-
PDF
- Split View
-
Views
-
Cite
Cite
Sung-Suk Suh, Kyu-Ri Choi, Ilha Lee, Revisiting Phase Transition during Flowering in Arabidopsis, Plant and Cell Physiology, Volume 44, Issue 8, 15 August 2003, Pages 836–843, https://doi.org/10.1093/pcp/pcg109
Close - Share Icon Share
Abstract
Single-phase transition during flowering has been suggested by Hempel and Feldman (1994) [Planta 192: 276]. When early flowering ecotypes of Arabidopsis were microscopically observed, a long day signal simultaneously induced the acropetal (bottom to top) production of flower primordia and the basipetal (top to bottom) differentiation of paraclades (axillary flowering shoots) from the axils of pre-existing leaf primordia. However, this model could not account for the production of an extra number of secondary shoots in the TERMINAL FLOWER 1 overexpressor line or AGL20 overexpressor line in Columbia background with a functional allele of FRIGIDA. We report here that Columbia with a functional allele of FRIGIDA under long days and Columbia under short days show an inflorescence-producing phase between the vegetative and the flower-producing phases, supporting two-step phase transition during flowering. In addition, a late-flowering mutant, fwa shows an inflorescence phase but fca, fy and fve follow a single-phase transition, suggesting flowering time mutations have different effects on phase transition during flowering.
(Received April 1, 2003; Accepted June 17, 2003)
Introduction
Higher plants pass through a series of growth phases that are discernible by morphological characteristics such as leaf shape and trichome distribution (Poethig 1990, Schultz and Haughn 1993, Telfer et al. 1997). The most dramatic phase change in plant life cycle is a transition from vegetative to reproductive growth that is regulated by a complex network of flowering time genes (reviewed in Simpson and Dean 2002, Mouradov et al. 2002). In Arabidopsis, the vegetative shoot apical meristem produces rosette leaves that are closely appressed due to lack of internode elongation. However, during the transition to reproductive growth, four obvious morphological changes occur at the shoot apex. Internode elongation occurs and results in the ‘bolting’ of an inflorescence shoot. Leaf development is partially suppressed, so the cauline leaves developed after the transition to flowering are smaller than the rosette leaves. The primordia of paraclade, which develop into secondary shoots/coflorescences, are initiated on the axils of cauline leaves. Finally flowers that are not associated with bracts are produced at the flank of a shoot apical meristem (Schultz and Haughn 1991).
To date, two models to explain morphological changes during floral phase transition have been suggested. One is a model of two-step phase transition, which proposes that the shoot apical meristem first undergoes a transition from a rosette leaf-producing vegetative phase (V phase) to a coflorescence-producing inflorescence phase (I phase) and thereafter undergoes a transition from an inflorescence phase to a flower-producing phase (F phase) (Schultz and Haughn 1991, Mandel et al. 1992). The other is a single-phase transition model, which proposes that a shoot apical meristem directly changes from a vegetative to a flower-producing phase and the cauline leaves and paraclades are developed simultaneously with flowers after the transition to flowering (Hempel and Feldman 1994).
The model of two-step phase transition is based on macroscopic morphology of the wild-type Arabidopsis shoot; nodes with cauline leaves and coflorescences are observed between nodes with rosette leaves and nodes with flowers only after floral transition (Schultz and Haughn 1991). This model is also supported by the phenotype of mutations in flower meristem identity genes such as LEAFY (LFY) and APETALA 1 (AP1). Although lfy and ap1 mutants show normal vegetative growth, they show the conversion of flowers into inflorescence, suggesting the failure of the second transition from I phase to F phase (Schultz and Haughn 1991, Schultz and Haughn 1993, Irish and Sussex 1990, Mandel et al. 1992, Bowman et al. 1993).
In contrast, the single-phase transition model is based on the microscopic observation of the shoot apex during floral transition (Hempel and Feldman 1994). When Arabidopsis plants grown for 30 d in short days were transferred to long days, the shoot apical meristem produced flower primordia immediately and paraclades were differentiated from the axils of pre-existing leaf primordia in a basipetal direction. Concurrent with the paraclades activation, paraclade-associated leaf primordia were partially suppressed to develop as small cauline leaves. The fact that the uppermost paraclade primordium was the most highly developed and the biggest was suggested as the evidence of basipetal differentiation of paraclades (Hempel and Feldman 1994). Such morphological characteristics were also observed in plants grown continuously under long day conditions. Therefore, Hempel and Feldman (1994) proposed a single-phase transition model because acropetal initiation of flowers and basipetal initiation of paraclades occur simultaneously during floral transition.
