-
PDF
- Split View
-
Views
-
Cite
Cite
Ana M C Heureux, Jodi N Young, Spencer M Whitney, Maeve R Eason-Hubbard, Renee B Y Lee, Robert E Sharwood, Rosalind E M Rickaby, The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae, Journal of Experimental Botany, Volume 68, Issue 14, 8 September 2017, Pages 3959–3969, https://doi.org/10.1093/jxb/erx179
Close - Share Icon Share
Abstract
The haptophyte algae are a cosmopolitan group of primary producers that contribute significantly to the marine carbon cycle and play a major role in paleo-climate studies. Despite their global importance, little is known about carbon assimilation in haptophytes, in particular the kinetics of their Form 1D CO2-fixing enzyme, Rubisco. Here we examine Rubisco properties of three haptophytes with a range of pyrenoid morphologies (Pleurochrysis carterae, Tisochrysis lutea, and Pavlova lutheri) and the diatom Phaeodactylum tricornutum that exhibit contrasting sensitivities to the trade-offs between substrate affinity (Km) and turnover rate (kcat) for both CO2 and O2. The pyrenoid-containing T. lutea and P. carterae showed lower Rubisco content and carboxylation properties (KC and kCcat) comparable with those of Form 1D-containing non-green algae. In contrast, the pyrenoid-lacking P. lutheri produced Rubisco in 3-fold higher amounts, and displayed a Form 1B Rubisco kCcat–KC relationship and increased CO2/O2 specificity that, when modeled in the context of a C3 leaf, supported equivalent rates of photosynthesis to higher plant Rubisco. Correlation between the differing Rubisco properties and the occurrence and localization of pyrenoids with differing intracellular CO2:O2 microenvironments has probably influenced the divergent evolution of Form 1B and 1D Rubisco kinetics.
Introduction
The CO2-fixing enzyme Rubisco (EC 4.1.1.39) evolved in the Archaean Eon when the atmosphere lacked O2, and CO2 was estimated to be 50-fold higher than current levels (Berner and Canfield, 1989; Berner, 2006; Tabita et al., 2008). With the evolution of O2-producing photosynthesis around the early Proterozoic (2.5 Gya), atmospheric O2 increased while the CO2 concentration declined (Canfield, 2005) (Fig. 1). The diminishing atmospheric CO2:O2 ratio negatively influenced Rubisco catalysis, as its photosynthetic CO2-fixing function is competitively inhibited by O2 to produce 2-phosphoglycolate (2-PG) (Tcherkez et al., 2006; Whitney et al., 2011; Sharwood, 2017). Recycling of 2-PG to 3-phosphoglycerate (3-PGA) by photorespiration consumes energy and loses fixed CO2. A further limitation to Rubisco function in the modern atmosphere is a low affinity for CO2 and a slow catalytic rate that necessitates high Rubisco concentrations to support adequate rates of photosynthesis (Reinfelder, 2011; Whitney et al., 2011; Long et al., 2015; Sharwood et al., 2016b).
Rubisco evolution and catalysis. Geological history of the past versus present atmospheric [CO2] (gray) and percentage atmospheric O2 (% v/v) (black; modified from Berner and Canfield, 1989; Badger et al., 2002; Whitney et al., 2011) highlighting the estimated appearance of key primary producers (horizontal lines) (Yoon et al., 2002, 2004; Liu et al., 2010) their differing Form 1B or ID Rubisco lineages they produce, and the predicted timing when algal carbon-concentrating mechanisms (CCMs; gray shading) evolved (Badger et al., 2002; Moritz and Griffiths, 2013).
The catalytic limitations of Rubisco are exacerbated in aquatic ecosystems due to restraints on aqueous CO2 availability because of slow rates of gas diffusion in water (~10000 times slower than in air) and reliance on mixing of the water column (Badger et al., 1998). In addition, increased partitioning of inorganic carbon to HCO3– with higher pH in aquatic systems diminishes aqueous CO2 availability. There is evidence of catalytic adaptation by Rubisco in algae to the changing atmospheric CO2 (and O2) conditions over geological time scales (Young et al., 2012). Recent work, however, showed that the characteristic faster CO2 fixation rates (kCcat) and lower CO2 affinities (i.e. higher Km for CO2; KC) observed in Form 1A and Form 1B Rubisco [e.g. in Chlamydomonas with a pyrenoid-based CO2-concentrating mechanism (CCM)] (Badger et al., 1998; Ghannoum et al., 2005; Sharwood et al., 2016a) are not shared by diatom Form 1D Rubisco (Hanson, 2016; Young et al., 2016; see also Fig. 3A). This has led to calls for a more expansive analysis of Rubisco’s natural kinetic diversity so that we can fully understand the correlative interactions between specificity for CO2 as opposed to O2 (SC/O), kCcat, and KC. The one-dimensional, linear correlations previously proposed (Tcherkez et al., 2006; Savir et al., 2010) may actually vary with photosynthetic taxa (Tcherkez, 2013, 2016; Hanson, 2016; Sharwood, 2017).
In photosynthetic organisms, the CCM arose multiple times in response to a declining atmospheric CO2:O2 ratio as a means to increase the CO2/O2 environment around Rubisco (Fig. 1). Data on the anatomical, biochemical, and genomic detail for CCMs in vascular plants with C4 and Crassulacean acid metabolism (CAM) physiologies are highly detailed (von Caemmerer and Furbank, 2016). The high CO2 environment reduces Rubisco oxygenation and the associated energy costs of photorespiration, allowing the plant to work with lower stomatal conductance and reduced amounts of Rubisco (Sage et al., 2012). These features allow more efficient use of water, nitrogen, and light, and permit these plants to survive in more arid and nutrient-limited environments (Sage, 2002; Ghannoum et al., 2005; Lara and Andreo, 2011; Long et al., 2015). The CCM in plants also allowed Form 1B Rubisco to evolve a higher kCcat at the expense of a higher KC (i.e. lower CO2 affinity) with little or no effect on SC/O (Sharwood et al., 2016a, b). Curiously this kCcat–KC trade-off is not shared by Form 1D Rubisco from diatoms where relatively higher KC values have been retained as a consequence of other environmental pressures (low nutrient and extracellular CO2 availability) that pose limitations to resource investment into Rubisco (Young et al., 2016). It is likely that resources other than CO2, such as nitrogen and light availability, have a strong influence on CCM evolution and regulation (Raven et al., 2008, 2012).
Understanding how microalgal Rubisco catalysis has differentially evolved remains limited by our understanding of the structural components and effectiveness of the CCM in microalgae. The last few years have seen significant advances in our understanding of CCM in the model freshwater green alga, Chlamydomonas reinhardtii (Engel et al., 2015; Wang et al., 2015; Yamano et al., 2015; Mackinder et al., 2016; Mangan et al., 2016; Wang et al., 2016). To what extent this knowledge is translatable to the CCM of the structurally differing and evolutionarily distinct marine microalgae (e.g. diatoms and haptophytes) remains unclear (Bedoshvili et al., 2009; Hopkinson et al., 2011, 2013). Currently a completed nuclear haptophyte genome is available for the Isochrysidale Emiliania huxleyi (Read et al., 2013) and a draft genome for the Prymnesiale Chrysochromulina tobin (Hovde et al., 2015). Although less understood, the CCMs of marine microalgae are known typically to employ a pyrenoid, Rubisco activase, carbonic anhydrase (CA), and inorganic carbon (Ci) transporters to elevate CO2 levels around Rubisco (Hopkinson et al., 2011; Reinfelder, 2011; Loganathan et al., 2016). The pyrenoid is a proteinaceous body that appears electron dense when examined by TEM and contains most, sometimes all, of the cellular Rubisco (Engel et al., 2015; Mackinder et al., 2016).
