Blackeny, A.B., Harris, P.J., Henry, R.J. and Stone, B.A. (
Blumenkrantz, N. and Asboe-Hansen, G. (
Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J. and Rutter, W.J. (
Conrad, H.E. (
Darley, C.P., Forrester, A.M. and McQueen-Mason, S.J. (
Dickinson, D.B. (
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. (
Dygert, S., Li, L.H., Florida, D. and Thoma, J.A. (
Fernando, D.D., Owens, J.N., Yu, X. and Ekramoddoullah, A.K.M. (
Ferguson, C., Teeri, T.T., Siika-aho, M., Read, S.M. and Bacic, A. (
Franklin-Tong, V.E. (
Futamura, N., Mori, H., Kouchi, H. and Shinohara, K. (
Handford, M.G., Baldwin, T.C., Goubet, F., Prime, T.A., Miles, J., Yu, X. and Dupree, P. (
Hepler, P.K., Vidali, L. and Cheung, A.Y. (
Herth, W., Franke, W.W., Bittiger, H., Kuppel, A. and Keilich, G. (
Hird, D.L., Worrall, D., Hodge, R., Smartt, S., Paul, W. and Scott, R. (
Hrmova, M. and Fincher, G.B. (
Hrmova, M., Harvey, A.J., Wang, J., Shirley, N.J., Jones, G.P., Stone, B.A., Høj, P.B. and Fincher, G.B. (
Huber, D.J. and Nevins, D.J. (
Joseleau, J.-P., Chambat, G. and Chumpitazi-Hermoza, B. (
Jungfermann, C., Ahlers, F., Grote, M., Gubatz, S., Steuernagel, S., Thom, I., Wetzels, G. and Wiermann, R. (
Kotake, T., Li, Y.Q., Takahashi, M. and Sakurai, N. (
Kotake, T., Nakagawa, N., Takeda, K. and Sakurai, N. (
Li, H., Bacic, A. and Read, S.M. (
Li, Y.Q., Kotake, T., Sakurai, N., Zhao, N.M. and Liu, Q. (
Li, Y.Q. and Linskens, H.F. (
Li, Y.Q., Moscatelli, A., Cai, G. and Cresti, M. (
Logemann, J., Schell, J. and Willmitzer, L. (
Mascarenhas, J.P. (
Mascarenhas, J.P. (
Rae, A.L., Harris, P.J., Bacic, A. and Clarke, A.E. (
Sakurai, N. (
Schlüpmann, H., Bacic, A. and Read, S.M. (
Simmons, C.R. (
Stieglitz, H. and Stern, H. (
Suen, D.F., Wu, S.S.H., Chang, H.C., Dhugga, K.S. and Huang, A.H.C. (
Taylor, L.P. and Hepler, P.K. (
Timotiwu, P.B. and Sakurai, N. (
Vidali, L., Yokota, E., Cheung, A.Y., Shimmen, T. and Hepler P.K. (
Vithanage, H.I.M.V., Gleeson, P.A. and Clarke, A.E. (