-
PDF
- Split View
-
Views
-
Cite
Cite
Parviz Khajehdehi, Mohammad Mojerlou, Saeed Behzadi, Ghanbar Ali Rais‐Jalali, A randomized, double‐blind, placebo‐controlled trial of supplementary vitamins E, C and their combination for treatment of haemodialysis cramps, Nephrology Dialysis Transplantation, Volume 16, Issue 7, July 2001, Pages 1448–1451, https://doi.org/10.1093/ndt/16.7.1448
Close - Share Icon Share
Abstract
Background. Muscle cramps that improve after carnitine or vitamin E therapies are common in haemodialysis (HD) patients. Because vitamin C participates in carnitine biosynthesis, and its levels are reduced in uraemia, subclinical vitamin C depletion may contribute to HD cramps. Our aim was to determine the effects of vitamins C, E and their combination on the frequency and intensity of HD cramps.
Methods. In this placebo‐controlled, double‐blind study, 60 HD‐patients were randomized into four therapeutic groups. Each group (n=15) received six identical capsules daily for 8 weeks, containing one of the following: vitamin E (400 mg), vitamin C (250 mg), their combination, or placebo.
Results. The frequency and intensity of HD cramps decreased significantly in all three vitamin groups compared with the placebo group at the end of the trial, and compared with the pre‐treatment values. At the end of the trial, vitamins E, C, their combination, and placebo produced cramp reductions of 54, 61, 97 and 7%, respectively. The percentage cramp reduction had no significant correlation with age, sex, aetiology of end‐stage renal disease, serum electrolytes or HD duration, but showed a positive correlation (r=0.33, P=0.01) with the type of therapy. No vitamin‐related adverse effects were encountered during the trial.
Conclusion. Short‐term treatment with the combination of vitamins E and C is safe and effective in reducing HD cramps; however, its safety for prolonged therapy has yet to be evaluated in HD patients.
Introduction
In haemodialysis (HD) patients, painful involuntary muscular contractions, called cramps and found typically in the lower extremities, are common [1–3]. Most commonly, HD patients complain of waking in the night with severe pain due to cramps, which interferes with functioning in normal life [3–6]. To deal with this problem, many approaches have been proposed, but none has been conclusively effective, and some have been associated with serious side effects [6–8]. Considering the potential toxicity of quinine, vitamin E has been recommended as the initial treatment of choice for HD cramps [7]. However, given the fact that muscle cramps due to carnitine deficiency improve after carnitine therapy in HD patients, and that vitamin C levels have been reported to be low in uraemia, it is possible that subclinical vitamin C depletion might contribute to HD cramps [9–15]. This study was initiated to determine whether ascorbic acid is effective against HD cramps, and whether it is more effective when combined with vitamin E.
Subjects and methods
Our patients underwent two to three HD sessions per week on polysulfune membranes in the three main teaching hospitals of the Shiraz University of Medical Sciences in southern Iran. Patients with the following criteria were selected:
Those signing a written consent to participate in a 8‐week trial of vitamin E and C.
Patients who had at least two muscle cramps (sudden and recurrent tonic or clonic painful involuntary muscle contractions, usually in lower extremities, occurring most commonly at night and interfering with sleep and normal life) per week.
Patients who were stable on HD without any episode of hypotension at least for 3 months.
Those being adequately dialysed with a total weekly Kt/V of ≥3.6.
Patients without renal stones who had neither personal nor family history of nephrolithiasis.
Those who were not receiving any drugs with muscle cramp‐producing or ‐relieving properties.
Patients who had not been scheduled for kidney transplantation during the course of the trial.