In C. reinhardtii, HCO3– transport occurs via thylakoids and Ci transporters that work in association with pyrenoid CAs to elevate CO2 around Rubisco (Karlsson et al., 1998; Trimborn et al., 2007; Lee et al., 2013; Wang et al., 2015; Yamano et al., 2015). Physiological and genetic evidence in model diatoms imply that HCO3– is pumped into the chloroplast stroma and diffuses into the pyrenoid where it is converted to CO2 by CA to elevate the [CO2] around Rubisco (Hopkinson et al., 2011, 2016). Recent identification of a thylakoid lumen-localized CA in P. tricornutum further suggests that the pyrenoid-penetrating thylakoids probably provide an important CO2 supply within the pyrenoids (Kikutani et al., 2016).
What remains unclear is how the pyrenoid structure influences CCM efficiency. In C. reinhardtii, the pyrenoid contains a starch sheath (Moroney et al., 2011; Engel et al., 2015) while in diatoms it can comprise a lipid membrane (Bedoshvili et al., 2009), lack a delimiting structure (Bendif et al., 2011), vary in number and shape, and differ in the presence/structure of traversing thylakoids (Badger et al., 1998). To better understand the relationships between Rubisco kinetics, content, and pyrenoid biology in marine microalgae, we have expanded on our previous study of diatom Rubisco (Young et al., 2016) to include three marine haptophytes that contain bulging (Pleurochrysis carterae), immersed [Tisochrysis lutea, formerly Isocrysis sp. strain CS-177 (Bendif et al., 2013)], or no pyrenoid (Pavlova lutheri) within their chloroplast and varying numbers and location of pyrenoid-traversing thylakoids (Fig. 2A).
Microalgae pyrenoid and CCM composition. (A) TEM images were compiled from the literature to represent the range of pyrenoids presented in this. To represent a pyrenoid lacking Pavlovale, we use Pavlova viridis from Bendif et al. (2011) (Protist, 162, Bendif EM, Probert I, Hervé A, Billard C, Goux D, Lelong C, Cadoret JP, Véron B. Integrative taxonomy of the Pavlovophyceae (Haptophyta): a reassessment, 738–761, ©2011, with permission from Elsevier) as the TEM image clearly represents the lack of pyrenoid. Pavlova lutheri is visualized in the same study; however, the TEM image does not show the chloroplast (Ch) lacking a pyrenoid as clearly. TEM image of P. carterae from Beech and Wetherbee (1988) [republished with permission of the International Phycological Society from Observations on the flagellar apparatus and peripheral endoplasmic reticulum of the coccolithophorid, Pleurochrysis carterae (Prymnesiophyceae), Beech PL, Wetherbee R, Phycologia 27, 1988; permission conveyed through Copyright Clearance Center, Inc.] illustrates pyrenoids (Py) bulging toward the center of the cell, and the two species T. lutea (Bendif et al., 2014) (Journal of Applied Phycology, Erratum to: On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta), 26, 2014, 1617, Bendif EM, Probert I, Schroeder DC, de Vargas C. With permission of Springer) and P. tricornutum (Allen et al., 2011) (Allen AE, Moustafa A, Montsant A, Eckert A, Kroth PG, Bowler C. Evolution and functional diversification of fructose bisphosphate aldolase genes in photosynthetic marine diatoms. Molecular Biology and Evolution 2012, 29, 367–279, by permission of Oxford University Press) show pyrenoids immersed within the chloroplast. (B) Summary of published experimental evidence for the presence of a CCM in the species with a pyrenoid. Evidence for a CCM is detectable by: (i) inhibition of CO2 assimilation by the impermeable acetazolamide (AZA) or membrane-permeable ethoxyzolamide (EZA) CA inhibitors (Burns and Beardall, 1987; Okazaki et al., 1992; Badger et al., 1998; Hopkinson et al., 2013); (ii) stimulation of CA activity following cell illumination (Badger et al., 1998); (iii) whether the intercellular Ci pool is higher than the external environment (Badger et al., 1998); or (iv) the preliminary detection of δ-CA using methods described in the Materials and methods.
Materials and methods
Algae culturing
Cultures of the haptophytes P. lutheri (CS-182), P. carterae (CS-287), T. lutea [CS-177; original strain name Isochrysis sp. (Bendif et al., 2013)], and the diatom, Phaeodactylum tricornutum (CS-29) were obtained from the Australian National Algae Culture Collection CSIRO (https://www.csiro.au/en/Research/Collections/ANACC) and grown at 20 °C in 0.2 μm filtered and autoclaved seawater containing f/2 (Guillard and Ryther, 1962) or GSe (P. carterae; Blackburn et al., 2001) nutrients, vitamins, and trace metals. The cultures were grown in polycarbonate culture flasks under 150 ± 50 μmol photons m–2 s–1 illumination on a 16:8 h light:dark cycle.
Pyrenoid morphology and CCM characterization.
Details of pyrenoid morphology and estimates of Ci and CA pools for the microalgae were compiled from the literature (Manton and Peterfi, 1969; Billard and Gayral, 1972; Green, 1975; Green and Pienaar, 1977; Borowitzka and Volcani, 1978; Hori and Green, 1985; Badger et al., 1998; Bendif et al., 2011, 2013). A preliminary screen for putative δ-CA genes was carried out using PCR. δ-CA is a functional carbonic anhydrase, as demonstrated in vitro by Del Prete et al. (2014) and Lee et al. (2013). It is regulated by CO2, and is thus important in inorganic carbon acquisition (Lane and Morel, 2000a).
Genomic DNA was extracted as described (Richlen and Barber, 2005) and candidate δ-CA genes were amplified by PCR using primers Δfwd (5'-GTTGGCGAGACGTACGAGGTGCACTGG-3') and Δrev (5'- GCGATCGACCTGCCAGGTGATGGG-3') that were designed to the conserved C-terminal amino acid sequences VGETYEVHW and PITWQVDR, respectively. The ~370 bp DNA product amplified from P. carterae and Isochrysis galbana (for comparison with T. lutea) was sequenced by Source BioScience (Oxford, UK). Confirmation that the absence of δ-CA from our P. tricornutum (strain CCAP1055/1 a monoclonal culture derived from a fusiform cell in May 2003 from strain CCMP632) was further supported by analysis for δ-CA homologs within the fully sequenced genome of P. tricornutum (Bowler et al., 2008) and its predicted protein products. Similarly, the absence of δ-CA was further confirmed by a search of the Pavlovales sp. CCMP2436 (JGI) genome sequence.
