-
PDF
- Split View
-
Views
-
Cite
Cite
Simon Davies, Patrik Španel, David Smith, A new ‘online’ method to measure increased exhaled isoprene in end‐stage renal failure, Nephrology Dialysis Transplantation, Volume 16, Issue 4, April 2001, Pages 836–839, https://doi.org/10.1093/ndt/16.4.836
Close - Share Icon Share
Abstract
Background. Isoprene is the most abundant hydrocarbon present in breath, and recent reports indicate that breath concentrations increase following haemodialysis. The purpose of this study was to establish whether selected ion flow tube mass spectrometry (SIFT‐MS), a newly established technique in breath analysis, may be used to quantify breath isoprene in haemodialysis patients in the clinical setting. SIFT‐MS is compared and contrasted with the established gas chromatography mass spectrometric technique for this purpose.
Methods. Three consecutive exhalations from 19 haemodialysis patients (12 males, seven females) undergoing a morning dialysis shift were analysed just prior to commencing treatment. Within 5 min of completing their usual dialysis regimen, using polysulphone membranes, the breath of each patient was analysed again. Additional contemporary samples were obtained from 17 normal controls. Breath isoprene was quantified using SIFT‐MS, a method previously validated quantitatively using neat isoprene.
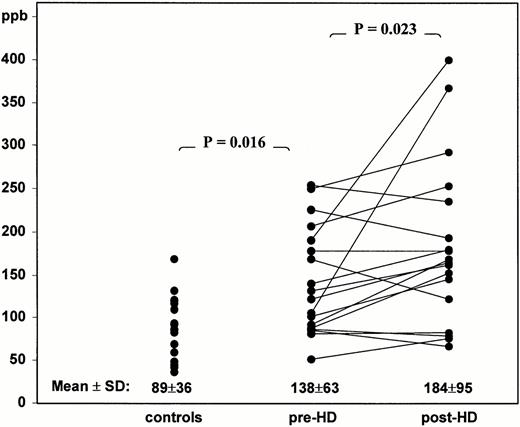
Results. Successful measurements of breath isoprene were obtained for each subject within 2 min, with minimum disruption to a busy dialysis environment. The coefficient of variation of triplicate measurements of breath isoprene was <10%. Prior to dialysis, the mean (±SD) breath isoprene concentration (138±63 parts per billion (ppb)) was significantly greater than for normal controls (89±36 ppb; P=0.016). Immediately following treatment, breath isoprene increased significantly to 184±95 ppb (P=0.023).
Conclusions. SIFT‐MS permits the accurate and rapid measurement of breath isoprene in haemodialysis patients in the clinical setting. The previously reported increase in breath isoprene following dialysis treatment is confirmed. SIFT‐MS is the ideal analytical tool to investigate this phenomenon further.
Introduction
Isoprene is present in the breath of all individuals and is the major breath hydrocarbon in healthy people [1]. Techniques used previously to quantify isoprene in human breath have been based almost exclusively on gas chromatography mass spectrometry (GCMS) [2], although there have been some problems with this method as discussed below. We have thus developed the selected ion flow tube mass spectrometric technique (SIFT‐MS) [3,4]. This method allows precise quantification of trace gases, such as isoprene, in breath with only single exhalations and in real time [5].
Isoprene is a by‐product of cholesterol synthesis, during the conversion of mevalonate to mevanolate‐5‐pyrophosphate and isopentenyl pyrophosphate [1]. Breath isoprene concentrations fall when measures are taken to suppress cholesterol synthesis, such as treatment with 3‐hydroxy‐3‐methylglutaryl (HMG)‐CoA‐reductase inhibitors or when the diet is supplemented with extra exogenous cholesterol [6]. Rapid increases in breath isoprene have also been observed in the context of acute tissue injury, such as myocardial infarction and lung injury associated with exposure to ozone [7,8]. It was demonstrated recently that isoprene increases after a haemodialysis treatment session [9].
The purpose of this study was to establish whether our new technique, SIFT‐MS, could be used to quantify breath isoprene in haemodialysis patients. In particular, we wished to demonstrate that this method could provide reliable and immediate results in the clinical setting, as a prelude to investigating the cause of elevated breath isoprene levels in haemodialysis patients in more detailed clinical studies.