Sixty out of 239 patients undergoing regular HD in our three main teaching university hospitals fulfilled the above‐mentioned criteria. There were 31 men and 29 women, aged 50.8±15.2 years, with a duration on HD of 28.2±27.7 months (mean±SD). All 60 HD‐patients were given one tablet of multi‐vitamins daily that was replaced by one tablet of vitamin B complex. After 1 month on vitamin B complex they were randomized into the four therapeutic groups, as follows. Each group (n=15) received one tablet of vitamin B complex plus one of the following treatments: vitamin E (400 mg), vitamin C (250 mg), their combination or placebo, daily for 8 weeks. Each patient received six capsules daily that were identical in size, colour and weight, and were made and supplied under secret codes by the Shiraz School of Pharmacology. Both clinicians and patients were blind to the type of therapy during the trial. Frequency of cramps was recorded daily, for 1 week before the start of trial and up to the end of the trial. The intensity of cramps was recorded daily only during the week before the trial and on the final week of the trial. Cramp intensity was scored using following scale: no pain, mild, discomforting, distressing, horrible and excruciating, which were given scores ranging from one to six, respectively. All patients were interviewed and examined twice weekly during HD sessions to assess compliance with the therapies and to detect vitamin‐related side effects. Each patient served as self‐control, and her/his pre‐ and post‐therapy values were compared. Ultrasonography of genitourinary systems was performed just before and at the end of the trial. In all patients, clinical manifestations of scurvy including perifolicular hyperkeratotic papules with fragmented and buried hairs, perifollicular haemorrhage, purpura coalescing to become ecchymoses, haemorrhages in muscle and joints, gingivitis, loosening of teeth, and splinter haemorrhage in the nail beds, were examined at the time of randomization.
Statistical analysis
Data are presented as means±SD. For comparisons among the four therapeutic groups, standard analysis of variance (ANOVA) with Bonferroni/Dunn post‐hoc correction was used. Student's t‐tests and χ2 tests were used for comparing means and percentages when appropriate. Multiple regression analysis was used to correlate the percentage of cramp reduction obtained at end of the trial with age, sex, HD duration, aetiology of end‐stage renal disease, serum electrolytes, and the type of therapy that each group received.
Results
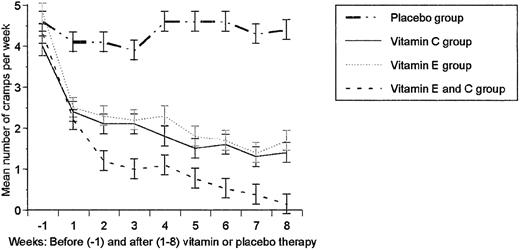
Table 1 compares patient characteristics among the four therapeutic groups at randomization. No statistically significant differences were found and there were no differences in pre‐ and post‐dialysis serum creatinine, blood urea nitrogen and blood pressure. Haemoglobin and haematocrit values remained unchanged during the course of the study. The frequency of HD cramps at the different time points (1 week pre‐trial and for 8 weeks during the trial) is shown in Figure 1. HD cramp frequency decreased progressively and significantly in all three groups receiving vitamins compared with the placebo group and the pre‐treatment values at all weekly time points. The decline in frequency of cramps was significantly more prominent in the combination group than in the two groups receiving vitamin C or E alone at all weekly time points. At the end of trial, the percentage cramp reduction was 54, 61, 97 and 7% in vitamin E, vitamin C, combination and placebo groups, respectively. The percentage cramp reduction had no significant correlation with the age, sex, aetiology of end‐stage renal disease, serum electrolytes or HD duration, but showed a positive correlation with the type of therapy (r=0.33, P=0.01). Table 2 compares the intensity score of cramps among the four therapeutic groups just before therapy and at the end of the trial. The intensity score decreased significantly in all three vitamin groups compared with the placebo group and with pre‐treatment values, with the strongest effect shown in the combination group (receiving vitamin C and E). All patients except for two had leg cramps characterized by sudden tonic or colonic involuntary contractions of the gastrocnemius muscle; the remaining two had cramps in the biceps and finger muscles. In addition to the gastrocnemius muscle, concomitant contractions in other muscles, mainly in the upper extremities, were present as follows: 11, nine, three and two patients had concomitant cramps of finger, biceps, intercostal and neck muscles, respectively. No vitamin‐related adverse effects or urinary stone formation were encountered during the trial. No clinical features of scurvy were found in any patient at randomization.
There was no significant difference in the frequency of haemodialysis cramps among the four therapeutic groups at randomization. However, cramp frequency decreased progressively and significantly in all three vitamin groups compared with the placebo group and baseline values at all weekly time points (P≤0.001 and P≤0.02, respectively). The decline in the frequency of cramps was more prominent in the combination group (vitamins E and C), which was significantly lower than in either of the groups receiving vitamin C or E alone, at all weekly time points (P≤0.01 and P≤0.04, respectively).