A BLAST search of the genomes was carried out using the δ-CA protein sequence from Thalassiosira pseudonana (BAO52718) and Thalassiosira weissflogii (AAV39532), both centric diatoms, and Fragilariopsis cylindrus CCMP1102 (OEU11320), a pennate diatom, as query sequences. The BLAST search yielded no hits. Together with our PCR, we concluded that there were no δ-CA homologs in this strain of P. tricornutum. A BLAST search was also carried out on the genome of Pavlovales sp. CCMP2436 (JGI), an environmental isolate using the δ-CA protein sequence from T. pseudonana (BA052718) and the haptophytes Emiliania huxleyi (ABG37687), I. galbana (EC146202, EC142695), and Chrysochromulina sp. CCMP291 (KO021563, KO028292). Although this genome is not fully curated and the culture has not been taxonomically described, the preliminary search yielded no hits.
Rubisco extraction and kinetic assessment
Algal cells were harvested via centrifugation (2000 g for 10 min) and the pelleted cells snap-frozen in liquid nitrogen and stored at –80 °C until assay. The crude soluble cell extracts were obtained by rupturing cells using a French press as described previously (Young et al., 2016). As detailed in the same study, Rubisco content was quantified by [14C]CABP (2-carboxyarabinitol 1,5-bisphosphate) binding within the crude extract and concentrations of soluble protein were quantified using the Bradford assay against BSA. Rubisco catalytic parameters: maximum carboxylation rate (kCcat) and half-saturation constants for CO2 and O2 (KC and KO, respectively) were measured at 25 °C using 14CO2 fixation assays employing crude extract that had been activated for 10–15 min at 25 °C with 10 mM MgCl2 and 10 mM NaHCO3. The CO2 concentrations in the 14CO2 assays were calculated using the Henderson–Hasselbalch equation and the parameters detailed in (Sharwood et al., 2016a). Measurements of SC/O were made using Rubisco rapidly purified from ~1 g of pelleted algal cells as described (Young et al., 2016).
Simulating the influence of microalgae Rubisco on C3 plant photosynthesis
The carboxylase activity-limited assimilation rates were simulated according to Farquhar et al. (1980) using the equation:
assuming a CO2 solubility in H2O (sc) of 0.0334M bar–1, an O of 267 μM, a Rubisco content (B) of 20 µmol catalytic sites m–2, and a non-photorespiratory CO2 assimilation rate (Rd) of 2 µmol m–2 s–1. Under higher chloroplast CO2 pressures (Cc), the photosynthetic rate becomes light- (or electron transport rate, ETR-) limited and is modeled according to the equation:
assuming an electron transport rate (J) of 150 µmol m–2 s–1.
Results and Discussion
The differing pyrenoid morphologies within the microalgae studied
A central objective of this study was to examine the correlations between pyrenoid morphology, evidence of a CCM, and the content and catalysis of Rubisco in microalgae. As summarized in Fig. 2A, T. lutea possesses a pyrenoid immersed in the center of the plastid with 1–2 thylakoids traversing the center of the pyrenoid (Bendif et al., 2013; Borowitzka and Volcani, 1978). In contrast, the pyrenoid in P. carterae bulges out from the plastid toward the center of the cell, with 5–6 continuous thylakoids traversing the plastid and pyrenoid (Manton and Peterfi, 1969; Beech and Wetherbee, 1988). Immersed versus bulging pyrenoids differ in the location within the cell, relative separation from the plastid (i.e. the presence of a lipid membrane has been suggested from TEM observations), and the connectivity to plastid thylakoids. A lipid membrane has been observed around the immersed/semi-immersed pyrenoids of two other members of the Isochrysidales—Chrysotila lamellosa (Billard and Gayral, 1972; Green and Parke, 1975) and Isochrysis galbana (Green and Pienaar, 1977). However, Bendif et al. (2013) did not detect a membrane around the pyrenoid of T. lutea nor has one been observed around pyrenoids of the bulging morphotype in any haptophyte species, including P. carterae. In the Pavlovophyceae, the pyrenoids are often bulging towards the exterior of the cell—albeit not in P. lutheri where no pyrenoid is apparent (Green, 1975; Burris, 1981; Bendif et al., 2011). Phaeodactylum tricornutum was included in this study and, like many members of the lineage, has pyrenoids that are fully immersed within the chloroplast with 1–2 pyrenoid-traversing thylakoids (Borowitzka and Volcani, 1978; Bedoshvili et al., 2009) (Fig. 2A).
Experimental evidence for a CCM
A key component of a CCM is the enzyme CA that catalyzes the rapid interconversion between CO2 and HCO3–. In marine primary producers, the CA activity of a CCM is particularly beneficial for accessing CO2 from the high oceanic HCO3– concentrations. The effects of two CA inhibitors, the membrane-permeable ethoxyzolamide (EZA) and the relatively impermeable acetazolamide (AZA), are commonly used to test for CCM activity (Okazaki et al., 1992). This is undertaken by examining the influence of EZA and AZA on the affinity of photosynthesis for plasma membrane-based inorganic carbon (Ci) (Okazaki et al., 1992; Badger et al., 1998) and light-stimulated CA activity (Burns and Beardall, 1987).
Using modern cell biology tools, there have been significant advances in understanding the CCM components in microalgae (Engel, 2015) which includes the discovery of novel CA isoforms and their intercellular localization (Jin et al., 2016; Kikutani et al., 2016). While a comparable detailed analysis of haptophyte CCM components is beyond the scope of this work, Fig. 2B summarizes the known CCM features in the microalgae studied here. EZA treatment reduces the affinity for Ci in photosynthesis for the pyrenoid-containing I. galbana (as a proxy for T. lutea), P. tricornutum, and P. carterae species, with the external CA inhibitor AZA also affecting the Ci affinity in P. carterae (Badger et al., 1998; Chen et al., 2006; Hopkinson et al., 2013). We note that other diatoms with fully immersed pyrenoids have been shown to be sensitive to AZA (Hopkinson et al., 2013). The influence of EZA or AZA on the photosynthetic carbon assimilation rate in P. lutheri, the species lacking a pyrenoid, remains untested. Similarly, the light-stimulated CA activity found in P. tricornutum and T. lutea has yet to be examined in P. carterae and P. lutheri (Fig. 2B). It is estimated that the CCMs associated with immersed pyrenoids can increase intracellular Ci pools ~6-fold higher than that permissible by passive diffusion (Fig. 2B (Burns and Beardall, 1987; Colman and Rotatore, 1995; Badger et al., 1998).