Quantification of breath isoprene using SIFT‐MS and comparison with GCMS
The principles of the SIFT‐MS technique, which utilizes chemical ionization in a flow tube to identify and quantify trace gases present in complex mixtures, such as are found in breath, has been described in detail elsewhere, including its application to breath analysis in patients with renal failure [3,4,10]. Briefly, precursor ions are injected into fast‐flowing helium carrier gas where they thermalize to the gas temperature and then react with metabolites, e.g. isoprene (C5H8), in the sample of breath, which is introduced into the carrier gas downstream via a calibrated capillary leak. O2+ precursor ions are used because the dominant product ions, C5H8+ and C5H7+, do not coincide with the product ions of the reactions of O2+ with any of the common breath gases. The chosen breath trace gas can be quantified in a single exhalation without collection into bags and without pre‐concentration. SIFT‐MS analyses provide absolute concentrations of isoprene present in breath as sampled at constant (usually atmospheric) pressure and at a known flow rate. The measured concentrations are expressed in parts per billion (ppb), 22.4 ppb being equivalent to 1 nmol/l at normal temperature and pressure. Detailed descriptions of the measurement of breath isoprene using SIFT‐MS, including calibration and the distribution of concentrations in normal controls are now published [5]. Use of this method coupled with the appropriate software can produce immediate results.
GCMS has been the most commonly used technique for the detection and quantification of breath metabolites, including isoprene [2,11–14]. In this technique it is usual to pre‐concentrate the breath metabolites first, either by cryogenic trapping or by trapping onto some form of adsorption trap [2,9,11,14,15]. This can result in a loss of precision in quantification, because it is necessary for accurate quantification to measure the volume of the breath sample that is collected and this is not a trivial procedure. The metabolites are then released from the trap and introduced into the chromatographic column, where they separate and are individually ionized, and the characteristic ‘cracking pattern’ of each metabolite is recognized and the signal level determined. Ultraviolet detection of specific metabolites has also been used [11]. These methods require careful calibration of the instrument for each metabolite if accurate quantification is to be achieved. This has led to some methodological difficulties in separating N‐pentane, the breath hydrocarbon linked previously to oxidative stress, from isoprene, which is far more abundant [12]. A more recent method that also uses GCMS has been described by Hypsler et al. [14]. This involves the direct comparison of prepared standard mixtures of isoprene in air with breath samples, but in this method also the isoprene is collected onto an adsorption trap before it is released into the GCMS for quantification. The results obtained for breath isoprene quantification in healthy subjects are in good agreement using SIFT‐MS and the method of Hypsler et al. The important distinction is that SIFT‐MS analyses are performed rapidly and ‘online’ to the patient, and thus the results are immediate. The rate of data acquisition is also considerably greater for SIFT‐MS analyses and several breath metabolites can be quantified simultaneously [16]. Finally, there is no question of failure to separate isoprene from other alkanes, e.g. pentane, that might be present in breath.
Subjects and methods
Control subjects and patients
We have established and published previously the normal range for breath isoprene in the healthy population using the SIFT‐MS technique [5]. However, for the purposes of the present study, additional samples were obtained from 17 healthy volunteers (eight males, nine females; age range 24–62 years) on the same day as the patients to allow direct comparison. None of the controls were taking drugs that might interfere with lipid metabolism. A total of 19 patients (12 males, seven females; age range 35–80 years) with end‐stage renal failure (ESRF) were investigated, all of whom had been treated for at least 3 months with haemodialysis. All were receiving three treatment sessions per week using polysulphone membranes (Fresenius Medical Care) and bicarbonate buffer, with a median Kt/Vurea of 1.2. Dialysis sessions lasted 4 h (range 3–5 h). Breath samples were obtained immediately prior to morning dialysis treatment, following the longest inter‐dialytic period. Post‐treatment samples were collected within 5 min of completing a routine dialysis session in 18 of these individuals (one patient completed dialysis too late for inclusion). All patients and subjects gave their informed consent, and the study was approved by the local ethics committee. Statistical comparisons were made using non‐parametric statistics due to the skewed distribution of breath isoprene concentration in the normal population [5], using the Mann–Whitney test to compare pre‐dialysis patients with controls and Wilcoxon paired analysis for patients before and after dialysis.
Breath sampling
For the purposes of the study, the SIFT‐MS instrument was situated in a room adjacent to the hospital haemodialysis facility. Each subject was asked to exhale through a 1‐cm diameter disposable tube over a period of a few seconds, then to inhale through the same tube and repeat the exhalation/ inhalation cycle twice more. A small constant flow of air is continuously sampled from the breathing tube by a heated, calibrated capillary, directly into the SIFT‐MS instrument. In this way, three breath profiles, each of ∼8 s duration, of several metabolites, including isoprene, ammonia, acetone and ethanol, were obtained. During each exhalation phase, the levels of isoprene reach a plateau [5], taken as the equilibration with alveolar air, and the final concentrations were taken as the average of these three readings. The concentration of isoprene in the ambient air was sampled between each exhalation and always found to be below the current limit of detection of SIFT‐MS, i.e. <10 ppb.