Comparison of patient characteristics at randomization
| Patient characteristics | Sixty haemodialysis patients randomized into four therapeutic groups | Total (n=60) | P value | ||||||
| Vitamin E (n=15) | Vitamin C (n=15) | Vitamins E and C (n=15) | Placebo (n=15) | ||||||
| Age, years (mean±SD) | 49.7±18.4 | 56.1±16.5 | 47.5±12.1 | 49.4±12.9 | 50.8±15.2 | NS | |||
| Sex, female/male ratio | 8/7 | 6/9 | 8/7 | 7/8 | 29/31 | NS | |||
| HD duration, months (mean±SD) | 20.7±12.7 | 32.6±31.5 | 31.7±21.1 | 27.6±39.0 | 28.2±27.7 | NS | |||
| ESRD due to diabetes (n) | 6 | 4 | 5 | 3 | 18 | NS | |||
| ESRD due to glomerulopathy (n) | 5 | 6 | 5 | 7 | 23 | NS | |||
| ESRD due to tubular disease (n) | 2 | 4 | 2 | 4 | 12 | NS | |||
| ESRD of unknown aetiology (n) | 2 | 1 | 3 | 1 | 7 | NS | |||
| Sodium, mEq/l (mean±SD) | 141.4±2.6 | 141.8±3.0 | 140.6±2.9 | 140.8±4.8 | 141.1±3.4 | NS | |||
| Potassium, mEq/l (mean±SD) | 4.6±0.44 | 4.5±0.45 | 4.8±0.26 | 4.7±0.38 | 4.6±0.39 | NS | |||
| Calcium, mg/dl (mean±SD) | 9.3±0.33 | 9.4±0.44 | 9.3±0.44 | 9.4±0.50 | 9.3±0.42 | NS | |||
| Phosphorous, mg/dl (mean±SD) | 4.4±0.85 | 4.5±0.54 | 4.7±0.32 | 4.5±0.81 | 4.5±0.66 | NS | |||
| Haemoglobin, g/dl (mean±SD) | 10.6±0.99 | 11.1±1.0 | 10.9±1.0 | 11.0±0.97 | 10.9±0.99 | NS | |||
| Haematocrit, % (mean±SD) | 32.2±3.1 | 34.0±3.1 | 33.2±3.4 | 33.1±2.9 | 33.1±3.1 | NS | |||
| Frequency of cramps (mean±SD) | 4.0±1.9 | 4.8±2.1 | 4.4±1.1 | 4.4±1.7 | 4.4±1.7 | NS | |||
| Cramp intensity score (mean±SD) | 3.5±1.6 | 2.8±1.4 | 3.5±1.6 | 3.1±1.6 | 3.2±1.6 | NS | |||
| Total weekly Kt/V (mean±SD) | 4.0±0.26 | 3.9±0.34 | 4.0±0.24 | 3.9±0.27 | 3.9±0.28 | NS | |||
| Patient characteristics | Sixty haemodialysis patients randomized into four therapeutic groups | Total (n=60) | P value | ||||||
| Vitamin E (n=15) | Vitamin C (n=15) | Vitamins E and C (n=15) | Placebo (n=15) | ||||||
| Age, years (mean±SD) | 49.7±18.4 | 56.1±16.5 | 47.5±12.1 | 49.4±12.9 | 50.8±15.2 | NS | |||
| Sex, female/male ratio | 8/7 | 6/9 | 8/7 | 7/8 | 29/31 | NS | |||
| HD duration, months (mean±SD) | 20.7±12.7 | 32.6±31.5 | 31.7±21.1 | 27.6±39.0 | 28.2±27.7 | NS | |||
| ESRD due to diabetes (n) | 6 | 4 | 5 | 3 | 18 | NS | |||
| ESRD due to glomerulopathy (n) | 5 | 6 | 5 | 7 | 23 | NS | |||
| ESRD due to tubular disease (n) | 2 | 4 | 2 | 4 | 12 | NS | |||
| ESRD of unknown aetiology (n) | 2 | 1 | 3 | 1 | 7 | NS | |||
| Sodium, mEq/l (mean±SD) | 141.4±2.6 | 141.8±3.0 | 140.6±2.9 | 140.8±4.8 | 141.1±3.4 | NS | |||
| Potassium, mEq/l (mean±SD) | 4.6±0.44 | 4.5±0.45 | 4.8±0.26 | 4.7±0.38 | 4.6±0.39 | NS | |||