The δ isoform of CA (or TWCA1), whose expression in the diatom T. weissflogii is modulated by extracellular CO2 levels (Morel et al., 1994; Lane and Morel, 2000a, b) and in marine dinoflagellates functions as an external CA (Lapointe et al., 2008), holds the potential as a key component of a CCM in microalgae. The catalytic activity and inhibition of δ-CA demonstrate the functionality of the CA in the diatom Thalassiosira pseudonana and the haptophyte Emiliania huxleyi (Soto et al., 2006; Lee et al., 2013; Del Prete et al., 2014). Using sequence homology searches, we were able to detect δ-CA homologs in genome data sets for P. carterae and T. lutea, but not in P. tricornutum or P. lutheri (using the Pavlovale sp. CCMP2436 genome as a proxy). Importantly the absence of detectable sequence homology does not disqualify these microalgae from producing δ-CA or alternative CA isoforms, especially considering that new CAs are still being discovered (Jin et al., 2016; Kitkanti et al., 2016). Indeed, a number of other CA types are expressed in P. tricornutum that include one localized in the pyrenoid (Tachibana et al., 2011), an extracellular CA (Hopkinson et al., 2013), and a θ-type CA located in the thylakoid lumen (Jin et al., 2016). Our BLAST search of the Pavlovale sp. CCMP2436 genome supports the absence of a δ-CA in P. lutheri; however, further investigation is required (e.g. whether P. lutheri contains other forms of CA). Overall, the existing evidence suggests that the presence of a pyrenoid coincides with the presence and activity of CA (Fig. 2B), consistent with their role in the microalgae CCM.
The carboxylation properties of haptophyte Rubisco
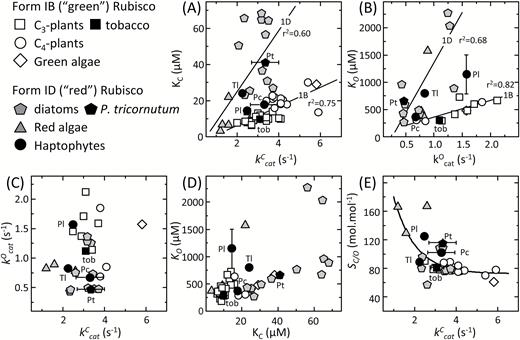
Form 1B Rubisco from organisms operating a CCM characteristically show higher rates of maximum carboxylation kCcat and a reduction in CO2 affinity (i.e. an increase in KC) than the Rubisco from their non-CCM relatives. For example, the Rubisco from C4 plants typically have a higher kCcat and higher KC than C3 plant Rubisco (Sage, 2002; Savir et al., 2010; Sharwood et al., 2016a, c; Tcherkez, 2016). As shown in Fig. 3A, the KC diversity among Form 1B vascular plant Rubisco spans a limited range in values relative to the Form 1D Rubisco from diatoms (Hanson, 2016; Young et al., 2016). Moreover, the relationship between KC and kCcat at 25 °C for the Form 1D Rubisco differs from Form 1B Rubisco (Fig. 3A).
The diversity in the kinetic properties of haptophyte Rubisco at 25 °C. Comparative relationships between the kinetic properties measured in this study for Rubisco from P. lutheri (Pl), P. carterae (Pc), T. lutea (Tl), the diatom P. tricornutum (Pt), and from tobacco (Tob) with those of other Form 1B and 1D Rubiscos (see key) as curated by Young et al. (2016). The plotted maximal carboxylation and oxygenation turnover rates (kCcat, kOcat), relative specificity for CO2 over O2 (SC/O). and the Michaelis constants (Km) for CO2 and O2 (KC, KO) are from Table 1. Linear regressions are shown for the differing (A) kCcat–KC and (B) kOcat–KO relationships displayed for Form 1B and 1D Rubiscos. No statistically significant relationships were evident among correlative analyses of (C) kCcat and kOcatKC or between (D) KC and KO. (E) An exponential relationship was apparent when comparing the kinetic trade-off between SC/O with kCcat with the differing phylogenetic Rubisco groupings aggregated at differing positions along the gradient
For comparison of different CCM effectiveness on Rubisco kinetics within organisms containing the 1D Rubisco, we examined the kinetics of the Form 1D Rubiscos from freshly lysed P. carterae, T. lutea, and P. lutheri cells. The Rubisco activity in the cellular extract was stable at 25 °C for at least 20 min following extraction (see Supplementary Fig. S1 at JXB online). The CO2–Mg2+ activation status of the extracted Rubisco varied between 50% and 60%, comparable with that seen in the cellular extract of diatoms (Young et al., 2016). To ensure full activation of all eight catalytic sites in each L8S8 molecule, the cellular extract was incubated for 10–15 min at 25 °C in buffer containing 10 mM MgCl2 and 10 mM NaHCO3 before assaying kCcat under varying CO2 concentrations by 14CO2 fixation. By this approach, the values of kCcat and KC extrapolated from fitting the data to the Michaelis–Menten equation were reproducible between replicate cellular preparations (Table 1).
Rubisco kinetic parameters measured at 25 °C
| Species . | kCcat (s–1) . | KC (µM) . | KO (µM) . | Sc/o (mol mol–1) . | KC21% O2 (µM) . | kOcat (s–1) . | kOcat/KO (mM–1 s–1) . | CE . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | k C cat/KC (mM –1 s –1 ) . | k C cat K C 21%O2 (mM –1 s –1 ) . |
| Haptophytes | |||||||||
| P. carterae | 3.3 ± 0.4 | 17.7 ± 1.5 | 366 ± 60 | 102 ± 1 | 30.6 | 0.7 | 1.8 | 186 | 108 |
| T. lutea | 2.2 ± 0.1 | 24.1 ± 0.5 | 800 ± 55 | 89 ± 1 | 32.2 | 0.8 | 1.0 | 91 | 68 |
| P. lutheri | 2.5 ± 0.1 | 14.5 ± 1.6 | 1146 ± 212 | 125 ± 2 | 17.8 | 1.6 | 1.4 | 172 | 140 |
| Diatoma | |||||||||
| P. tricornutum | 3.3 ± 0.5 | 41.1 ± 1.3 | 664 ± 54 | 116 ± 2 | 57.6 | 0.5 | 0.7 | 80 | 55 |
| C3planta | |||||||||
| N. tabacum | 3.1 ± 0.3 | 9.7 ± 0.1 | 283 ± 15 | 81 ± 1 | 18.9 | 1.1 | 3.9 | 319 | 164 |
| Species . | kCcat (s–1) . | KC (µM) . | KO (µM) . | Sc/o (mol mol–1) . | KC21% O2 (µM) . | kOcat (s–1) . | kOcat/KO (mM–1 s–1) . | CE . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | k C cat/KC (mM –1 s –1 ) . | k C cat K C 21%O2 (mM –1 s –1 ) . |
| Haptophytes | |||||||||
| P. carterae | 3.3 ± 0.4 | 17.7 ± 1.5 | 366 ± 60 | 102 ± 1 | 30.6 | 0.7 | 1.8 | 186 | 108 |
| T. lutea | 2.2 ± 0.1 | 24.1 ± 0.5 | 800 ± 55 | 89 ± 1 | 32.2 | 0.8 | 1.0 | 91 | 68 |
| P. lutheri | 2.5 ± 0.1 | 14.5 ± 1.6 | 1146 ± 212 | 125 ± 2 | 17.8 | 1.6 | 1.4 | 172 | 140 |
| Diatoma | |||||||||
| P. tricornutum | 3.3 ± 0.5 | 41.1 ± 1.3 | 664 ± 54 | 116 ± 2 | 57.6 | 0.5 | 0.7 | 80 | 55 |
| C3planta | |||||||||
| N. tabacum | 3.1 ± 0.3 | 9.7 ± 0.1 | 283 ± 15 | 81 ± 1 | 18.9 | 1.1 | 3.9 | 319 | 164 |
CE, carboxylation efficiency.