Results
Reproducibility and practicability of the technique
One of the particular advantages of the SIFT‐MS technique is the ease of sample acquisition. Satisfactory samples were obtained from all subjects with a turnaround time of 2 min. With the ability to analyse samples on‐line using specialized software, absolute measurements of breath isoprene concentration are immediately available. Patients found the technique minimally invasive and it was possible to sample an entire shift of dialysis patients without disrupting the treatment session. The method was very reproducible, with an intra‐patient (within a triplicate sample) variation of <10%.
Comparison of haemodialysis patients with normal controls
The mean (±SD) breath isoprene concentration for the 17 parallel normal controls was 89±36 ppb (see the distribution in Figure 1). This did not differ significantly from our previously published normal range of 83±45 ppb. We found no obvious correlation with age or sex, and all the normal volunteers were non‐smokers. Pre‐dialysis breath isoprene concentrations in 19 haemodialysis patients were modestly but significantly higher (138±63 ppb; P=0.016) when compared with controls. There was no obvious relationship to age, sex or smoking status (five were smokers). None were taking HMG‐CoA‐reductase inhibitors. It can be seen in Figure 1 that the distribution has a significant tail at the upper end of the range.
Summary of the distribution and median concentrations of breath isoprene in healthy controls (same day and time), and ESRF patients pre‐ and post‐haemodialysis treatment. Paired pre‐ and post‐dialysis concentrations are indicated by lines joining individual values.
Breath isoprene levels in haemodialysis patients before and after dialysis
Following haemodialysis treatment, there was a significant increase in the mean breath isoprene concentration (184±95 ppb; P=0.023). The change in breath isoprene distribution can be seen in Figure 1, demonstrating a further increase in the upper tail. It can also be seen in Figure 1 that in the majority of cases, the isoprene concentration increased, although there was considerable variation between individuals. Other metabolites, such as ammonia, decreased during dialysis as reported previously [10].
Discussion
This is the first report of the use of SIFT‐MS to quantify breath isoprene in patients undergoing haemodialysis. We have demonstrated it is a reproducible technique that can be applied in the clinical setting to give immediate results. We have shown that breath isoprene concentrations are higher in haemodialysis patients than normal controls, and have confirmed recent observations that they increase during dialysis [9]. This analytical technique will be a powerful tool in the further investigation of this phenomenon, the cause of which is currently uncertain.
Of the number of methods now available to measure breath isoprene concentration [2,11–14], the principal advantages of the SIFT‐MS technique described here are the avoidance of pre‐concentration of the breath sample, the ease and speed of sample collection, and the immediacy of the availability of the result. The values obtained for the normal range agree well with those using alternative techniques such as microextraction and GCMS [14], the mean concentration being 80 ppb (≈3.5 nmol/l). The precision of SIFT‐MS analysis compares much more favourably than with the other techniques.
The reason for higher breath isoprene levels in haemodialysis patients compared with controls is not clear and will require further investigation. This difference was not observed previously and, although statistically significant, is not large. Breath isoprene concentration is influenced by cholesterol metabolism; in patients with renal failure this will be affected by co‐morbid diseases, diet and nutritional status, and the case‐mix may well vary between studies. So far, these studies have involved small numbers of patients, and it has not been attempted to attribute inter‐patient variability to clinical factors. Published data on cholesterol synthesis in ESRF is contradictory [17,18], and further studies, in the absence or presence of drugs that suppress cholesterol synthesis, are required. It is possible that previous investigators, using methodologies unable to separate different alkanes, may have been unable to establish this difference, but this is not the case for SIFT‐MS. Other possible explanatory factors might include age [13], although this has not been found to influence breath isoprene in non‐uraemic adults [12]. While isoprene is an important constituent of cigarette smoke, it only increases breath isoprene for 5 min following inhalation, and otherwise breath concentrations in chronic smokers are not increased [19]. Whilst some of our patients were smokers, we saw no relationship to breath isoprene concentrations. A diurnal variation in breath isoprene, between sleep and wakefulness, has been observed [14,20]. Breath isoprene concentration is highest at night, reflecting the increase in cholesterol synthesis that occurs at this time. To avoid this confounding factor, all our patients were studied at the same time of day, during the morning dialysis shift when fully awake. We have not seen significant daytime variation in breath isoprene in our previous studies with normal controls. We have also investigated the influence of fasting and feeding on breath isoprene in normal individuals, and found no significant effect [21]. Finally, the possibility that isoprene excretion is impaired in renal failure must also be considered, and residual renal function may have varied between studies. Little is known about the renal excretion of isoprene, but the patients in this study were anuric, so if this is normally an important route for elimination, then much higher pre‐dialysis levels would have been anticipated.