| Calcium, mg/dl (mean±SD) | 9.3±0.33 | 9.4±0.44 | 9.3±0.44 | 9.4±0.50 | 9.3±0.42 | NS | |||
| Phosphorous, mg/dl (mean±SD) | 4.4±0.85 | 4.5±0.54 | 4.7±0.32 | 4.5±0.81 | 4.5±0.66 | NS | |||
| Haemoglobin, g/dl (mean±SD) | 10.6±0.99 | 11.1±1.0 | 10.9±1.0 | 11.0±0.97 | 10.9±0.99 | NS | |||
| Haematocrit, % (mean±SD) | 32.2±3.1 | 34.0±3.1 | 33.2±3.4 | 33.1±2.9 | 33.1±3.1 | NS | |||
| Frequency of cramps (mean±SD) | 4.0±1.9 | 4.8±2.1 | 4.4±1.1 | 4.4±1.7 | 4.4±1.7 | NS | |||
| Cramp intensity score (mean±SD) | 3.5±1.6 | 2.8±1.4 | 3.5±1.6 | 3.1±1.6 | 3.2±1.6 | NS | |||
| Total weekly Kt/V (mean±SD) | 4.0±0.26 | 3.9±0.34 | 4.0±0.24 | 3.9±0.27 | 3.9±0.28 | NS | |||
NS, no significant difference among the four groups; ESRD, end‐stage renal disease.
Comparison of patient characteristics at randomization
| Patient characteristics | Sixty haemodialysis patients randomized into four therapeutic groups | Total (n=60) | P value | ||||||
| Vitamin E (n=15) | Vitamin C (n=15) | Vitamins E and C (n=15) | Placebo (n=15) | ||||||
| Age, years (mean±SD) | 49.7±18.4 | 56.1±16.5 | 47.5±12.1 | 49.4±12.9 | 50.8±15.2 | NS | |||
| Sex, female/male ratio | 8/7 | 6/9 | 8/7 | 7/8 | 29/31 | NS | |||
| HD duration, months (mean±SD) | 20.7±12.7 | 32.6±31.5 | 31.7±21.1 | 27.6±39.0 | 28.2±27.7 | NS | |||
| ESRD due to diabetes (n) | 6 | 4 | 5 | 3 | 18 | NS | |||
| ESRD due to glomerulopathy (n) | 5 | 6 | 5 | 7 | 23 | NS | |||
| ESRD due to tubular disease (n) | 2 | 4 | 2 | 4 | 12 | NS | |||
| ESRD of unknown aetiology (n) | 2 | 1 | 3 | 1 | 7 | NS | |||
| Sodium, mEq/l (mean±SD) | 141.4±2.6 | 141.8±3.0 | 140.6±2.9 | 140.8±4.8 | 141.1±3.4 | NS | |||
| Potassium, mEq/l (mean±SD) | 4.6±0.44 | 4.5±0.45 | 4.8±0.26 | 4.7±0.38 | 4.6±0.39 | NS | |||
| Calcium, mg/dl (mean±SD) | 9.3±0.33 | 9.4±0.44 | 9.3±0.44 | 9.4±0.50 | 9.3±0.42 | NS | |||
| Phosphorous, mg/dl (mean±SD) | 4.4±0.85 | 4.5±0.54 | 4.7±0.32 | 4.5±0.81 | 4.5±0.66 | NS | |||
| Haemoglobin, g/dl (mean±SD) | 10.6±0.99 | 11.1±1.0 | 10.9±1.0 | 11.0±0.97 | 10.9±0.99 | NS | |||
| Haematocrit, % (mean±SD) | 32.2±3.1 | 34.0±3.1 | 33.2±3.4 | 33.1±2.9 | 33.1±3.1 | NS | |||
| Frequency of cramps (mean±SD) | 4.0±1.9 | 4.8±2.1 | 4.4±1.1 | 4.4±1.7 | 4.4±1.7 | NS | |||
| Cramp intensity score (mean±SD) | 3.5±1.6 | 2.8±1.4 | 3.5±1.6 | 3.1±1.6 | 3.2±1.6 | NS | |||
| Total weekly Kt/V (mean±SD) | 4.0±0.26 | 3.9±0.34 | 4.0±0.24 | 3.9±0.27 | 3.9±0.28 | NS | |||
| Patient characteristics | Sixty haemodialysis patients randomized into four therapeutic groups | Total (n=60) | P value | ||||||
| Vitamin E (n=15) | Vitamin C (n=15) | Vitamins E and C (n=15) | Placebo (n=15) | ||||||