The rate of oxygenation (kOcat) was calculated using the equation kOcat=(kCcat×KO)/(KC×SC/O)×KC at 25 °C under ambient atmospheric O2 levels; KC21%O2 was calculated as KC(1+[O2]/KO) assuming an O2 solubility of 0.00126 mol (l bar)–1 and an atmospheric pressure of 1.013 bar resulting in an [O2] value of 267 µM in solution.
Values shown are average of measurements from n≥3 (±SD) biological repeat samples.
aData from Young et al. (2016).
Rubisco kinetic parameters measured at 25 °C
| Species . | kCcat (s–1) . | KC (µM) . | KO (µM) . | Sc/o (mol mol–1) . | KC21% O2 (µM) . | kOcat (s–1) . | kOcat/KO (mM–1 s–1) . | CE . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | k C cat/KC (mM –1 s –1 ) . | k C cat K C 21%O2 (mM –1 s –1 ) . |
| Haptophytes | |||||||||
| P. carterae | 3.3 ± 0.4 | 17.7 ± 1.5 | 366 ± 60 | 102 ± 1 | 30.6 | 0.7 | 1.8 | 186 | 108 |
| T. lutea | 2.2 ± 0.1 | 24.1 ± 0.5 | 800 ± 55 | 89 ± 1 | 32.2 | 0.8 | 1.0 | 91 | 68 |
| P. lutheri | 2.5 ± 0.1 | 14.5 ± 1.6 | 1146 ± 212 | 125 ± 2 | 17.8 | 1.6 | 1.4 | 172 | 140 |
| Diatoma | |||||||||
| P. tricornutum | 3.3 ± 0.5 | 41.1 ± 1.3 | 664 ± 54 | 116 ± 2 | 57.6 | 0.5 | 0.7 | 80 | 55 |
| C3planta | |||||||||
| N. tabacum | 3.1 ± 0.3 | 9.7 ± 0.1 | 283 ± 15 | 81 ± 1 | 18.9 | 1.1 | 3.9 | 319 | 164 |
| Species . | kCcat (s–1) . | KC (µM) . | KO (µM) . | Sc/o (mol mol–1) . | KC21% O2 (µM) . | kOcat (s–1) . | kOcat/KO (mM–1 s–1) . | CE . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | k C cat/KC (mM –1 s –1 ) . | k C cat K C 21%O2 (mM –1 s –1 ) . |
| Haptophytes | |||||||||
| P. carterae | 3.3 ± 0.4 | 17.7 ± 1.5 | 366 ± 60 | 102 ± 1 | 30.6 | 0.7 | 1.8 | 186 | 108 |
| T. lutea | 2.2 ± 0.1 | 24.1 ± 0.5 | 800 ± 55 | 89 ± 1 | 32.2 | 0.8 | 1.0 | 91 | 68 |
| P. lutheri | 2.5 ± 0.1 | 14.5 ± 1.6 | 1146 ± 212 | 125 ± 2 | 17.8 | 1.6 | 1.4 | 172 | 140 |
| Diatoma | |||||||||
| P. tricornutum | 3.3 ± 0.5 | 41.1 ± 1.3 | 664 ± 54 | 116 ± 2 | 57.6 | 0.5 | 0.7 | 80 | 55 |
| C3planta | |||||||||
| N. tabacum | 3.1 ± 0.3 | 9.7 ± 0.1 | 283 ± 15 | 81 ± 1 | 18.9 | 1.1 | 3.9 | 319 | 164 |
CE, carboxylation efficiency.
The rate of oxygenation (kOcat) was calculated using the equation kOcat=(kCcat×KO)/(KC×SC/O)×KC at 25 °C under ambient atmospheric O2 levels; KC21%O2 was calculated as KC(1+[O2]/KO) assuming an O2 solubility of 0.00126 mol (l bar)–1 and an atmospheric pressure of 1.013 bar resulting in an [O2] value of 267 µM in solution.
Values shown are average of measurements from n≥3 (±SD) biological repeat samples.
aData from Young et al. (2016).
Significant kinetic diversity at 25 °C was observed among each haptophyte Rubisco relative to P. tricornutum (model diatom species) and Nicotiana tabacum (tobacco, model plant Rubisco used in kinetic comparisons; Whitney et al., 2001; Sharwood et al., 2016a, b) (Table 1). The kCcat of P. carterae, P. tricornutum, and tobacco were similar and each ~50% higher than those of T. lutea and P. lutheri Rubisco. Comparable levels of variation in KC were also observed among the haptophyte Rubiscos (14.5–24.1 μM) that are notably lower and spanning a smaller range than the KC values of diatom Rubiscos (22–70 μM; Fig. 3A). This suggests haptophyte Rubisco may experience a lower CO2 microenvironment relative to diatoms. This is probably the case for Rubisco in P. lutheri that lacks a pyrenoid (Burris, 1981; Bendif et al., 2011) and whose Rubisco has the lowest KC and highest SC/O (i.e. a greater selectivity for CO2 over O2; Table 1).
A correlative analysis of haptophyte Rubisco kinetics
A comparison of Rubisco kinetics of each haptophyte identified contrasting relationships when compared with the 25 °C properties of Form 1B and 1D L8S8 Rubisco from a range of eukaryotic phototrophs (Fig. 3A–E; data compiled in Supplementary Table S1). An examination of the KC–kCcat relationship for ‘green’ Form 1B (vascular plants, CCM-positive green algae) and ‘non-green’ Form 1D (diatoms, haptophytes, and red algae) Rubisco suggests they follow differing trajectories (Fig. 3A). As suggested previously, this might arise from lineage-dependent variation in ribulose bisphosphate (RuBP) enolization energies and/or mechanistic differences in their multistep carboxylation chemistry (Tcherkez, 2013, 2016; Young et al., 2016).
With regard to haptophyte Rubisco, the KC–kCcat relationship of P. lutheri and P. carterae Rubisco appeared to align more closely with Form 1B Rubisco (Fig. 3A). Consequently, their carboxylation efficiencies under both anaerobic (kCcat/KC) and ambient O2 (kCcat/KC21%O2) are higher than those of T. lutea Rubisco (Table 1) whose KC–kCcat relationship closely aligns with red algae and diatom Form 1D Rubiscos (Fig. 3A), and lower carboxylation efficiency of P. tricornutum Rubisco (Table 1). These findings suggest that there may be differences in the CCM effectiveness between the bulging pyrenoids in P. carterae relative to the immersed pyrenoids of diatoms and T. lutea. It also raises questions regarding how differences in the CCM and/or cellular metabolism in phytoplankton with immersed pyrenoids have led to the evolution of atypical Form 1D Rubisco kinetics.