The increase in breath isoprene that was found in our patients after dialysis confirms a recent study reporting a similar rise in isoprene, but no change in breath pentane [9]. Again, the cause of this increase is only a matter for speculation at this stage, but it should be emphasized that it is most unusual to see an increase in any metabolite during dialysis treatment. It seems unlikely that the increase of breath isoprene that occurred following dialysis reflects an increase in cholesterol synthesis. Indeed, there is evidence that the metabolic precursor mevalonate drops significantly during a treatment session [17]. Alternative possible mechanisms that warrant investigation include biocompatibility of dialysis membranes, haemodynamic stress and inflammation. SIFT‐MS provides the ideal technique to explore these possibilities in more detail.
Correspondence and offprint requests to: Dr S. J. Davies, Department of Nephrology, North Staffordshire Hospitals Trust, Princes Road, Hartshill, Stoke‐on‐Trent ST4 7LN, UK.
This work was supported by the North Staffordshire Medical Institute, the Engineering and Physical Sciences Research Council, UK, and by the Grant Agency of the Czech Republic under project number 203/97/P130. We thank the Royal Society for the award of a Joint Project Grant that supports the essential collaboration between Professor Smith and Dr Španel.
References
Springfield JR, Levitt MD. Pitfalls in the use of breath pentane measurements to assess lipid peroxidation.
Smith D, Spanel P. Application of ion chemistry and the SIFT technique to the quantitative analysis of trace gases in air and on breath.
Spanel P, Smith D. Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath.
Spanel P, Davies S, Smith D. Quantification of breath isoprene using the selected ion flow tube mass spectrometric analytical method.
Stone BG, Besse TJ, Duane WC, Evans CD, DeMaster EG. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men.
Mendis S, Sobotka PA, Euler DE. Expired hydrocarbons in patients with acute myocardial infarction.
Foster WM, Jiang L, Stetkiewicz PT, Risby TH. Breath isoprene: temporal changes in respiratory output after exposure to ozone.
Capodicasa E, Trovarelli G, De Medio GE et al. Volatile alkanes and increased concentrations of isoprene in exhaled air during hemodialysis.
Davies S, Spanel P, Smith D. Quantitative analysis of ammonia on the breath of patients in end‐stage renal failure.
Jones AW, Lagesson V, Tagesson C. Determination of isoprene in human breath by thermal desorption gas chromatography with ultraviolet detection.
Mendis S, Sobotka PA, Euler DE. Pentane and isoprene in expired air from humans: gas‐chromatographic analysis of single breath.
Nelson N, Lagesson V, Nosratabadi AR, Ludvigsson J, Tagesson C. Exhaled isoprene and acetone in newborn infants and in children with diabetes mellitus.
Hyspler R, Crhova S, Gasparic J, Zadak Z, Cizkova M, Balasova V. Determination of isoprene in human expired breath using solid‐phase microextraction and gas chromatography‐mass spectrometry.
Schubert JK, Muller WP, Benzing A, Geiger K. Application of a new method for analysis of exhaled gas in critically ill patients.
Smith D, Spanel P. The novel selected‐ion flow tube approach to trace gas analysis of air and breath.
Scoppola A, De Paolis P, Menzinger G, Lala A, Di Giulio S. Plasma mevalonate concentrations in uremic patients.
Sutherland WH, Walker RJ, Ball MJ, Stapley SA, Corboy J, Robertson MC. Cholesterol precursor concentration in plasma from patients with chronic renal failure or kidney grafts.
Euler DE, Dave SJ, Guo H. Effect of cigarette smoking on pentane excretion in alveolar breath.
Taucher J, Hansel A, Jordan A, Fall R, Futrell JH, Lindinger W. Detection of isoprene in expired air from human subjects using proton‐transfer‐reaction mass spectrometry.





Comments