Although the single-phase transition model fits in the wild-type Arabidopsis, especially for early-flowering ecotypes such as Columbia (Col), Landsberg erecta (Ler) and Nossen (No), it could not explain the extended length of coflorescence producing I phase in certain mutants and transgenic plants. The most dramatic extension of an inflorescence phase was observed in the transgenic plants that overexpress TERMINAL FLOWER 1 (TFL1) (Ratcliffe et al. 1998, Ratcliffe et al. 1999). The tfl1 mutant shows a shorter vegetative phase as well as a terminal flower phenotype which is indicative of a shorter I phase (Schultz and Haughn 1991, Shannon and Meeks-Wagner 1991). Consistent with this, 35S::TFL1 shows an extension of not only a V phase but also an I phase (Ratcliffe et al. 1998). Based on the phenotype of 35S::TFL1, Ratcliffe et al. (1998) divided the reproductive phase of growth into two distinct I phases according to the activity of the shoot apical meristem. During the first inflorescence phase (I1 phase), the shoot apical meristem produces cauline leaves that subtend secondary flowering shoots (paraclades) whereas during the second inflorescence phase (I2 phase), flowers are produced on the flanks of the shoot apical meristem.
The microscopic analyses of the shoot apex during flowering were performed mainly in early-flowering ecotypes of Arabidopsis such as Col, Ler and No (Hempel and Feldman 1994, Hempel and Feldman 1995, Hempel et al. 1998). Recently the genetic basis of late-flowering phenotypes in winter annual strains such as Stockholm and San feliu-2 was revealed (Lee et al. 1993, Clarke and Dean 1994, Lee et al. 1994, Koornneef et al. 1994). The two dominant genetic loci, FRIGIDA (FRI) and FLOWERING LOCUS C (FLC) largely account for the flowering phenotype of winter annual ecotypes. FLC encoding a MADS box gene acts as a repressor of floral transition and the transcript level of FLC is quantitatively related to the timing of flowering (Sheldon et al. 1999, Michaels and Amasino 1999a). It was also shown that the dominant FRI allele increases FLC transcript levels whereas vernalization, a long period of cold treatment for flowering, decreases FLC levels (Michaels and Amasino 1999a, Sheldon et al. 2000). The early flowering ecotypes Col and Ler have a 16 bp deletion in the FRI locus and Ler additionally has a recessive FLC locus that causes early flowering (Lee et al. 1994, Johanson et al. 2000). The plant lines containing functional FRI and FLC (FRI FLC) show delay in the phase transitions of all stages of the plant life cycle as well as in flowering. However, if AGAMOUS-LIKE 20 (AGL20) is overexpressed as in agl20-101D, all of the phase transitions in FRI FLC are accelerated (Lee et al. 2000). AGL20 is also called SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CO 1) because it was isolated in a screen for suppressors of CONSTANS (CO) overexpressor line (Onouchi et al. 2000, Samach et al. 2000).
Because the two models for floral transition seem to be contradictory, we have re-evaluated the two models by microscopic analyses using different genotypes and different physiological conditions. Our results show that a late-flowering winter annual ecotype undergoes two-step phase transition but an early-flowering ecotype undergoes a single-phase transition during flowering under long days. However, a vernalization-treated late-flowering ecotype undergoes a single-phase transition under long days. In contrast to this, under non-inductive short days an early flowering ecotype undergoes two-step phase transition. This result shows that the phase transition mode depends on the strength of floral signals and the competence of plants to the floral signals. We also report that the effects on the mode of phase transition are different among late-flowering mutants.
Results
Mode of phase transition in long day grown agl20-101D FRI FLC during flowering
The mutant agl20-101D was obtained by activation tagging mutagenesis using the FRI FLC line as a wild type (Lee et al. 2000). Overexpression of AGL20 in agl20-101D FRI FLC caused an acceleration of all stages of plant development as well as flowering time. Interestingly, agl20-101D FRI FLC produced a similar total number of leaves as Col wild type under long days (Table 1). agl20-101D FRI FLC produced on average 5.3 rosette leaves and 5.6 cauline leaves while Col produced 9.5 rosette leaves and 2.5 cauline leaves. Although the total numbers of leaves produced from the two plants before flowering were similar, agl20-101D FRI FLC produced approximately half the number of rosette leaves and twice the number of cauline leaves compared to Col. The single-phase change model is based on the timing of the cauline leaf initiation which is proposed to occur during the vegetative phase (Hempel and Feldman 1994). Thus, we wondered if the cauline leaves of agl20-101D FRI FLC were also produced before floral transition.