Somewhat analogous to their differing KC–kCcat relationships, both Form 1B and 1D enzymes showed differing linear correlations between KO and kOcat (Fig. 3B) but no identifiable correlations between their kCcat/kOcat (Fig. 3C) and KC/KO (Fig. 3D) relationships. This finding is consistent with increasing experimental evidence that changes in the carboxylation and oxygenation properties are not coupled in an obligatory manner (Savir et al., 2010; Whitney et al., 2011; Sharwood et al., 2016a; Sharwood, 2017). This unfastening of carboxylation and oxygenation has enabled significant Rubisco kinetic diversity to have evolved in nature, in particular with regard to CO2/O2 selectivity (SC/O) whose correlative trade-off with kCcat follows a diffuse exponential relationship (Fig. 3E; Sharwood, 2017) rather than the linear response previously postulated (Tcherkez et al., 2006; Savir et al., 2010). Within the kCcat–SC/O relationship, the haptophyte Rubisco localizes in a region comparable with diatom Rubisco between the high SC/O, low kCcat of Form 1D red algae Rubisco and the lower SC/O, higher kCcat of Form 1B Rubisco (Fig. 3E).
Interpreting the differing O2 sensitivities of haptophyte Rubisco
While the CCMs of phototrophic organisms function to elevate the CO2:O2 ratio around Rubisco through increased CO2 supply, it is unclear how the ratio is dependent on complementary mechanisms to lower O2. In many organisms employing a CCM, the O2-generating components are located away from Rubisco. For example, the Rubisco-containing bundle sheath cell (BSC) chloroplasts in C4 plants within NADP-malic enxyme (NADP-ME) subtypes characteristically lack the O2-evolving PSII complexes (Sage et al., 2014; von Caemmerer and Furbank, 2016). Similarly, the thylakoids traversing the pyrenoid of the red algae Porphyridium cruentum lack PSII (McKay and Gibbs, 1990), while in the dinoflagellate Gonyaulax polyedra during times of high carbon fixation the Rubisco is spatially relocated to pyrenoids near the cell center away from the O2-evolving light-harvesting reactions (Nassoury et al., 2001). Similarly in cyanobacteria, the carboxysomes localize to the cell interior away from the PSII thylakoids lining the cell periphery (Liberton et al., 2011). These strategies for spatially separating O2 production away from Rubisco appear to be key components of the CCM. Unfortunately, measuring the O2 concentration in bundle sheath cell chloroplasts, cyanobacteria carboxysomes, or inside the pyrenoid of algae remains an insurmountable challenge.
As indicated above, the oxygenation properties of Rubisco show significant natural variation. Drawing correlations between their O2 sensitivity (i.e. KO) and CCM efficiency is therefore quite challenging. An additional complexity is that the extent of O2 solubility is reduced by the highly proteinaceous matrix of pyrenoids, chloroplasts, and carboxysomes (Tcherkez, 2016) and by the increasing pressures experienced by microalgae down the water column. Interestingly the higher KO of T. lutea, P. tricornutum, and P. lutheri Rubisco lend to limiting the influence of changing O2 concentrations on carboxylation efficiency relative to Rubisco from tobacco and P. carterae (Fig. 4). This implies that the variable O2 concentrations experienced by diatoms and haptophytes within the water column might have influenced their Rubisco kinetic evolution. Furthermore, pyrenoid morphology and ultrastructure may also have influenced the evolved oxygenase properties. For example, P. lutheri Rubisco exhibits a low affinity for O2 (KO ~1150 μM), implying that its Rubisco experiences higher O2 concentrations than the Rubisco from algae possessing immersed (e.g. T. lutea, P. tricornutum, KO ~650–800 μM) or bulging (P. carterae, KO ~366 μM) pyrenoids (Table 1).
The differential effect of O2 on Rubisco carboxylation efficiency. Variation in the response of carboxylation efficiency (CE; kCcat/KC) to O2 levels (O) for Rubisco from tobacco (tob, vascular plant control), the diatom P. tricornutum (Pt, dotted line), and the haptophytes P. lutheri (Pl, solid gray line), P. carterae (Pt, solid black line), and T. lutea (Tl, dashed black line). Lines were fitted to the equation CE=kCcat/{KC×[1+(O/KO)]} using the parameters listed in Table 1. Arrows indicate the differing O2 levels in fresh water and the ocean surface [assuming ~3.5% (w/v) salinity] at an atmospheric pressure of 1.013 bar.
These observations could be interpreted to suggest that the pyrenoids, in particular those with a bulging morphology, might be lowering the O2 environment to augment the CO2:O2 ratio around Rubisco. Possibly internally bulging pyrenoids may locate Rubisco closer to the reducing chemistry of the cytosol or the mitochondria and their respired CO2. Other mechanisms for altering pyrenoid CO2:O2 include reducing thylakoid number within the pyrenoid, reducing the O2-producing PSII activity (McKay and Gibbs, 1990), or employing pyrenoid tubules for diffusion (Engel et al., 2015). Challenging these hypotheses is the observed high KO (~2000 μM, indicating an insensitivity to O2) for Rubisco from the pyrenoid-containing diatom Thalassiosira weissflogii (Young et al., 2016) and that multiple thylakoids traverse the pyrenoid of P. carterae, albeit with untested levels of PSII activity (Manton and Peterfi, 1969).
The influence of a CCM on the Rubisco requirement in haptophytes
A characteristic feature of the CCM in C4 plants is that it reduces the requirement for Rubisco, allowing for increased nitrogen use efficiency (Ghannoum et al., 2005). In diatoms, the Rubisco content was found to correlate with KC, suggesting that the allocation of resources into the enzyme may depend on CCM efficiency (Young et al., 2016). For example, the low KC of Rubisco from Phaeodactylum and Chaetoceros diatom species correlated with increased investment in Rubisco content, while in Thalassiosira and Skeletonema species it was hypothesized that resources were instead allocated to the CCM rather than Rubisco to saturate the enzymes low CO2 affinity (Young et al., 2016).
Among the three haptophyte species examined here, we identified a negative relationship between increasing CO2 affinity (i.e. reducing KC) and increasing Rubisco content (dashed line, Fig. 5). The trajectory of this relationship poorly correlated with the Rubisco content and KC of P. tricornutum. While it is known that Rubisco content in diatoms can be influenced by growth stage (Losh et al., 2013), our measurements comprised replicate algae samples from cultures growing under non-nutrient limiting conditions and resulted in reproducible measures of Rubisco content (Fig. 5). While future experiments are aimed at examining these properties from a wider range of microalgae species, it is apparent that in the pyrenoid-lacking P. lutheri cells there is ~3- to 4-fold higher investment of soluble cellular protein in Rubisco (Fig. 5). Likewise, the Rubisco content in hornworts also shows a comparable correlation with the presence/absence of pyrenoids (Hanson et al., 2002). Similarly, in C3 plants, Rubisco comprises a larger resource investment [25–50% (w/w) total soluble protein] relative to C4 plants where the CCM and higher kCcat reduces the amount of Rubisco required [i.e. 8–15% (w/w) of the soluble cellular protein (Ghannoum et al., 2005; Sharwood et al., 2016a, c)].