| Age, years (mean±SD) | 49.7±18.4 | 56.1±16.5 | 47.5±12.1 | 49.4±12.9 | 50.8±15.2 | NS | |||
| Sex, female/male ratio | 8/7 | 6/9 | 8/7 | 7/8 | 29/31 | NS | |||
| HD duration, months (mean±SD) | 20.7±12.7 | 32.6±31.5 | 31.7±21.1 | 27.6±39.0 | 28.2±27.7 | NS | |||
| ESRD due to diabetes (n) | 6 | 4 | 5 | 3 | 18 | NS | |||
| ESRD due to glomerulopathy (n) | 5 | 6 | 5 | 7 | 23 | NS | |||
| ESRD due to tubular disease (n) | 2 | 4 | 2 | 4 | 12 | NS | |||
| ESRD of unknown aetiology (n) | 2 | 1 | 3 | 1 | 7 | NS | |||
| Sodium, mEq/l (mean±SD) | 141.4±2.6 | 141.8±3.0 | 140.6±2.9 | 140.8±4.8 | 141.1±3.4 | NS | |||
| Potassium, mEq/l (mean±SD) | 4.6±0.44 | 4.5±0.45 | 4.8±0.26 | 4.7±0.38 | 4.6±0.39 | NS | |||
| Calcium, mg/dl (mean±SD) | 9.3±0.33 | 9.4±0.44 | 9.3±0.44 | 9.4±0.50 | 9.3±0.42 | NS | |||
| Phosphorous, mg/dl (mean±SD) | 4.4±0.85 | 4.5±0.54 | 4.7±0.32 | 4.5±0.81 | 4.5±0.66 | NS | |||
| Haemoglobin, g/dl (mean±SD) | 10.6±0.99 | 11.1±1.0 | 10.9±1.0 | 11.0±0.97 | 10.9±0.99 | NS | |||
| Haematocrit, % (mean±SD) | 32.2±3.1 | 34.0±3.1 | 33.2±3.4 | 33.1±2.9 | 33.1±3.1 | NS | |||
| Frequency of cramps (mean±SD) | 4.0±1.9 | 4.8±2.1 | 4.4±1.1 | 4.4±1.7 | 4.4±1.7 | NS | |||
| Cramp intensity score (mean±SD) | 3.5±1.6 | 2.8±1.4 | 3.5±1.6 | 3.1±1.6 | 3.2±1.6 | NS | |||
| Total weekly Kt/V (mean±SD) | 4.0±0.26 | 3.9±0.34 | 4.0±0.24 | 3.9±0.27 | 3.9±0.28 | NS | |||
NS, no significant difference among the four groups; ESRD, end‐stage renal disease.
Comparison of cramp intensity scores among 60 haemodialysis patients randomized into the four therapeutic groups shown just before therapy and at the end of the trial
| Parameter | Vitamin E (n=15) | Vitamin C (n=15) | Vitamins C and E (n=15) | Placebo (n=15) | ||||||||
| Before | After | Before | After | Before | After | Before | After | |||||
| Intensity score of cramps (mean±SD) | 3.5±1.6 | 2.1±1.2 | 2.8±1.4 | 2.0±0.76 | 3.5±1.6 | 1.1±0.35 | 3.1±1.6 | 3.1±1.9 | ||||
| P value | 0.00003a | 0.03b, 0.02c | 0.04a | 0.01b, 0.04c | 0.00003a | 0.00003b | ||||||
| Parameter | Vitamin E (n=15) | Vitamin C (n=15) | Vitamins C and E (n=15) | Placebo (n=15) | ||||||||
| Before | After | Before | After | Before | After | Before | After | |||||
| Intensity score of cramps (mean±SD) | 3.5±1.6 | 2.1±1.2 | 2.8±1.4 | 2.0±0.76 | 3.5±1.6 | 1.1±0.35 | 3.1±1.6 | 3.1±1.9 | ||||
| P value | 0.00003a | 0.03b, 0.02c | 0.04a | 0.01b, 0.04c | 0.00003a | 0.00003b | ||||||
Intensity of cramps was scored using the following scale: no pain, mild, discomforting, distressing, horrible and excruciating, which were given scores of 1–6, respectively.
aDifference between pre‐ and post‐treatment values in each therapeutic group; b and c, different from placebo and combination (vitamins E and C) groups, respectively.
Comparison of cramp intensity scores among 60 haemodialysis patients randomized into the four therapeutic groups shown just before therapy and at the end of the trial
| Parameter | Vitamin E (n=15) | Vitamin C (n=15) | Vitamins C and E (n=15) | Placebo (n=15) | ||||||||
| Before | After | Before | After | Before | After | Before | After | |||||
| Intensity score of cramps (mean±SD) | 3.5±1.6 | 2.1±1.2 | 2.8±1.4 | 2.0±0.76 | 3.5±1.6 | 1.1±0.35 | 3.1±1.6 | 3.1±1.9 | ||||
| P value | 0.00003a | 0.03b, 0.02c | 0.04a | 0.01b, 0.04c | 0.00003a | 0.00003b | ||||||
| Parameter | Vitamin E (n=15) | Vitamin C (n=15) | Vitamins C and E (n=15) | Placebo (n=15) | ||||||||
| Before | After | Before | After | Before | After | Before | After | |||||
| Intensity score of cramps (mean±SD) | 3.5±1.6 | 2.1±1.2 | 2.8±1.4 | 2.0±0.76 | 3.5±1.6 | 1.1±0.35 | 3.1±1.6 | 3.1±1.9 | ||||
| P value | 0.00003a | 0.03b, 0.02c | 0.04a | 0.01b, 0.04c | 0.00003a | 0.00003b | ||||||
Intensity of cramps was scored using the following scale: no pain, mild, discomforting, distressing, horrible and excruciating, which were given scores of 1–6, respectively.
aDifference between pre‐ and post‐treatment values in each therapeutic group; b and c, different from placebo and combination (vitamins E and C) groups, respectively.
Discussion
Numerous advances have been made in the medical management of the end‐stage renal disease of HD patients. However, painful nocturnal cramps interfering with normal life still remain a common complication of HD [1–6]. Many approaches for the treatment of HD‐related cramps have been proposed, but most have been associated with serious side effects, and none have been conclusively effective [6–8]. Considering the potential toxicity of quinine, vitamin E has been recommended as the initial treatment of choice for HD cramps [7]. Accordingly, in this study, short‐term vitamin E supplementation was safe and effective for HD cramps, but was less effective than the combination of vitamins C and E.
It has been shown that vitamin C levels are lower in HD patients than in healthy controls, and due to its beneficial effects against lipid metabolism abnormalities and oxidative stress, it has been recommended for HD patients [15–20]. However, muscle cramps due to carnitine deficiency have been shown to improve after carnitine therapy in HD patients, and experimental studies provide compelling evidence that vitamin C participates in carnitine biosynthesis [9–14]. Since HD patients are generally placed on vitamin C‐restricted diets, it is possible that a subclinical vitamin C deficit may contribute to carnitine deficiency and the development of muscle cramps in HD‐patients [9–20]. Yet, to our knowledge, vitamin C has never been used for the treatment of muscle cramps in HD patients. Although none of our patients had clinical features of scurvy, we found for the first time that vitamin C therapy significantly decreased the frequency and intensity of muscle cramps in HD patients. Although the percentage cramp reduction by vitamin C was higher than for vitamin E, and was sustained for 8 weeks, the difference never reached statistical significance.
The initial concerns of our study were to trust the efficacy of vitamin C and combined vitamin E and C supplementation against HD cramps. We showed for the first time that combined vitamin E and C supplementation was conclusively effective against them. Combination therapy was significantly more effective than treatment with either vitamin E or C given alone. This synergistic effect of vitamin C and E, the reduction in cramps after treatment with various drugs, and the fact that a small percentage (3%) of our patients did not improve after combination therapy, all suggest that HD cramps represent a multifactorial derangement. Nonetheless, the improvement in HD cramps after vitamin C and E could be due to their antioxidant properties. It also may be secondary to the beneficial effect of vitamin C on carnitine biosynthesis, or may be due to undefined mechanisms [14,18–20]. Since we were not able to measure oxidant–antioxidant status or serum levels of carnitine and vitamins, the extent of the contribution of each of the aforementioned mechanisms to the beneficial effect of vitamin C and E in HD cramps cannot be determined in this study.
Prolonged vitamin therapy may be associated with serious adverse effects, and several authors have indicated that vitamin C doses in HD patients should not exceed 200 mg/day. Vitamin C therapy is known to produce hyperoxaluria, oxalate containing urinary stones, and renal damage [21–23]. However, the short‐term vitamin supplementation in the present trial was well tolerated in our patients and no vitamin‐related side effects, including urinary stone formation, were seen during the trial. Our findings indicate that short‐term therapy with a combination of vitamin E and C is effective and safe in reducing HD cramps. The safety of prolonged therapy has yet to be evaluated in HD patients.
Correspondence and offprint requests to: Prof P. Khajehdehi, House 53, Lane 10‐Jangali, Mirza‐Kouchak‐Khan‐Jangli Highway, Shiraz 71959, Iran.
References
Romagnoli GF, Di‐Landro D, Catalano C et al. Short‐term outcome of diabetic patients in renal replacement therapy.
De‐Vecchi AF, Scalamogna A, Colombini M et al. Well being in patients on CAPD and hemodialysis.
Lok P. Stressors, coping mechanisms and quality of life among dialysis patients in Australia.
Chou CT, Wasserstein A, Schumacher HR, Fernandez P. Musculoskeletal manifestations in hemodialysis patients.
Riley JD, Antony SJ. Leg cramps: Differential diagnosis and management.
Roca AO, Jarjoura D, Blend D et al. Dialysis leg cramps. Efficacy of quinine versus vitamin E.
Mandal AK, Abernathy T, Nelluri SN, Stitzel V. Is quinine effective and safe in leg cramps?
Ahmad S, Robertson HT, Golper TA et al. Multicenter trial of L‐carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects.
Goral S. Levocarnitine and muscle metabolism in patients with end‐stage renal disease.
Sakurauchi Y, Matsumoto Y, Shinzato T et al. Effects of L‐carnitine supplementation on muscular symptoms in hemodialyzed patients.
Feinfeld DA, Kurian P, Cheng JT et al. Effect of oral L‐carnitine on serum myoglobin in hemodialysis patients.
Bellinghieri G, Savica V, Mallamace A et al. Correlation between increased serum and tissue L‐carnitine levels and improved muscle symptoms in hemodialyzed patients.
Bakaev VV, Efremov AV, Tityaev II. Low levels of dehydroascorbic acid in uraemic serum and the partial correction of dehydroascorbic acid deficiency by haemodialysis.
Saionji K, Sato T, Higurashi H, Iizuka K. Homeostasis of antioxidant status in hemodialysis patients.
Bohm V, Tiroke K, Schneider S, Sperschneider H, Stein G, Bitsch R. Vitamin C status of patients with chronic renal failure, dialysis patients and patients after renal transplantation.
Hultqvist M, Hegbrant J, Nilsson‐Thorell C et al. Plasma concentrations of vitamin C, vitamin E and/or malondialdehyde as markers of oxygen free radical production during hemodialysis.
Descombes E, Hanck AB, Fellay G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation.
Khajehdehi P. Effect of vitamins on the lipid profile of the patients on regular hemodialysis.
Levine M. New concepts in the biology and biochemistry of ascorbic acid.
Rolton HA, McConnell KM, Modi‐KS, Macdougall AI. The effect of vitamin C intake on plasma oxalate in patients on regular haemodialysis.





Comments