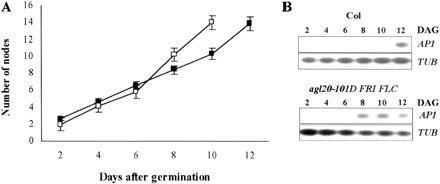
To address this question, we compared the rates of node (leaves and flowers) production and the timing of floral transition in Col and agl20-101D FRI FLC (Fig. 1). The rates of node production were determined by counting leaf primordia and flower meristem with scanning electron microscopic analysis (Fig. 1A). Interestingly, both of the two plants showed biphasic node production curves: the early phase of slow node production and the late phase of fast node production (Fig. 1A). It was also noteworthy that the rates of node production were similar in both plants if comparing the same phase. During the early phase, the rates of node production were 1.1 and 1.0 nodes d–1 but during the late phase, the rates were 1.9 and 1.8 nodes d–1 for Col and agl20-101D FRI FLC respectively. However, the timing of phase shifting from the early to the late phase was different between Col and agl20-101D FRI FLC. In Col, the shifting occurred at 10 d after germination when the production of rosette leaves was finished and cauline leaves were being produced. On the other hand, in agl20-101D FRI FLC, the shifting occurred right after the production of the last rosette leaves at 6 d after germination. Thus, all of the cauline leaves of agl20-101D FRI FLC were produced during the late phase of fast node production.
The change in node production rates usually occurs after floral initiation in mustard plants (Gonthier et al. 1987, Corbesier et al. 1996). Thus, we checked if the shifting from early to late phase was due to floral initiation in Col and agl20-101D FRI FLC. We compared the onset of AP1 expression in Col and agl20-101D FRI FLC because AP1 is commonly used as a molecular marker for floral determination: AP1 starts to be expressed approximately 1–2 d after photoinduction for flowering (Hempel et al. 1997, Hempel et al. 1998, Kardailsky et al. 1999). AP1 was first detected at 12 d and 8 d after germination in Col and agl20-101D FRI FLC respectively (Fig. 1B). Thus, in both plants, AP1 was first detected 2 d after the shifting from early to late phase. This result strongly supports the view that shifting from early to late phase occurred due to floral induction. In addition, it suggests that most of the cauline leaves, if not all, in agl20-101D FRI FLC were produced after floral transition, which is contradictory to the single-phase transition model.
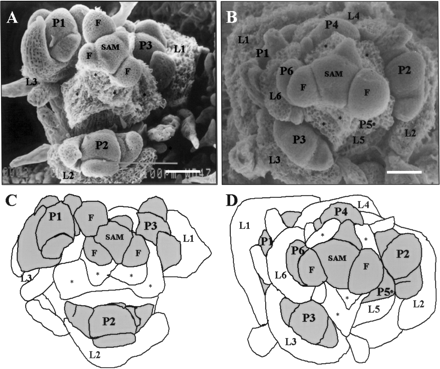
Finally, we checked the direction of paraclade differentiation in agl20-101D FRI FLC by scanning electronic microscopy. Paraclade is a structure subtended by a cauline leaf. According to the single phase-transition model, paraclades are differentiated from the axils of pre-existing leaf primordia in a basipetal (top to bottom) direction after floral transition (Hempel and Feldman 1994). Simultaneously, pre-existing leaf primordia develop as cauline leaves. Because cauline leaves of agl20-101D FRI FLC were produced after floral initiation, we wondered whether paraclade differentiation of agl20-101D FRI FLC occurs basipetally or acropetally. As a control, long day grown Col plants were checked for the direction of paraclades differentiation (Fig. 2A, C). As previously reported, Col plants grown under long days showed basipetal differentiation of paraclades: the uppermost paraclade was the largest and the most developed whereas the lowermost paraclade was the smallest and the least developed (Hempel and Feldman 1994). In contrast to this, agl20-101D FRI FLC showed acropetal differentiation of paraclades (Fig. 2B, D). The uppermost paraclade was the least developed while the lowermost paraclade was the most developed in agl20-101D FRI FLC. Such an acropetal differentiation of paraclades supports again the view that the two-step phase transition occurred in agl20-101D FRI FLC during flowering.
Phase transition mode is changed by environmental conditions
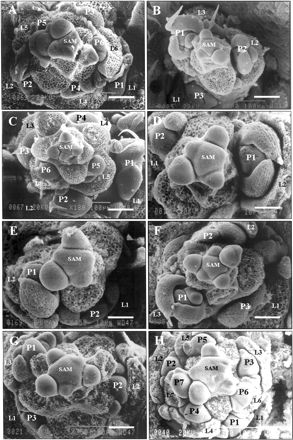
Because I phase and the flower-producing F phase may have been uncoupled in agl20-101D FRI FLC due to ectopic expression of AGL20, we wondered if the FRI FLC line without AGL20 overexpression also shows two-step phase transition. The FRI FLC line has been obtained by eight backcrosses into Col, so is a near-isogenic line of Col with a functional FRI gene (Michaels and Amasino 1999a). On the other hand, Col has a 16 bp deletion at the end of the first exon in the FRI gene, which causes a null mutation (Johanson et al. 2000). The microscopic morphology of the shoot apex was observed in FRI FLC undergoing floral transition. Similar to agl20-101D FRI FLC, the FRI FLC line showed acropetal differentiation of paraclades (Fig. 3A). The lowermost paraclade was the most highly developed and the level of development decreased gradually in the acropetal direction. In addition to the paraclade development, cauline leaf development also occurred acropetally. This result shows that two-step phase transition also occurs during flowering in the wild-type FRI FLC.
Vernalization accelerates flowering of FRI FLC dramatically. Four weeks vernalized FRI FLC produced similar numbers of rosette leaves and cauline leaves as Col grown under long days (Table 1). To check if the acceleration of flowering by vernalization affects the mode of the phase transition, the shoot apex of vernalized FRI FLC was microscopically observed (Fig. 3B). Four weeks vernalized FRI FLC showed basipetal differentiation of paraclades like the long day grown Col. This result shows that vernalization changes the mode of the phase transition as well as accelerating flowering.
The next question we asked was whether the photoperiod also affects the mode of the phase transition. Long day grown and short day grown Col plants were compared. Although long day grown Col showed basipetal differentiation of paraclades, short day grown Col plants showed acropetal differentiation (Fig. 3C). This result shows that the photoperiod also affects the mode of the phase transition. In addition, the results we obtained demonstrate that the mode of phase transition depends on the environmental conditions.
Mode of phase transition in the flowering time mutants
Previously, it was shown that the number of rosette and cauline leaves increases proportionally in all late-flowering mutants. However, the relative numbers of rosette and cauline leaves were different among the mutants (Koornneef et al. 1991, Koornneef et al. 1998). For example, the ratio of cauline/total leaf number is higher in co, gi and fwa mutants while the ratio is lower in fca, fve and fy mutants in general (Koornneef et al. 1991). This may suggest that different late-flowering mutants affect the phase transition mode differently. To test this, we analyzed fca, fve, fy and fwa mutants. As reported previously, fca, fy and fve showed low cauline/total leaf number but fwa showed a high cauline/total leaf number (Table 2). Rosette leaf numbers of fca-4, fy-1 and fve-1 were approximately 2 times higher than that of Ler wild type while the cauline leaf numbers were only 1.5 times higher. On the other hand, the numbers of both rosette and cauline leaves produced for fwa were three times higher than those of Ler (Table 2). Microscopic analysis of shoot apex was also performed for these mutants grown under long days (Fig. 3). Interestingly, fca-4, fy-1, fve-1 showed basipetal differentiation of paraclades as Ler (Fig. 3D, E, F, G). However, fwa-2 mutant showed acropetal differentiation of paraclades (Fig. 3H). Therefore, fwa-2 mutation changed the mode of phase transition, while the other mutations only delayed the flowering time without affecting the phase transition mode.
Discussion
Hempel and Feldman’s model of single-phase transition from vegetative to reproductive growth was widely accepted after they reported the results of microscopic analyses of a shoot apex undergoing floral transition (Hempel and Feldman 1994). However, there were many reports that were contradictory to a single-phase transition model (Schultz and Haughn 1993, Bowman et al. 1993, Ratcliffe et al. 1998, Ratcliffe et al. 1999). Using the same experimental approaches as Hempel and Feldman, we show here that plants alternatively undergo two-step transition depending on their environmental conditions and genotypes.
Although the single-phase transition during flowering was observed in the Col wild type, this model could not explain the extra cauline leaves produced in agl20-101D FRI FLC, a line which overexpresses AGL20 (Lee et al. 2000). According to the single-phase transition model, the extra cauline leaves of agl20-101D FRI FLC should have been produced during the vegetative phase but they were produced after floral induction. Two pieces of experimental evidence support such an interpretation. Firstly, the cauline leaves of agl20-101D FRI FLC were produced after a shift in the rates of leaf initiation, which is a strong indicator that floral induction has started (Fig. 1A). Secondly, AP1, a marker of floral determination, starts to be expressed while the cauline leaves are being produced (Fig. 1B). It should be noted that AP1 is also expressed in leaf/paraclade primordia with strong photoinduction (Hempel et al. 1997).
In addition, paraclade primordia with cauline leaves developed acropetally in agl20-101D FRI FLC, which is the opposite direction from that observed in Col undergoing a single-phase transition (Fig. 2). These results strongly support the presence of an I phase, during which inflorescence with cauline leaves develop, before the flower-producing phase, and hence supports the two-step phase transition model.
The occurrence of a two-step phase transition during flowering is not likely to be an exceptional case. For example, a change in the rate of leaf initiation from rosette leaf to cauline leaf production was also observed in 35S::TFL1 plants, which indicates that cauline leaves were produced after floral induction (Ratcliffe et al. 1998). In addition, 35S::AP1 35S::TFL1 double transgenic plants produced a similar number of rosette leaves but a higher number of cauline leaves than the wild type, suggesting that the extra number of cauline leaves should not have been developed during the vegetative phase (Ratcliffe et al. 1999). In order to explain the phenotype of 35S::TFL1, Ratcliffe et al. (Ratcliffe et al. 1998, Ratcliffe et al. 1999) also proposed the presence of an I phase. Therefore, two-step transition during flowering is more or less common.
The two-step transition during flowering was not limited to transgenic plants ectopically expressing certain genes or mutants. It also occurs naturally in the shoot apex of wild-type plants. The acropetal development of paraclades in FRI FLC definitely showed that two-step transition occurs in the wild type also (Fig. 3). In addition, if Col plants are grown under short days, they also show acropetal development of paraclades. In contrast, if FRI FLC lines are vernalized to activate flowering, basipetal development of paraclades proceeds, which indicates that they undergo single-phase transition (Fig. 3). Therefore, it depends on environmental conditions whether a single-phase transition or a two-step transition occurs. Our results suggest that a single-phase transition model fits only with the plants that undergo abrupt floral transition by strong floral signals.
Other evidence supporting a phase transition model based on two-step transition is observed in the phenotype of late-flowering mutants. Although fca and gi mutants produce a similar number of rosette leaves, gi produces a higher number of cauline leaves than fca (Koornneef et al. 1991). If there is only a single-phase transition from vegetative to reproductive growth, fca and gi should affect the number of rosette leaves and cauline leaves similarly. We interpret such a discrepancy as pointing to the presence of an I phase during which cauline leaves are produced. It is likely that fca and gi affect the transition from a vegetative to an I phase similarly but the transition from an I to a F phase differently. In general, the mutations of the genes in the photoperiod pathway affect more strongly the phase transition from I to F than the mutations of the genes in the autonomous pathway (Koornneef et al. 1991, Koornneef et al. 1998). Indeed, when we microscopically observed the shoot apex of the late flowering mutants, the mutant fwa which affects the photoperiod pathway showed acropetal development whereas the autonomous pathway mutants, fy, fca and fve showed basipetal development of paraclades (Fig. 3).
We propose that the presence of an I phase depends on the strength of floral signals and the competence of the shoot apex to respond to the floral signals. If the floral signals are strong enough and the shoot apex is competent to such signals, which happens in long day grown Col, a single-phase transition occurs. Since the fates of leaf primordia are not completely determined and the degree of determination is increased in a basipetal direction (Hempel and Feldman 1995, Hempel et al. 1998), the strong floral signals change the fate of leaf primordia to paraclades and such a change is greatest in the uppermost primordium. Thus, the basipetal development of paraclades from the existing leaf primordia is observed. Simultaneously, newly arising anlagen from the shoot apex develop as flowers under the influence of strong floral signals, hence a single-phase transition occurs in the absence of an I phase. However, FRI FLC lines express high levels of FLC which was proposed to strongly suppress the competence of the shoot apex (Michaels and Amasino 1999b). It is likely that under the suppression of meristem competence in FRI FLC, the fates of newly arising anlagen are changed gradually from a vegetative to a floral fate, hence the I phase, during which paraclades develop in an acropetal direction, proceeds before the F phase. Vernalization reduces the expression of FLC in FRI FLC and increases the competence of the shoot apex, thus causing a single-phase transition like Col plants grown under long days. Finally, Col plants under short days show two-step transition because they produce weak floral signals which cannot change the fate of leaf primordia.
In this study we reevaluated the current model of the single-phase transition during flowering in Arabidopsis and presented evidence showing that the mode of phase transition depends on both environmental conditions and genotypes. The revelation of the exact process of phase transition during flowering is potentially of critical importance to the understanding of floral induction events and the mechanisms by which flowering time genes act.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana were grown at 23±2°C, 60±10% relative humidity under long days (16 h light/8 h dark) or short days (8 h light/16 h dark), under cool white fluorescent lights (100 µmol m–2 s–1). For vernalization studies, seeds were imbibed on 0.75% phytagar containing half strength Murashige-Skoog (MS) medium (GIBCO-BRL) and incubated at 4°C for 4 weeks under short days. The FRI FLC line is a Columbia near isogenic line with FRI allele from San Feliu-2 obtained by eight backcrosses into Col as described before (Michaels and Amasino 1999a). The mutant agl20-101D FRI FLC was obtained from the activation tagging mutagenesis as described (Lee et al. 2000). The late-flowering mutants, fy-1, fca-4, fve-1 and fwa-2 are in the Ler background.
Scanning electron microscopy
For scanning electron microscopy, samples were collected, fixed, coated, and photographed as described by Bowman et al. (1989). The samples of late-flowering mutants were collected 3 d before the time when the inflorescence became visible in the most of the corresponding mutants.
RNA analysis
Total RNA was extracted as described before (Puissant and Houdebine 1990). The RT-PCR procedure and primers used for AP1 and TUB were previously described (Lee et al. 2000).
Acknowledgments
We thank C. Dean for providing fwa-2 seeds; R. Macknight for fca-4 seeds. This work was supported by a grant (code: PF003201-01) from the Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Korean government and by the Korea Science and Engineering Foundation through the Plant Metabolism Research Center, Kyung Hee University. S.-S. Suh and K.-R. Choi were supported by the Brain Korea 21 program.
Corresponding author: Email, ilhalee@plaza.snu.ac.kr; Fax, +82-2-872-1993.
Fig. 1 The rate of node production and floral transition times in Col and agl20-101D FRI FLC. (A) The rate of node production in Col and agl20-101D FRI FLC. Closed squares indicate Col, open squares indicate agl20-101D FRI FLC. The values represent the mean ± SD of 12 plants. (B) AP1 expression was determined by RT-PCR using RNA extracted from plants harvested every 2 d after germination. TUB was used as a quantitative control. AP1 expression is an indicator of floral determination.
Fig. 2 Morphogenesis at the shoot apex of Col and agl20-101D FRI FLC that undergo phase transition. Shoot apices shown are representative of apices from each genotype. A minimum of 10 apices was examined. (A, C) Shoot apex of Col (×320; bar = 37 µm). (B, D) Shoot apex of agl20-101D FRI FLC (×350; bar = 34 µm). Note the size and the degree of differentiation of paraclade primordia. In the downward direction, the size and the degree of differentiation of paraclades decrease in Col and increase in agl20-101D FRI FLC. Leaf primordia are numbered in ascending order (L1 = oldest leaf primordium). Developing paraclades are numbered in the direction of differentiation (P1 = most developed paraclade). L, leaf primordium; F, flower primordium; P, paraclade primordium; SAM, shoot apical meristem; *, organs discarded to reveal the structure.
Fig. 3 Morphogenesis at the shoot apex of plants that undergo phase transition. Shoot apices shown are representative of apices from each plant below. A minimum of 10 apices was examined. (A) FRI FLC without vernalization. Paraclade size increases in a basipetal direction (×250; bar = 48 µm). (B) FRIFLC treated with 4 weeks of vernalization. Paraclade size increases in an acropetal direction (×200; bar = 60 µm). (C) Col grown under short days. The paraclade size increases in a basipetal direction (×180; bar = 66 µm). (D) Ler (×400; bar = 30 µm), (E) fy-1 (×450; bar = 37 µm), (F) fca-4 (×300; bar = 40 µm), and (G) fve-1 (×350; bar = 34 µm) showed an increase in the paraclade size in an acropetal direction. (H) fwa-2 (×370; bar = 32 µm) showed an increase in the paraclade size in a basipetal direction. Leaf primordia are numbered in ascending order (L1 = oldest leaf primordium). Developing paraclades are numbered in the direction of differentiation (P1 = most developed paraclade). L, leaf primordium; F, flower primordium; P, paraclade primordium; SAM, shoot apical meristem; *, organs discarded to reveal the structure.
Flowering time of Col, agl20-101D FRI FLC and FRI FLC under different environmental conditions
| Plants | Treatment | Total leaves | RL | CL | n |
| Col | Long days | 11.21±0.29 | 8.78±0.26 | 2.31±0.37 | 38 |
| Short days | 52.08±0.44 | 46.24±0.82 | 5.76±0.50 | 15 | |
| agl20-101DFRI FLC | Long days | 11.31±0.57 | 5.73±0.51 | 5.54±0.35 | 42 |
| FRI FLC | Long days | 50.94±5.81 | 44.05±6.45 | 6.15±1.26 | 19 |
| Ver (2 weeks) | 36.75±1.51 | 30.23±2.55 | 6.53±0.51 | 13 | |
| Ver (4 weeks) | 14.41±0.64 | 11.52±0.76 | 2.86±0.42 | 35 |
| Plants | Treatment | Total leaves | RL | CL | n |
| Col | Long days | 11.21±0.29 | 8.78±0.26 | 2.31±0.37 | 38 |
| Short days | 52.08±0.44 | 46.24±0.82 | 5.76±0.50 | 15 | |
| agl20-101DFRI FLC | Long days | 11.31±0.57 | 5.73±0.51 | 5.54±0.35 | 42 |
| FRI FLC | Long days | 50.94±5.81 | 44.05±6.45 | 6.15±1.26 | 19 |
| Ver (2 weeks) | 36.75±1.51 | 30.23±2.55 | 6.53±0.51 | 13 | |
| Ver (4 weeks) | 14.41±0.64 | 11.52±0.76 | 2.86±0.42 | 35 |
Long days, 16 h light/8 h dark; short days, 8 h light/16 h dark. Ver, treated with vernalization. RL, number of rosette leaves produced when flowering. CL, number of cauline leaves produced when flowering.
Flowering time of Col, agl20-101D FRI FLC and FRI FLC under different environmental conditions
| Plants | Treatment | Total leaves | RL | CL | n |
| Col | Long days | 11.21±0.29 | 8.78±0.26 | 2.31±0.37 | 38 |
| Short days | 52.08±0.44 | 46.24±0.82 | 5.76±0.50 | 15 | |
| agl20-101DFRI FLC | Long days | 11.31±0.57 | 5.73±0.51 | 5.54±0.35 | 42 |
| FRI FLC | Long days | 50.94±5.81 | 44.05±6.45 | 6.15±1.26 | 19 |
| Ver (2 weeks) | 36.75±1.51 | 30.23±2.55 | 6.53±0.51 | 13 | |
| Ver (4 weeks) | 14.41±0.64 | 11.52±0.76 | 2.86±0.42 | 35 |
| Plants | Treatment | Total leaves | RL | CL | n |
| Col | Long days | 11.21±0.29 | 8.78±0.26 | 2.31±0.37 | 38 |
| Short days | 52.08±0.44 | 46.24±0.82 | 5.76±0.50 | 15 | |
| agl20-101DFRI FLC | Long days | 11.31±0.57 | 5.73±0.51 | 5.54±0.35 | 42 |
| FRI FLC | Long days | 50.94±5.81 | 44.05±6.45 | 6.15±1.26 | 19 |
| Ver (2 weeks) | 36.75±1.51 | 30.23±2.55 | 6.53±0.51 | 13 | |
| Ver (4 weeks) | 14.41±0.64 | 11.52±0.76 | 2.86±0.42 | 35 |
Long days, 16 h light/8 h dark; short days, 8 h light/16 h dark. Ver, treated with vernalization. RL, number of rosette leaves produced when flowering. CL, number of cauline leaves produced when flowering.
Flowering time of late flowering mutants and wild type
| Genotype | Treatment | Total leaves | RL | CL | n |
| Ler | Long days | 7.39±0.51 | 5.42±0.45 | 1.93±0.56 | 12 |
| fca-4 | Long days | 14.38±0.58 | 11.54±0.68 | 2.77±0.46 | 17 |
| fy-1 | Long days | 13.75±0.61 | 11.15±0.64 | 2.56±0.53 | 11 |
| fve-1 | Long days | 13.91±0.64 | 11.01±0.71 | 2.87±0.33 | 15 |
| fwa-2 | Long days | 23.75±0.52 | 16.46±0.67 | 7.21±0.40 | 15 |
| Genotype | Treatment | Total leaves | RL | CL | n |
| Ler | Long days | 7.39±0.51 | 5.42±0.45 | 1.93±0.56 | 12 |
| fca-4 | Long days | 14.38±0.58 | 11.54±0.68 | 2.77±0.46 | 17 |
| fy-1 | Long days | 13.75±0.61 | 11.15±0.64 | 2.56±0.53 | 11 |
| fve-1 | Long days | 13.91±0.64 | 11.01±0.71 | 2.87±0.33 | 15 |
| fwa-2 | Long days | 23.75±0.52 | 16.46±0.67 | 7.21±0.40 | 15 |
RL, number of rosette leaves produced before flowering. CL, number of cauline leaves produced when flowering.
Flowering time of late flowering mutants and wild type
| Genotype | Treatment | Total leaves | RL | CL | n |
| Ler | Long days | 7.39±0.51 | 5.42±0.45 | 1.93±0.56 | 12 |
| fca-4 | Long days | 14.38±0.58 | 11.54±0.68 | 2.77±0.46 | 17 |
| fy-1 | Long days | 13.75±0.61 | 11.15±0.64 | 2.56±0.53 | 11 |
| fve-1 | Long days | 13.91±0.64 | 11.01±0.71 | 2.87±0.33 | 15 |
| fwa-2 | Long days | 23.75±0.52 | 16.46±0.67 | 7.21±0.40 | 15 |
| Genotype | Treatment | Total leaves | RL | CL | n |
| Ler | Long days | 7.39±0.51 | 5.42±0.45 | 1.93±0.56 | 12 |
| fca-4 | Long days | 14.38±0.58 | 11.54±0.68 | 2.77±0.46 | 17 |
| fy-1 | Long days | 13.75±0.61 | 11.15±0.64 | 2.56±0.53 | 11 |
| fve-1 | Long days | 13.91±0.64 | 11.01±0.71 | 2.87±0.33 | 15 |
| fwa-2 | Long days | 23.75±0.52 | 16.46±0.67 | 7.21±0.40 | 15 |
RL, number of rosette leaves produced before flowering. CL, number of cauline leaves produced when flowering.
Abbreviations
References
Bowman, J.L., Smyth, D.R. and Meyerowitz, E.M. (
Bowman, J.L., Alvarez, J., Weigel, D., Meyerowitz, E.M. and Smyth, D.R. (
Clarke, J.H. and Dean, C. (
Corbesier, L., Gadisseur, I., Silvestre, G., Jacqmard, A. and Bernier, G. (
Gonthier, R., Jacqmard, A. and Bernier, G. (
Hempel, F.D. and Feldman, L.J. (
Hempel, F.D. and Feldman, L.J. (
Hempel, F.D., Weigel, D., Mandel, M.A., Ditta, G., Zambryski, P.C., Feldman, L.J. and Yanofsky, M.F. (
Hempel, F.D., Zambryski, P.C. and Feldman, L.J. (
Irish, V.F. and Sussex, I.M. (
Johanson, U., West, J., Lister, C., Michaels, S., Amasino, R. and Dean, C. (
Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J. and Weigel, D. (
Koornneef, M., Hanhart, C.J. and van der Veen, J.H. (
Koornneef, M., Blankestijn-de Vries, H., Hanhart, C., Soppe, W. and Peeters, T. (
Koornneef, M., Alonso-Blanco, C., Blankestijn-de Vries, H., Hanhart, C.J. and Peeters, A.J. (
Lee, I., Bleecker, A. and Amasino, R.M. (
Lee, I., Michaels, S.D., Masshardt, A.S. and Amasino, R.M. (
Lee, H., Suh, S., Park, E., Cho, E., Ahn, J.H., Kim, S., Lee, J.S., Kwon, Y.M. and Lee, I. (
Mandel, M.A., Gustafson-Brown, C., Savidge, B. and Yanofsky, M.F. (
Michaels, S.D. and Amasino, R.M. (
Michaels, S.D. and Amasino, R.M. (
Mouradov, A., Cremer, F. and Coupland, G. (
Onouchi, H., Igeno, M.I., Perilleux, C., Graves, K. and Coupland, G. (
Poethig, R.S. (
Puissant, C. and Houdebine, L.M. (
Ratcliffe, O.J., Amaya, I., Vincent, C.A., Rothstein, S., Carpenter, R., Coen, E.S. and Bradley, D.J. (
Ratcliffe, O.J., Bradley, D.J. and Coen, E.S. (
Samach, A., Onouchi, H., Gold, S.E., Ditta, G.S., Schwarz-Sommer, Z., Yanofsky, M.F. and Coupland, G. (
Schultz, E.A. and Haughn, G.W. (
Schultz, E.A. and Haughn, G.W. (
Shannon, S. and Meeks-Wagner, D.R. (
Sheldon, C.C., Burn, J.E., Perez, P.P., Metzger, J., Edwards, J.A., Peacock, W.J. and Dennis, E.S. (
Sheldon, C.C., Rouse, D.T., Finnegan, E.J., Peacock, W.J. and Dennis, E.S. (
Simpson, G.G. and Dean, C. (