Rubisco content is reduced in pyrenoid-containing phytoplankton. The Rubisco content (quantified by [14C]CABP binding and expressed as a percentage of the cellular soluble protein) in cells grown at 20 °C under saturating nutrients was higher (11.4 ± 1.2%) in the pyrenoid-lacking P. lutheri cells relative to that in P. carterae (3.0 ± 0.8%), T. lutea (3.5 ± 0.9%), and P. tricornutum (3.2 ± 0.6%).
How suited is phytoplankton Rubisco to supporting photosynthesis in C3 plants?
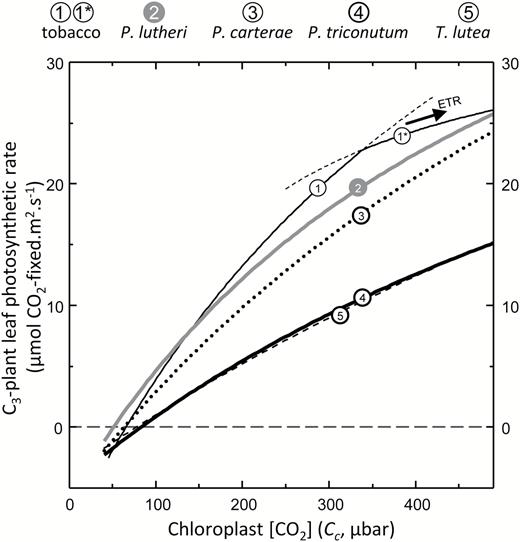
Improving the catalytic efficiency of Rubisco is a key target for improving the rate of photosynthesis and growth in key C3 crops such as rice and wheat (Long et al., 2015; Sharwood et al., 2016b). This has led to considerable interest in identifying whether the natural catalytic diversity of Rubisco can be exploited to deliver improvements in crop Rubisco performance. The faster Rubisco from Synechococcus PCC7942 (cyanobacteria) and the photosynthetic bacterium Rhodospirillum rubrum are not able to support faster C3 plant growth, even under elevated CO2, due to their low carboxylation efficiencies under ambient O2 (kCcat/KC21%O2) and their low SC/O (Sharwood, 2017). In comparison, the higher kCcat/KC21%O2 and SC/O of the Form 1D Rubisco from Griffithsia monilis (filamentous red algae) would support faster rates of photosynthesis in C3 crops with the potential to improve productivity by up to 30% (Long et al., 2015). Realizing this benefit is impeded by the incompatible assembly requirements of Form 1D Rubisco in plant chloroplasts (Whitney et al., 2001). Nevertheless, it is hoped solutions to improving crop Rubisco may be achieved through increased understanding of natural kinetic diversity among all Rubisco forms (Whitney et al., 2011; Sharwood, 2017). We therefore used the modeling approach of Farquhar et al. (1980) to simulate how each microalga would influence C3 photosyntesis under varying chloroplast CO2 concentrations (Cc; Fig. 6). The low kCcat/KC21%O2 of T. lutea (and P. tricornutum) Rubisco (Table 1) impeded their simulated effect on C3 photosythesis at 25 °C. In contrast, the high SC/O of P. lutheri Rubisco improved the simulated photosynthetic rates relative to tobacco Rubisco under low Cc but not above 150 μbar of CO2 due to its lower kCcat and kCcat/KC21%O2 (Table 1). This finding suggests hat future kinetic surveys to identify microalgae Rubisco better suited to operating in C3 plant chloroplasts might best focus on microalgae that lack a pyrenoid and a CCM.
The varying potential of phytoplankton Rubisco in a C3 leaf. The influence of each Rubisco analyzed in Table 1 on CO2 assimilation rates (A) at 25 °C in a C3 leaf as a function of Cc was modeled according to Farquhar et al. (1980) as described in the Materials and methods. For the tobacco Rubisco, the photosynthetic rate became light limited (indicated by 1*) at Cc>320 μbar.
Conclusions and future directions
Due to their importance at the base of the marine food chain, global biogeochemical cycling, and interpretation of paleo-chemical signals in marine sediments, it is essential that we better understand the diversity and function of the algae CCMs. In this pilot study, we provide preliminary evidence for correlations between Rubisco content, kinetics, and pyrenoid morphology within the chloroplast of different haptophytes and the diatom P. tricornutum. Recent work has highlighted the lack of knowledge on the components and variable efficiency of the CCM across environmentally important microalgae (Hanson, 2016; Hopkinson et al., 2016; Young et al., 2016). Previous models of the algal CCM relied on correlations with distant photosynthetic organisms and limited Rubisco kinetic data. Elucidating the diversity and biological relevance of CCMs in these species will provide the groundwork necessary for understanding primary production in the world’s oceans.
Our study provides evidence for the potential to use the binding affinities of Rubisco as a probe to gauge the intracellular CO2:O2 ratio around Rubisco and which might include an oxygen exclusion function by the pyrenoid. Future correlative analyses of Form 1D and Form 1B Rubisco kinetics from microalgae lacking pyrenoids and with differing pyrenoid morphologies are needed to yield a more robust functional understanding of the intrapyrenoid microenvironment and the natural diversity in carbon fixation in both terrestrial and aquatic ecosystems. Although challenging to measure, these Rubisco analyses are essential for understanding both (i) the different evolutionary histories of Form I Rubisco whose kinetics appear to have divergently evolved and (ii) the extent to which the competing carboxylation and oxygenation properties can be decoupled. Refining the existing assumptions about diversity and trends in photosynthetic evolution are paramount. Included in such endeavors are whether the non-canonical correlation between KC and kCcat is limited to Form 1D Rubisco from microalgae with immersed pyrenoids (Fig. 3A), if resource allocation to Rubisco is elevated in species with a less effective CCM (Fig. 5), and to what extent increases in kCcat/KC21%O2, SC/O, and KO (i.e. a reduced O2 sensitivity) can be used as a proxy to gauge the effectiveness of microalgae CCMs.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Measurement of Rubisco activation status and stability in vitro at 25 °C.
Table S1. Rubisco kinetics at 25 °C as shown in Fig. 3.
Acknowledgements
AMCH was funded through a Clarendon Scholarship, Oxford and ANU visiting scholar (CE140100015). Funding for JNY and SMW was provided through Australian Research Council Grant CE14010001. RES was funded through the ARC DECRA scheme (DE13010760) and REMR was funded through an ERC Starting Grant (SP2-GA-2008-200915).
References
Author notes
Present address: University of Hawai’i at Hilo, College of Agriculture, Forestry and Natural Resource Management, 200 W. Kawili St, Hilo, HI 96720, USA.



![Rubisco evolution and catalysis. Geological history of the past versus present atmospheric [CO2] (gray) and percentage atmospheric O2 (% v/v) (black; modified from Berner and Canfield, 1989; Badger et al., 2002; Whitney et al., 2011) highlighting the estimated appearance of key primary producers (horizontal lines) (Yoon et al., 2002, 2004; Liu et al., 2010) their differing Form 1B or ID Rubisco lineages they produce, and the predicted timing when algal carbon-concentrating mechanisms (CCMs; gray shading) evolved (Badger et al., 2002; Moritz and Griffiths, 2013).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/68/14/10.1093_jxb_erx179/7/m_erx17901.jpeg?Expires=1716434880&Signature=RHEWHzot4bKuz5b8UNy1afJjys0Ik6ccVodchAmK~o2KPqrndYK-UW1DwsnVsdLgTE1ORPg22-Hp2odyGxYOqvaLpfTad~coUefvBUDy0UzQYTysW8SeTvueMZlZ8APlA97d1HRE2YnuhJwwsVG~kVLDjT5cKXvmkzlLpjOGVzSQhhP5QzkTZCz0ri2AyUNUlQtPNesaS45DpoCTHgHV2rr72IamKNksLXDkWba6KG0Grc3hW5XoocqOY-BGAIcjkALNQQ0xf8CRmE44UC657X64HtllAmFbhju59cIEDpBVossm4NBkfJBvFNdM4vxAA-3OeftLkHcFZxKPWYcBfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Microalgae pyrenoid and CCM composition. (A) TEM images were compiled from the literature to represent the range of pyrenoids presented in this. To represent a pyrenoid lacking Pavlovale, we use Pavlova viridis from Bendif et al. (2011) (Protist, 162, Bendif EM, Probert I, Hervé A, Billard C, Goux D, Lelong C, Cadoret JP, Véron B. Integrative taxonomy of the Pavlovophyceae (Haptophyta): a reassessment, 738–761, ©2011, with permission from Elsevier) as the TEM image clearly represents the lack of pyrenoid. Pavlova lutheri is visualized in the same study; however, the TEM image does not show the chloroplast (Ch) lacking a pyrenoid as clearly. TEM image of P. carterae from Beech and Wetherbee (1988) [republished with permission of the International Phycological Society from Observations on the flagellar apparatus and peripheral endoplasmic reticulum of the coccolithophorid, Pleurochrysis carterae (Prymnesiophyceae), Beech PL, Wetherbee R, Phycologia 27, 1988; permission conveyed through Copyright Clearance Center, Inc.] illustrates pyrenoids (Py) bulging toward the center of the cell, and the two species T. lutea (Bendif et al., 2014) (Journal of Applied Phycology, Erratum to: On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta), 26, 2014, 1617, Bendif EM, Probert I, Schroeder DC, de Vargas C. With permission of Springer) and P. tricornutum (Allen et al., 2011) (Allen AE, Moustafa A, Montsant A, Eckert A, Kroth PG, Bowler C. Evolution and functional diversification of fructose bisphosphate aldolase genes in photosynthetic marine diatoms. Molecular Biology and Evolution 2012, 29, 367–279, by permission of Oxford University Press) show pyrenoids immersed within the chloroplast. (B) Summary of published experimental evidence for the presence of a CCM in the species with a pyrenoid. Evidence for a CCM is detectable by: (i) inhibition of CO2 assimilation by the impermeable acetazolamide (AZA) or membrane-permeable ethoxyzolamide (EZA) CA inhibitors (Burns and Beardall, 1987; Okazaki et al., 1992; Badger et al., 1998; Hopkinson et al., 2013); (ii) stimulation of CA activity following cell illumination (Badger et al., 1998); (iii) whether the intercellular Ci pool is higher than the external environment (Badger et al., 1998); or (iv) the preliminary detection of δ-CA using methods described in the Materials and methods.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/68/14/10.1093_jxb_erx179/7/m_erx17902.jpeg?Expires=1716434880&Signature=HBFnMshIPhWZn26f0XoZNiFRTonMosVzBKJ68r2nZwjgWnKl30ygqyRZp4eTn0blXYptED8Uqac~-SIIi4oIrQ-AympxmbD2P6yFYr-m-Iue6JuWu6tkPIkfQfuFjeGDQXQ3ozfUmMDepFTaljsjmcvrtv-KRzEwmN44c-TUSvh4QkSUA03IPVVflxvrQu-QYGsM64A8nmxK6wg4jxpf6ncQCg9zFfN8kB7aKzUZhRvyRVHauMbebJmkI~2ny-eHwE61NlP8Y8hNVkgpG3M85e1yLIhlNFfVlJ12YIK5gXkIMqPIieFq7tLa-UnHfZzdqf9APqRAdgQ5xNN~6Cp-Zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![The differential effect of O2 on Rubisco carboxylation efficiency. Variation in the response of carboxylation efficiency (CE; kCcat/KC) to O2 levels (O) for Rubisco from tobacco (tob, vascular plant control), the diatom P. tricornutum (Pt, dotted line), and the haptophytes P. lutheri (Pl, solid gray line), P. carterae (Pt, solid black line), and T. lutea (Tl, dashed black line). Lines were fitted to the equation CE=kCcat/{KC×[1+(O/KO)]} using the parameters listed in Table 1. Arrows indicate the differing O2 levels in fresh water and the ocean surface [assuming ~3.5% (w/v) salinity] at an atmospheric pressure of 1.013 bar.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/68/14/10.1093_jxb_erx179/7/m_erx17904.jpeg?Expires=1716434880&Signature=1S5Vy523yuBjwyPCnWxK-BRwTpzkdRTPpPgWP2zhss~37eRtaS7n51lWfgQA7i2bqQShyx2kr70H46td~SUh-ElW7TEzcW2Y0nEyT9hmzDtnrgYqf3v9LeZ1G1jkWLlcYRkBxON8kJWnYmnn7w~EsrsfLVT9oNa8PhT1UlBfP6GO8~dSk9owzuxja59vTgByyXkCgQjgzzq1FIa0GMk5J0qHieNoMu4SbBPMhoLsRhAiGBMVJsZ6J2ZvXCH8sTlylPadErmg7s~sghjyICenHXCn~tabU652agqYovyjxvJdXdh4KIpnp4GMhg-dOfAPQlzns2Z~zT6HyUqayMWOvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Rubisco content is reduced in pyrenoid-containing phytoplankton. The Rubisco content (quantified by [14C]CABP binding and expressed as a percentage of the cellular soluble protein) in cells grown at 20 °C under saturating nutrients was higher (11.4 ± 1.2%) in the pyrenoid-lacking P. lutheri cells relative to that in P. carterae (3.0 ± 0.8%), T. lutea (3.5 ± 0.9%), and P. tricornutum (3.2 ± 0.6%).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/68/14/10.1093_jxb_erx179/7/m_erx17905.jpeg?Expires=1716434880&Signature=FENV01rMeVy9rFtAVs1fmXwQ7aMiHHQztFRy~p86YhzkQW8B4ncGRufhLxz4Ai4pQiVy~HmRdJCEs1JZXggUds7-e5TeWLi4XWsPcI9AhmMpi3jZRH4Ev0D6u75xAFVf9TBbDHmTFdIcyBoJYnaaenpdhTSxSsqCIAkChOeUEBeG-SkBla~3KVUjDmzROof-afUueeGp1zw7r5TVYj~ck8qruWlk~r2RO47ZR4YtNIOf3J4841t8h1-o-~Wrp2Gp5MI41IP~8FANnHtoSV3GDg8os2i7al0rVj-WcMaB7ystZzZ7FXMGb6w7DHocRrhbjILlt28RH2iiTJ11iFt9sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments