-
PDF
- Split View
-
Views
-
Cite
Cite
F. Vinagre, C. Vargas, K. Schwarcz, J. Cavalcante, E. M. Nogueira, J. I. Baldani, P. C. G. Ferreira, A. S. Hemerly, SHR5: a novel plant receptor kinase involved in plant–N2-fixing endophytic bacteria association, Journal of Experimental Botany, Volume 57, Issue 3, February 2006, Pages 559–569, https://doi.org/10.1093/jxb/erj041
Close - Share Icon Share
Abstract
Endophytic nitrogen-fixing bacteria have been isolated from graminaceous plants such as maize, rice, and sugarcane. They are thought to promote plant growth, not only by fixing nitrogen, but also by the production of plant hormones. The molecular mechanisms involved in this interaction are not yet clear. In this work, the identification of a receptor-like kinase (RLK), named SHR5, which may participate in signal transduction involved in the establishment of plant–endophytic bacteria interaction is described for the first time. SHR5 seems to be part of a novel subclass of RLKs present in a wide range of plant species. The expression of this gene is down-regulated in sugarcane plants associated exclusively with beneficial endophytic bacteria and is not a general response caused by micro-organisms or abiotic stress. In addition, more successful sugarcane–endophytic bacteria associations have a more pronounced decrease in SHR5 expression, suggesting that SHR5 mRNA levels in plant cells are inversely related to the efficiency of the association.
Introduction
High levels of biological nitrogen fixation (BNF) contribution have already been described in some graminaceous plants (Boddey and Döbereiner, 1995), including sugarcane (Saccharum spp) (Urquiaga et al., 1992). Nitrogen-fixing bacteria have been isolated from sugarcane tissues, including Gluconacetobacter diazotrophicus, Herbaspirillum spp, Azospirillum spp, and Burkholderia tropicae (Cavalcante and Döbereiner, 1988; Baldani et al., 1992, 1996, 1997; Reis et al., 2004). Unlike rhizobium/leguminosae symbiosis, where bacteria are restricted to nodules, these diazotrophs are endophytic, colonizing intercellular spaces and vascular tissues of most plant organs without causing damage to the host (James and Olivares, 1998; Reinhold-Hurek and Hurek, 1998). They promote plant growth possibly by fixing nitrogen and also by the production of plant hormones (Sevilla et al., 2001). It is not yet clear which mechanisms are involved in the establishment of this particular type of endophytic interaction. The fact that distinct sugarcane genotypes have different rates of biological nitrogen fixation (BNF) suggests that plant genetic factors might be controlling the process of bacteria recognition, colonization, and/or nitrogen fixation (Urquiaga et al., 1992). Recently, ESTs that are preferentially or exclusively represented in cDNA libraries were identified from plants inoculated with G. diazotrophicus or H. rubrisubalbicans, suggesting that the plant is actively involved in the interaction (Nogueira et al., 2001). Several of the genes identified are possibly involved in different processes of plant/bacteria signalling (Vargas et al., 2003).
Plants have a large family of receptor-like kinases (RLKs) that have been implicated in mechanisms of perception and transduction of extracellular signals into the cell (reviewed by Shiu and Bleecker, 2001a). Phylogenetic analysis of the RLKs in A. thaliana revealed that more than 400 genes encode putative plant receptor kinases, defined as proteins with a signal sequence, an amino-terminal domain with a single transmembrane region, and a carboxyl-terminal cytoplasmic kinase domain (Shiu and Bleecker, 2001b). There are several classes of plant RLKs, distinguished according to their extracellular domains, which can potentially bind an array of molecules (Shiu and Bleecker, 2001a). The largest plant RLK class is characterized by the leucine-rich repeats (LRR) motif in the ectodomain. Nevertheless, out of the 216 LRR-RLKs in A. thaliana, only 10 or so have known functions, and only four have been extensively studied (Diévart and Clark, 2004). Members of this plant subfamily were known to play roles in diverse processes related to plant growth/development, stress, defence against pathogens, and symbiosis (Shiu et al., 2004).
In this study, the identification of a novel sugarcane gene, named SHR5, involved in the association with endophytic nitrogen-fixing bacteria is described. Sequence analyses suggest that the SHR5 gene encodes a protein that belongs to a subclass of the LRR-RLK protein family with a biological function not yet described and present in a wide range of different species. Gene expression studies show that SHR5 expression is drastically reduced in plants associated with the diazotrophic endophytes. They also suggest that it is not a general stress response to micro-organisms, but that it seems to be specific for beneficial associations. In addition, the results indicate that SHR5 mRNA levels in plant cells are related to the efficiency of the association between sugarcane and endophytic bacteria. As far as is known, this study is the first report on a plant RLK that is involved in the association between plants and endophytic N2-fixing bacteria.
Materials and methods
In vitro plant growth and micro-organism treatments
Sugarcane plantlets free of micro-organisms were obtained by sterile meristem culture and micropropagated according to the method of Hendre et al. (1983). In vitro-grown SP70-1143 rooted sugarcane plantlets were inoculated as described by James et al. (1994) using 0.1 ml of 106–107 bacterial suspension. Controls were inoculated with medium only. Seven days after the inoculation, plants were harvested and examined for bacterial colonization by the Most Probable Number (MPN) estimation, according to the methods of Reis et al. (1994). All plants were maintained at 30 °C with an irradiance of 60 μmol photons m−2 s−1 for 12 h d−1. The sugarcane genotypes used in this work were: SP70-1143 (high inputs of N from BNF), Chunee (low inputs of N from BNF), B-4362 (susceptible to H. rubrisubalbicans), SP70-3370 (susceptible to Leifsonia xyli subsp. xyli), and SP71-799 (susceptible to Puccinia melanocephala). The endophytic diazotrophic bacteria used were Gluconacetobacter diazotrophicus (PAL5 strain), Herbaspirillum seropedicae (HRC54 strain), H. seropedicae nif− (M2 strain, unable to fix nitrogen), H. rubrisubalbicans (HCC103 strain), and Azospirillum brasilensis (Sp245 strain). Inoculations with Agrobacterium tumefaciens (A281 strain) and Leifsonia xyli subsp. xyli (CTC B07 strain) bacteria were performed with in vitro-grown SP70-1143 and SP70-3370 varieties, respectively. In vitro-grown sugarcane plantlets (SP71-799 variety) free of micro-organisms were transferred to a greenhouse, then inoculated 15 d later with approximately 106 spores solution of Puccinia melanocephala. The material was harvested 15 d later, with the inoculated plants showing rust disease symptoms. Stalks of field-grown sugarcane SP86-155 and SP80-1842 varieties with and without mosaic virus disease and leaf scald disease, respectively, were germinated in a greenhouse and shoots were harvested 15 d later.
Other plant treatments
In vitro-grown SP70-1143 rooted sugarcane plantlets, free of micro-organisms, were subjected to different treatments: (i) heat shock treatment at 45 °C or 50 °C for 15 min; (ii) saline stress treatment for 16 h by the addition of 1% or 2% NaCl to the medium; and (iii) auxin treatment for 7 d by the addition of 2.42 μg ml−1 of indole acetic acid (IAA) or naphthalene acetic acid (NAA) to the medium. All plants were maintained at 30 °C with an irradiance of 60 μmol photons m−2 s−1 for 12 h d−1.
RNA extraction and cDNA synthesis
For each expression analysis experiment, three to five plantlets were pooled and total RNA was extracted according to Logeman et al., (1987). First-strand cDNA was prepared by reverse transcription of 5 μg of DNase I-treated RNA using the ‘First-Strand cDNA Synthesis Pharmacia Kit’ and Not-dT as primer, according to the manufacturer's instructions. For cDNA-AFLP experiments, PolyA+ RNA was isolated using Dynabeads® Oligo (dT)25 mRNA Purification kit according to the manufacturer's instructions (Dynal., Hamburg, Germany).
cDNA-AFLP
cDNA amplified fragment length polymorphism (cDNA-AFLP) analysis was performed according to Bachem et al. (1996) with modifications. Double-stranded cDNA was digested with the restriction enzymes SacI and MseI (New England Biolabs). Sequences of primers and adaptors used for AFLP reactions were: SacI adapters, 5′-CTCGTAGACTGCTACAAGCT-3′/3′-CATCTGACGCATGT-5′; MseI adapters, 5′-GACGATGAGTCCTGAG-3′/3′-TACTCAGGACTCAT-5′; SacI pre-amplification primer, 5′-CTCGTAGACTGCGTACAAG-3′; MseI pre-amplification primer, 5′-GACGATGAGTCCTGAGTAA-3′; SacI selective amplification primer, 5′-GACTGCGTACAAGCTC+NN-3′; and MseI selective amplification primer, 5′-GATGAGTCCTGAGTAA+NN-3′. The selective SacI primers were end-labelled using [γ-33P]dATP. The pre-amplification PCR conditions were: 30 s at 94 °C, 1 min at 60 °C, and 1 min at 72 °C (28 cycles). The selective touch-down PCR conditions were: 30 s at 94 °C, 1 min at 65 °C (–0.7 °C cycle−1), and 1 min at 72 °C (13 cycles), 30 s at 94 °C, 1 min at 56 °C, and 1 min at 72 °C (18 cycles). Selective amplification products were separated on 5% polyacrylamide gel run at 55 W and 50 °C until bromphenol blue dye reached the end. Gels were dried onto 3MM Whatman paper (Whatman, Maidstone, UK) and positionally marked before exposing to Kodak Biomax MR film for 24 h. The bands of interest were cut from the gel with a surgical blade, eluted, and re-amplified with the pre-amplification primers. The re-amplified cDNAs were subcloned using the pGEM-T vector system (Promega, Madison, USA) and at least three individual clones were sequenced using automated sequencing. The sequences were included for further analysis only when they were identical. Database searches were performed at the NCBI World Wide Web server using the Basic Local Alignment Search Tool (BLAST) network service (Altschul et al., 1997). Each transcript-derived fragment (TDF) sequence was compared against all sequences in the non-redundant database using the BLASTX program, in the EST database using the BLASTN program and in the S. officinarum database of TIGR Gene Indices using the BLASTN program.
SHR5 sequence analysis
3′-Nested PCR was performed to clone the 3′ end of the SHR5 cDNA. First strand cDNA was prepared from micro-organism-free sugarcane plantlets as described above. cDNA was used as a template for first-round PCR with an oligo dT anchor primer and a 5′ SHR5 gene-specific primer. Pfx Taq polymerase (Invitrogen) was used in the following cycling protocol: incubation at 94 °C for 5 min followed by 30 cycles of 94 °C for 1 min, at 60 °C for 1 min, and at 68 °C for 3 min, and finished at 68 °C for 5 min. Five μl of the first-round reaction was used in a second reaction with a second gene-specific primer designed to anneal 3′ to the first gene-specific primer under the same PCR conditions. TDF gene-specific primers were based on cDNA-AFLP 179 bp identified sequence. Primers used for the first-round PCR were TDF5a 5′-ACTTGAAGATCCTCTGGGCAT-3′ and NotI dT 5′-TGGAAGAATTCGCGGCCGCAGGAAT(18)-3′, and for the second-round PCR: TDF5b 5′-GCCTGACTCAGTTGGAAGATC-3′ and NotI 5′-TGGAAGAATTCGCGGCCGCAG-3′. A final fragment of 2366 bp (SHR5 3′) was amplified and cloned into the pGEM-T vector (Promega) and its sequence was determined using automated sequencing. Searches for the full-length cDNA sequence of SHR5 were performed at the S. officinarum database of TIGR Gene Indices using BLASTN (Altschul et al., 1997). The clone CA116269 (GenBank accession no.) was identified as SHR5, and its 5′ cDNA sequence was used to construct the entire SHR5 cDNA (GenBank accession no. DQ067098). A search for SHR5-related sequences was performed at the NCBI World Wide Web server using BLASTX in the non-redundant database. The best hits were used for further analysis. Derived protein sequences were analysed using BLAST2seq (Tatusova and Madden, 1999) to determine the percentage of similarity between the sequences. Pfam (Bateman et al., 2004) and SMART (Letunic et al., 2004) were used to identify protein domain architectures. Hydropathy analysis was generated by the Kyte–Doolittle algorithm (Kyte and Doolittle, 1982). Multiple sequence alignments were carried out using CLUSTAL×(Thompson et al., 1997). Phylogenetic analyses were conducted and viewed using MEGA version 2.1 (Kumar et al., 2001) based on the Neighbor–Joining method.
Semi-quantitative RT-PCR
Ten μl of the first-strand cDNA reaction diluted four times was used in a standard 50 μl PCR reaction (10 mM TRIS-Cl pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, and 2.5 U Taq polymerase) with 200 ng of the specific primers. The primers used were: SHR5.fwr (5′-TGACCGAGCACTCTTTGGTAA-3′) and the 3′-UTR based SHR5.rev (5′-TCGAATTAATCCAGCAGCAGC-3′), or ubi1 (5′-ATGCAGATCTTTGTGAAGAC-3′) and ubi2 (5′-TTACTGACCACCACGAAGAC-3′). In order to carry out experiments on the exponential phase of RT-PCR reaction curves, standard curves were performed varying the amount of cDNA and the number of cycles of PCR reaction. PCR conditions were 94 °C for 5 min, followed by 25–35 cycles (94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min) and with 72 °C for 5 min. The polyubiquitin constitutive gene was used as an internal control in PCR reactions. Products of the PCR reactions were eletrophoretically separated on 1% agarose gel, visualized with ethidium bromide under UV light, and then transferred onto a nylon membrane and hybridized with a radioactively-labelled cDNA fragment from sugarcane SHR5 or a ubiquitin cDNA fragment from Arabidopsis thaliana. Densitometric analyses of bands obtained in PCR amplification were performed using Scion Image 4.0.2 software (Copyright© 2000 Scion Corporation). GraphPad Prism (GraphPad Software Inc, San Diego, CA, USA) was used to perform statistical analysis. When three or more groups were analysed, samples were compared using one-way analysis of variance (ANOVA) followed by Tukey test; when two groups were compared, the unpaired t-test was used. A P value <0.05 was considered significant.
Results
Isolation and sequence analysis of SHR5 cDNA
cDNA-AFLP experiments were carried out in order to detect sugarcane genes that were repressed and/or induced in association with endophytic bacteria. RNA from four groups of sugarcane plantlets inoculated or not inoculated with the following different species of endophytic bacteria: H. rubrisubalbicans (HR), H. seropedicae (HS+) and H. seropedicae mutant, which is unable to fix N2 (HS−), were compared. Twelve transcript-derived fragments (TDF) were isolated and sequenced (F Vinagre, unpublished results). A 179bp TDF, corresponding to SHR5, showed homology with LRR domains and was selected to be characterized further.
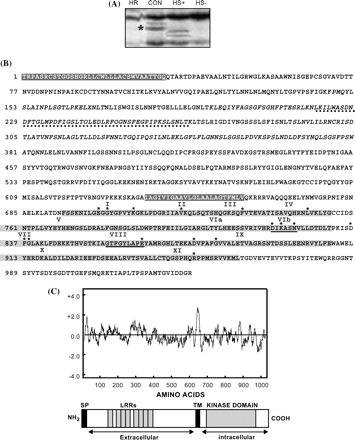
As shown in Fig. 1A, the SHR5 cDNA band is only detected in non-inoculated plants (CON), suggesting that SHR5 gene expression is being repressed in plants inoculated with any one of the endophytic bacteria mentioned above. As the SHR5 TDF corresponds to a small region of the SHR5 complete cDNA, based on its DNA sequence information an attempt was made to obtain a longer SHR5 cDNA sequence using two different approaches. A 2366 bp long cDNA corresponding to the 3′ end of SHR5 complete cDNA was obtained by 3′-Nested PCR. In addition, an EST corresponding to SHR5 was identified in the database of TIGR Gene Indices (GenBank accession no. CA116269) and its 5′ cDNA sequence was used to construct the SHR5 cDNA sequence (GenBank accession no. DQ067098). Although the first methionine was not sequenced in EST CA116269, it is very likely that only a few amino acids were missing, because the signal peptide was identified in the coding region and a comparison with other homologue proteins revealed a protein of similar size. The SHR5 cDNA has an estimated open reading frame of approximately 3084 bp, coding for a predicted protein of 1027 amino acids and a deduced molecular mass of approximately 107 kDa including the signal peptide (Fig. 1B). Analyses of structural properties of the SHR5 predicted protein using Pfam (Bateman et al., 2004) and SMART programs (Letunic et al., 2004) suggest that SHR5 encodes an RLK protein with four distinct regions: an N-terminal hydrophobic signal peptide, extracellular leucine-rich repeats (LRR), a transmembrane domain (TM), and a cytoplasmatic kinase domain (Fig. 1B, C). In the extracellular region, the first hydrophobic domain (black box) corresponds to the signal peptide (SP) possibly responsible for targeting the protein into the endoplasmic reticulum (Von Heijne, 1991). The SHR5 extracellular region contains eight predicted LRR domains. LRR domains can have a role in ligand recognition, protein–protein interactions and agonist binding, in proteins involved in signal transduction in eukaryotes (Baker et al., 1997). The second hydrophobic region, encompassing a segment of 23 amino acids, is located in the middle of the predicted protein sequence, corresponding to a single-membrane spanning region. Figure 1C represents a hydropathy plot generated by the Kyte–Doolittle algorithm (Kyte and Doolittle, 1982), where increased hydrophobicity is denoted by positive values showing the hydrophobic regions corresponding to the signal peptide and transmembrane domain. The kinase catalytic domain (from amino acid 694 to 962) was detected in the intracellular region of the protein. It is well known that, in their catalytic domain, serine/threonine/tyrosine protein kinases display amino acid sequence similarities that consist of 11 conserved subdomains (Hanks et al., 1988). In the predicted SHR5 protein, these 11 potential kinase subdomains are present (identified by roman numerals in Fig. 1B). Furthermore, the amino acid sequences in subdomains VIb (DIKASN) and VIII (GTFGYLAPE) are consistent with the consensus sequences, DLKXXN and G(T/S)XX(Y/F)XAPE, respectively, which are most common among serine/threonine kinases. This result suggests that SHR5 may have serine/threonine rather than tyrosine substrate specificity.
SHR5 identification and sequence analysis. (A) Visualization of a part of a cDNA-AFLP autoradiogram showing the TDF corresponding to SHR5 cDNA (asterisk). Four lanes are shown, corresponding to an amplified template from sugarcane plantlets of the B-4362 variety inoculated with endophytic bacteria. HR: H. rubrisubalbicans; HS+: H. seropedicae; HS−: H. seropedicae mutant unable to fix N2; CON: plants free of micro-organisms. (B) Predicted amino acid sequence of SHR5. Non-polar regions corresponding to the signal peptide sequence and transmembrane region are denoted by black boxes. Italicized amino acids indicate LRRs. The sequence corresponding to the TDF cDNA is dash underlined. Kinase domain is highlighted with grey. Roman numerals indicate the 11 characteristic subdomains of the protein kinases (Hanks et al., 1988). Amino acids highly conserved among protein kinases are indicated by asterisks. Residues that indicate serine/threonine specificity are underlined. (C) Structural features of the SHR5 protein. A hydropathy plot was deduced from the SHR5 amino acid sequence, where increased hydrophobicity is denoted by positive values. A linear schematic representation of SHR5 denotes the signal peptide (SP), LRRs, transmembrane (TM), and kinase domains identified by computational analysis.
Searches at the NCBI database were performed in order to identify protein sequences closely related to the extracellular domain, the kinase domain, and the entire SHR5 predicted protein. These three different analytical approaches revealed that the closer relatives to SHR5 are putative LRR-RLKs from rice (O. sativa) and A. thaliana, the kinase domain being the region with the most conserved homology. The closest homologue of SHR5 is a rice protein (GenBank accession no. XP_480586) that has 1030 amino acids and exhibits 77% identity and 86% similarity with SHR5. In A. thaliana, the closest SHR5 homologue (GenBank accession no. NP_176009) has 1032 amino acids and shows 53% identity and 68% similarity with SHR5.
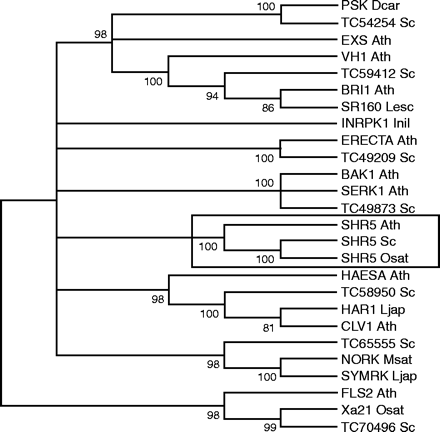
To classify SHR5 within the 15 LRR-RLK subfamilies established by Shiu et al. (2004), a phylogenetic tree using the kinase domain sequences of representative Arabidopsis members of each LRR-RLK subfamily was generated according to Shiu and Bleecker (2001b). With a bootstrap value of 100%, SHR5 formed a well-supported clade with LRR protein of the VIII-2 subfamily, indicating that SHR5 belongs to this LRR subfamily (data not shown). To obtain ideas about the possible biological roles of SHR5, another phylogenetic tree was generated based on kinase domains of the previously described plant LRR-RLK proteins involved in development, symbiosis, and host defence, as revised by Diévart and Clark (2004). In this analysis, other sugarcane LRR-RLK-related proteins were included (Fig. 2). Sugarcane SHR5, together with its homologues in rice and A. thaliana, formed a well-supported branch, separated from the other genes with known biological roles, with a bootstrap value of 100% (detailed with a box in Fig. 2). These data suggest that the SHR5 gene encodes for a novel LRR-RLK protein not yet associated with any biological role described for LRR-RLK proteins to date. The other sugarcane proteins were grouped with different classes of LRR-RLK, showing that sugarcane has a diverse collection of genes encoding for LRR receptor-like kinases.
Phylogenetic analyses of LRR-RLKs. Neighbor–Joining tree, including many LRR-RLKs of known function, sugarcane LRR-RLK-related sequences, sugarcane SHR5 and its homologues in A. thaliana and rice, was created using the MEGA program. To evaluate the confidence limits of the internal branches of the tree, a bootstrap analysis with 2000 replications was performed on the data set. The numbers next to the nodes give bootstrap percentages. This is a condensed tree with a cutoff value of 80. Accession numbers: BAK1_Ath (Q94F62); BRI1_Ath (AAC49810); CLV1_Ath (AAB58929); ERECTA_Ath (AAC49302); EXS_Ath (Q9LYN8); FLS2_Ath (AB010698); HAESA_Ath (AAA32859); HAR1_Ljap (BAC41331); INRPK1_Inil (AAB36558); NORK_Msat (CAD10807) PSK_Dcar (BAC00995); SERK1_Ath (CAB42254); SHR5_Ath (NP_176009); SHR5_Osat (BAD02994); SHR5_Sc (DQ067098); SR160_Lesc (Q8GUQ5); SYMRK_Ljap (AAM67418); VH1_Ath (Q9ZPS9); Xa21_Osat (A57676). Sugarcane (Saccharum spp) RLKs related sequences are shown with their corresponding TC identifiers (TIGR Gene Indices). Ath, A. thaliana; Dcar, Daucus carota; Inil, Ipomoea nil; Lesc, Lycopersicon esculentun; Ljap, Lotus japonicus; Msat, Medicago sativa; Osat, Oryza sativa; Sc, Saccharum spp.
SHR5 gene expression during association between sugarcane and endophytic nitrogen-fixing bacteria
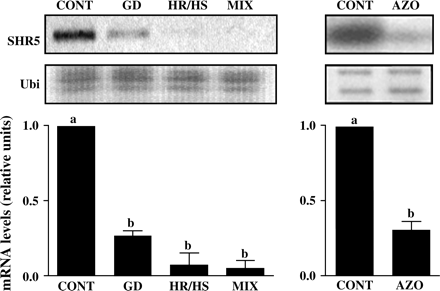
In order to determine how SHR5 mRNA expression is modulated during plant colonization by endophytic diazotrophic bacteria, sugarcane in vitro-grown plantlets of the SP70-1143 variety, free of micro-organisms, were inoculated with different species of endophytic diazotrophic bacteria. SP70-1143 is a sugarcane variety described to show high levels of BNF contribution (Urquiaga et al., 1992). SHR5 mRNA levels were investigated in these samples by semi-quantitative RT-PCR (Fig. 3). SHR5 mRNA levels decreased (0.265±0.035) in sugarcane plants associated with G. diazotrophicus PAL5 (GD), compared with mRNA levels in non-inoculated plants. Inoculation with Herbaspirillum spp., H. rubrisubalbicans HCC103 strain (HR), and H. seropedicae HRC54 strain (HS), showed an even more significant decrease in SHR5 mRNA levels, greater than 90% (0.075±0.075), when compared to the control. As field plants are naturally colonized by a mixture of different species of bacteria, sugarcane plants were also inoculated concomitantly with the three bacterial species mentioned above (GR; HR; HS). SHR5 mRNA levels decreased 95% in the inoculated plantlets (0.05±0.05) (Fig. 3). The analysis was also performed with in vitro-grown sugarcane plantlets inoculated with the A. brasilensis Sp245 strain (AZO), a nitrogen-fixing Azospirillum strain that can also be endophytic (James and Olivares, 1998). The inoculation with AZO led to a 69% decrease of SHR5 expression (0.31±0.06). In all the experiments, plant colonization by the inoculated bacteria was confirmed by the Most Probable Number (MPN) estimation (Materials and methods). Rates of colonization always ranged between log 4–6 cfu g−1 fwt (data not shown).
SHR5 gene expression during association between sugarcane and endophytic nitrogen-fixing bacteria. Sugarcane plantlets of the SP70-1143 variety were inoculated with: GD, G. diazotrophicus; HR/HS, Herbaspirillum spp; MIX, mixture of GD, HR, and HS; AZO, A. brasilensis; CONT, plants free of micro-organisms. Ubiquitin was used as an internal control. The graph represents the average of two experiments with SHR5 mRNA levels corrected for ubiquitin mRNA levels, comparing all groups to control levels. Error bars indicate ±standard error. Different letters indicate significant differences between groups.
The data show that SHR5 is widely repressed in plants colonized by the endophytic diazotrophic bacteria tested. In addition, the decreased mRNA expression is not specific to a bacterial species.
SHR5 gene expression in response to auxins
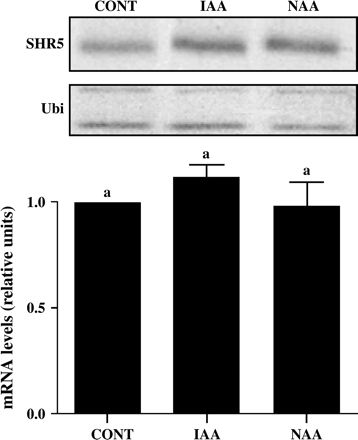
It is well known that endophytic nitrogen-fixing bacteria are capable of producing auxin (Fuentes-Ramirez et al., 1993) and that this can be one of the mechanisms by which these bacteria promote plant growth. Furthermore, it is known that RLKs are involved in hormone signalling in plants (Matsubayashi et al., 2002; Li et al., 2002). To verify if the auxin produced by these bacteria is directly modulating SHR5 gene expression, semi-quantitative RT-PCR were performed on plantlets grown for 7 d with two different auxins in the medium: indole acetic acid (IAA) and naphthalene acetic acid (NAA), both at concentrations similar to those secreted by bacteria in culture medium (Fuentes-Ramirez et al., 1993) (Fig. 4). SHR5 mRNA expression was not modified in either one of the treatments when compared with control mRNA expression (IAA: 1.115±0.065; NAA: 0.98±0.12). These results suggest that the mechanism responsible for modulation of SHR5 expression does not involve hormone produced by bacteria.
SHR5 gene expression in response to auxins. Sugarcane plantlets free of micro-organisms were cultivated in inoculation medium supplemented with 2,4 μg ml−1 of auxin (IAA or NAA) for 7 d. IAA, indole acetic acid; NAA, naphthalene acetic acid; CONT, plants cultivated in medium without hormones. Ubiquitin was used as an internal control. The graphs represent the average of two experiments with SHR5 mRNA levels corrected for ubiquitin mRNA levels. Error bars indicate ±standard error. Different letters indicate significant differences between groups.
SHR5 gene expression in response to association with endophytic nitrogen-fixing bacteria in different sugarcane genotypes
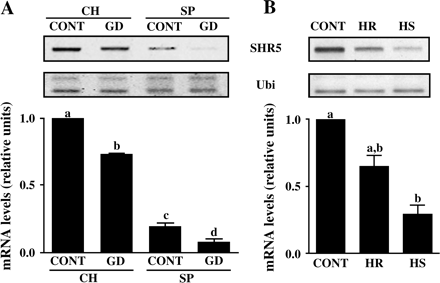
To evaluate if the efficiency of the association between sugarcane and the endophytic diazotrophic bacteria would affect SHR5 expression, sugarcane genotypes with different levels of BNF contribution were investigated. SHR5 mRNA levels were compared by semi-quantitative RT-PCR experiments in in vitro-grown plantlets of varieties with high BNF, SP70-1143 (SP), and low BNF, Chunee (CH), inoculated with the endophytic nitrogen-fixing bacteria G. diazotrophicus (GD) (Fig. 5). Interestingly, there was no difference in total bacteria colonization counts between the two different genotypes (data not shown). SHR5 mRNA expression was repressed in both genotypes (Fig. 5A). Nevertheless, it strikingly decreased in the SP70-1143 variety (0.08±0.02) compared with Chunee plantlets (0.735±0.005) (Fig. 5A). This result indicates that the two genotypes, which have different levels of BNF contribution, do not respond in the same way to the association with endophytic diazotrophic bacteria. Remarkably, SHR5 mRNA levels in SP70-1143 control plantlets are 81% lower (0.195±0.025) than in Chunee control plantlets, suggesting that the expression of SHR5 in SP70-1143 is normally kept lower than in the Chunee genotype. SHR5 expression was also investigated in a pathogenic interaction with a diazotrophic endophyte. The sugarcane B-4362 variety is known to develop the mottled stripe disease when associated with H. rubrisubalbicans (HR) (Olivares et al., 1997). This susceptibility is exclusive to this bacterial species, and is not triggered by other species of the Herbaspirillum genus. SHR5 mRNA levels were measured by semi quantitative RT-PCR in B-4362 in vitro-grown plantlets inoculated with two different Herbaspirillum species: H. rubrisubalbicans (HR) and H. seropedicae (HS) (Fig. 5B). The results showed that SHR5 expression was significantly diminished in healthy B-4362 plantlets colonized by HS (0.29±0.07) when compared with the control, while colonization by HR that led to development of disease revealed a non-significant decrease of SHR5 mRNA expression (0.65±0.08). Taken together, the data suggest that SHR5 mRNA levels in plant cells are related to the efficiency of the association between sugarcane and endophytic bacteria, meaning that the more beneficial the association is for the plant, the more pronounced the decrease is in SHR5 expression.
SHR5 gene expression in response to association with endophytic nitrogen-fixing bacteria in different sugarcane genotypes. (A) Sugarcane plantlets of the SP70-1143 variety (high BNF) and of Chunee specie (low BNF) inoculated with endophytic bacteria. (B) Sugarcane plantlets of the B-4362 variety (susceptible to H. rubrisubalbicans) were inoculated with endophytic bacteria. CH, Chunee; SP-, SP70-1143; GD, G. diazotrophicus; HR, H. rubrisubalbicans; HS, H. seropedicae; CONT, plants free of micro-organisms. Ubiquitin was used as an internal control. The graphs represent the average of two experiments with SHR5 mRNA levels corrected for ubiquitin mRNA levels, comparing all groups to control levels. Error bars indicate ±standard error. Different letters indicate significant differences between groups.
SHR5 expression in response to different micro-organisms and pathogenic interactions
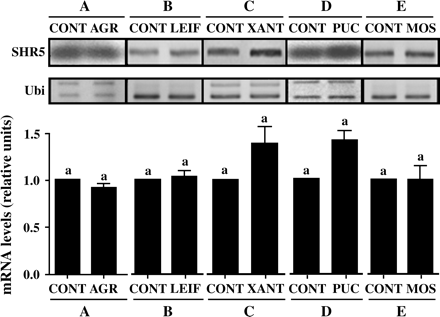
To verify if the modulation of SHR5 gene expression was associated only with beneficial endophytic diazotrophic bacteria as opposed to with any other micro-organism interaction, SHR5 mRNA levels were investigated in sugarcane plantlets inoculated with different micro-organisms. Agrobacterium tumefaciens A281 strain (AGR) is a nitrogen-fixing bacterium, pathogenic in dicots, which does not cause disease in sugarcane (Kanvinde and Sastry, 1990). Plantlets inoculated with AGR (0.92±0.04) showed no changes on SHR5 mRNA levels compared with control non-inoculated plants (Fig. 6A). Leifsonia xyli subsp. Xyli (LEIF) is a pathogenic bacterium responsible for the ratoon-stunting sugarcane disease, and exhibits colonization properties similar those seen in endophytic bacteria (Harrison and Davis, 1986). So far, L. xyli subsp. Xyli has only been identified in association with sugarcane and does not appear to be a soil-borne pathogen (Gillaspie and Teakle, 1989). Colonization of SP70-1143 with LEIF showed the same pattern of SHR5 expression (1.04±0.06) observed in plants free of micro-organisms (Fig. 6B). Sugarcane plants colonized by Xanthomonas albilineans, the pathogenic bacteria responsible for the leaf scald disease (Ricaud and Ryan, 1989), exhibited a small, but not significant increase of SHR5 expression (1.37±0.17), compared with control SHR5 mRNA levels (Fig. 6C). The modulation of SHR5 expression during sugarcane interaction with micro-organisms other than bacteria on SHR5 expression was also investigated. Inoculation of sugarcane plantlets of SP71-799 variety with Puccinia melanocephala (PUC) fungi, the causal agent of sugarcane rust disease (Purdy et al., 1983), exhibited a slight, but not significant, increase of SHR5 expression (1.41±0.11), compared with control SHR5 mRNA levels (Fig. 6D). Shoots from plants with the Sugarcane Mosaic Virus, SCMV (MOS) responsible for the Sugarcane Mosaic Virus Disease (Comstock and Lentini, 2002) showed no significant difference in SHR5 mRNA expression (0.995±0.155) in relation to shoots of virus-free plants (Fig. 6E). Taken together, these results indicate that SHR5 expression is modulated only in interactions between sugarcane and beneficial endophytic bacteria.
SHR5 expression in response to different micro-organisms and pathogenic interactions. (A) Sugarcane plantlets of SP70-1143 variety inoculated with: AGR, A. tumefaciens; CONT, plants free of micro-organisms. (B) Sugarcane plantlets of SP70-3370 variety inoculated with: LEIF, Leifsonia xyli subsp. Xyli; CONT, plants free of micro-organisms. (C) Sugarcane plants of SP80–1842 variety colonized by: XANT, Xanthomonas albilineans; CONT, plants free of X. albilineans. (D) Sugarcane plantlets of SP71-799 variety inoculated with: PUC, Puccinia melanocephala; CONT, plants free of micro-organisms. (E) Sugarcane plants of SP86-155 variety colonized by: MOS, sugarcane mosaic virus; CONT, plants free of mosaic virus. Ubiquitin was used as an internal control. The graphs represent the average of two experiments with SHR5 mRNA levels corrected for ubiquitin mRNA levels. Error bars indicate ±standard error. Different letters indicate significant differences between groups.
SHR5 expression in abiotic stress
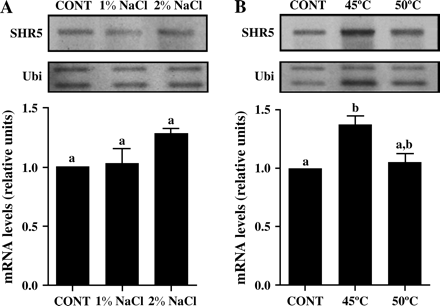
In order to investigate if SHR5 gene expression responds to abiotic stress, semi-quantitative RT-PCR analyses of SP70-1143 in vitro-grown sugarcane plantlets submitted to saline and temperature stresses were carried out (Fig. 7). For 16 h, neither the 1% NaCl nor the 2% NaCl saline treatments affected SHR5 expression significantly, in relation to non-treated plants (1.025±0.135 and 1280±0.05, respectively) (Fig. 7A). Plants kept at 50 °C for 15 min did not show any significant difference in SHR5 mRNA levels in relation to control (1.05±0.08). Plants incubated at 45 °C for 15 min showed an increase of 38% (1.375±0.075) in SHR5 expression when compared with plants kept in normal conditions (Fig. 7B). This modulation on SHR5 expression differs from the one observed with endophytic bacteria association, which leads to a decrease on gene expression.
SHR5 expression in abiotic stress. Sugarcane plantlets free of micro-organisms were submitted to: (A) Heat shock treatment at 45 °C or 50 °C for 15 min; (B) saline stress treatment at 1% or 2% NaCl for 16 h. CONT, plants were maintained at normal conditions. Ubiquitin was used as an internal control. The graphs represent the average of two experiments with SHR5 mRNA levels corrected for ubiquitin mRNA levels. Error bars indicate ±standard error. Different letters indicate significant differences between groups.
Discussion
In this study, the isolation of SHR5, a novel cDNA which encodes a putative, receptor-like protein kinase (RLK) from sugarcane is reported. The predicted SHR5 structural features, including a hydrophobic signal peptide, an extracellular domain, a hydrophobic membrane-spanning segment and a highly conserved kinase domain, strongly suggest that the encoded protein is a receptor protein kinase (Fig. 1). Computer analysis of the kinase domain revealed, among others, two subdomains: VIb (DIKASN) and VIII (GTFGYLAPE), suggesting that SHR5 has serine/threonine kinase substrate specificity. Most RLK genes described in plants, so far, encode for serine/threonine kinases (Shiu and Bleecker, 2001b). The SHR5 putative extracellular domain contains eight leucine-rich repeats (LRR), placing this receptor into the LRR-RLK family. Phylogenetic analysis of the SHR5 kinase domain classified it as belonging to the LRR VIII-2 subfamily (data not shown) (Shiu et al., 2004). So far, no studies have been reported associating any biological functions with the members of this subfamily. Even though the phylogeny used to classify RLKs into subfamilies was based on the kinase domain, it showed that members within each of the RLK subfamilies tended to have similar extracellular domains, such as the structural arrangement of LRRs (Shiu and Bleecker, 2001b). LRR motifs are thought to be involved in protein–protein interactions, and all its ligands described so far in plants are proteins, except for a plant steroid hormone (brassinolide) (Matsubayashi, 2003), although evidence suggests the involvement of a putative secreted brassinosteroid-binding protein in the binding of this hormone to its LRR receptor (Li et al., 2001). So far, LRR-RLK proteins have few reported ligands, and none of them belong to the LRR-VII subfamily. According to Pfam, SHR5 has unrelated sequences or ‘islands’ between their LRR motifs; the first ‘island’ being between the first and second LRR motifs. The second island is between the fourth and fifth LRRs. The BRI1 receptor also presents an LRR island that might mediate the binding of this receptor to its steroid hormone ligand, brassinosteroid (Wang et al., 2001). These data suggest that SHR5 LRR islands could also be related to ligand-binding. It is interesting to note that, in the phylogenetic analysis (Fig. 2), SHR5 was grouped with its homologues in other species, like the dicot A. thaliana, rather than with other sugarcane putative RLKs or other RLKs of known functions. This indicates that SHR5 might represent a new class of gene present in a wide range of different species. An example of conservation has already been reported in the LRR-RLK function between monocots and dicots (Yamamuro et al., 2000).
These data suggest that this new RLK might play a role in the association of plants with beneficial endophytic bacteria. It has been shown that the sugarcane variety SP70-1143, in association with the beneficial endophytic diazotrophic bacteria G. diazotrophicus (GD), Herbaspirillum spp (HR/HS), and Azospirillum brasilensis (AZO) (Fig. 3), exhibited significantly decreased levels of SHR5 mRNA. Sugarcane in the field is colonized by multiple species of endophytic bacteria (Baldani et al., 1997), and these results showed that inoculation with a mixture of different species of bacteria led to similar expression levels seen with individual species. The response of SHR5 expression to the association with the beneficial endophytic bacteria seems to be dependent on the plant genotype. SP70-1143 and Chunee sugarcane genotypes, described as having high and low biological nitrogen fixation (BNF) rates, respectively (Urquiaga et al., 1992), showed different modulation of SHR5 gene expression (Fig. 5A). When inoculated with GD, both genotypes showed a significant decrease in mRNA expression, but this decrease was considerably more pronounced in the SP70-1143 genotype. Comparing mRNA levels in plants free of endophytes (controls), it was observed that SHR5 is much less expressed (81%) in SP70-1143 than in Chunee, indicating that SHR5 steady-state mRNA levels are lower in plants with high BNF rates. Chunee is a wild species not used commercially and SP70-1143, which has an important commercial value in Brazil, is a sugarcane variety selected for its high yields. Historically, Brazilian sugarcane cultivars have been selected for their greater yield with low inputs of inorganic nitrogen fertilizer (Cavalcante and Döbereiner, 1988), which is possibly achieved by choosing genotypes that establish more efficient associations with diazotrophic micro-organisms. It is possible that a decreased expression of SHR5 in SP70-1143 is a consequence of this selection, as it is correlated with a more successful plant–endophytic bacteria interaction.
An intriguing question is how plants sense a beneficial micro-organism. Metabolites provided by the endophytes could be candidates to take part in this signalling. Incubation of sugarcane plantlets with two different auxins (IAA and NAA) in the medium caused no difference in SHR5 expression when compared with non-treated plantlets (Fig. 4). In addition, cDNA AFLP analyses showed that plants inoculated with a mutant H. seropedicae bacterium unable to fix N2 also exhibited decreased SHR5 gene expression (Fig. 1A). These data suggest that the SHR5 response to the association with beneficial endophytes may not be directly attributed to nitrogen fixation or auxin production by the bacteria, but possibly to signalling mechanisms recognized during the interaction with the beneficial micro-organism. Interestingly, it seems that the decrease in SHR5 expression is not only dependent on the interaction between the plant and potentially beneficial bacteria, but also that it is mainly controlled by the effective establishment of an efficient association. Inoculation of B-4362 with H. rubrisubalbicans (HR), which causes mottled stripe disease in this plant genotype, led to an insignificant decrease in SHR5 mRNA expression; meanwhile, inoculation of B-4362 with H. seropedicae (HS), which establishes a non-pathogenic interaction, caused a considerable decrease in SHR5 mRNA levels.
The modulation of SHR5 gene expression does not seem to be a general response to stress, since it does not respond to the abiotic stress imposed by saline and heat treatments. Only plants exposed to 45 °C showed increased SHR5 mRNA expression. This increase is possibly triggered by a different response pathway, since the association with endophytic bacteria decreases SHR5 mRNA levels. An LRR-RLK gene from Arabidopsis has been reported to be induced by abiotic stresses such as dehydration, high salt, and cold treatments (Hong et al., 1997), indicating that LRR-RLKs may be involved in multiple-stress signal transduction. In addition, the studies on modulation of SHR5 expression by interaction with other micro-organisms revealed that associations with non-pathogenic diazotrophic bacteria (A. tumefaciens), pathogenic endophytic bacteria (L. xyli subsp. Xyli and X. albilineans), pathogenic fungus (P. melanocephala) or a pathogenic virus (SCMV) did not alter SHR5 mRNA expression (Fig. 6), which strengthens the idea that SHR5 expression is only decreased in beneficial associations between sugarcane and endophytic bacteria.
It is interesting to point out that receptors related to host defence or symbiosis are structurally similar. Therefore, the possibility cannot be excluded that SHR5 might be involved in a plant defence mechanism and that the decrease in SHR5 gene expression may be a necessary process for the establishment of an efficient association between sugarcane and endophytic bacteria. It has been hypothesized that during the first interaction between plant and symbiotic bacteria, the latter is recognized as a potential pathogen and host defence mechanisms are activated. Later, when a beneficial association is established, those mechanisms are suppressed (Sikorski et al., 1999).
It is important to highlight that, only recently, genes encoding LRR-RLKs have been reported to be related to beneficial interactions between plant and micro-organisms. For example, NORK/SYMRK leguminous mutants are unable to establish symbiosis with arbuscular mycorrhizal (AM) fungi or rhizobia (Endre et al., 2002; Stracke et al., 2002). AM-like interactions were detected in early land plants whereas root nodule symbioses evolved later (Kistner and Parniske, 2002). Proteins that possess similarity with the NORK/SYMRK extracellular domain are found in Arabidopsis, monocots, and gymnosperms (Endre et al., 2002), suggesting that, during evolution, an ancient system of signal transduction was recruited for symbiosis establishment. Resembling NORK/SYMRK, SHR5 is also an LRR-RLK and orthologues are possibly found in monocots and Arabidopsis (Fig. 2). Some symbiosis-related RLKs, despite structural differences from SHR5, can have their mRNA levels decreased in plants colonized by their symbionts, as occurs with SHR5 gene expression (Lange et al., 1999; Radutoiu et al., 2003). Therefore, the data suggest that SHR5 may take part in a novel signalling cascade involved in the establishment of symbiotic-like interactions between plants and micro-organisms.
Despite the fact that modulation of SHR5 expression has been primarily by micro-organisms, it can be expected that SHR5 may also be involved in development processes, as a great number of the LRR-RLK genes described so far are associated to development. Overlaps between signalling pathways can occur among development and host defence signalling proteins (Gomez-Gomez et al., 1999; Montoya et al., 2002; Pastuglia et al., 2002). Moreover, HAR1/NARK LRR-RLK is thought to be involved in the regulation of the later stages of nodulation and also in root development, as HAR1/NARK non-inoculated mutants have a shortened root system and an enhanced number of lateral roots (Krusell et al., 2002; Nishimura et al., 2002; Searle et al., 2003; Wopereis et al., 2000).
Further studies are required in order to understand the mechanisms that involve the SHR5 signalling pathways. SHR5 could be involved directly in plant–bacteria signalling, recognizing molecules produced by bacteria or recognizing bacteria themselves, so it is also important to identify the SHR5 ligands. On the other hand, SHR5 could be involved downstream of a primary bacteria recognition event with its down-regulation being an intermediate step in the signalling pathway that leads to a successful plant–bacteria interaction. Future analysis of intracellular target molecules interacting with SHR5 could help elucidate these signalling mechanisms.
In this work, it has been reported that SHR5 encodes an LRR-RLK that seems to be related to the mediation of the beneficial association between plant and endophytic bacteria. Besides contributing to the understanding of mechanisms underlying endophytic association, this work may also provide tools for future agricultural applications. Genetic handling of genes that can lead to more efficient plant–bacteria associations could result in higher production yields, reduced input costs, and diminished negative environmental consequences due to the use of the excessive addition of N fertilizers.
We are grateful to Leonardo Mega França and Ana Cláudia de Jesus for technical assistance in DNA sequencing and plant culture, respectively. We also thank COPERSUCAR for providing sugarcane genotypes and plant material infected with P. melanocephala and mosaic virus disease and to Gonçalo Apolinário da Silva for providing plant material infected with L. xyli subsp. Xyli. We thank Ana Carolina Andrade for help in finalizing the manuscript and Erika M Korowin (USA) for language editing. FV is indebted to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a graduate fellowship. ASH and PCGF received support from a CNPq research grant. The study was partially supported by the project PronexII/CNPq and PADCT III.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ.
Bachem CW, van der Hoeven RS, de Bruijn SM, Vreugdenhil D, Zabeau M, Visser RG.
Baker B, Zambryski P, Staskawicz B, Dinesh-Cumar SP.
Baldani JI, Caruso L, Baldani VLD, Goi SR, Döbereiner J.
Baldani JI, Pot P, Kirchlaof G, et al.
Baldani VLD, Baldani JI, Olivares FL, Döbereiner J.
Bateman A, Coin L, Durbin R, et al.
Boddey RM, Döbereiner J.
Cavalcante VA, Döbereiner J.
Comstock JC, Lentini RS.
Diévart A, Clark SE.
Endre G, Kereszt A, Devei Z, Mihacea S, Kalo P, Kiss G.
Fuentes-Ramirez LE, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J.
Gillaspie AG, Teakle DS.
Gomez-Gomez L, Felix G. Boller T.
Hanks SK, Quinn AM, Hunter T.
Harrison NA, Davis MJ.
Hendre KR, Iyer RS, Kotwain M, Kluspe SS, Mascarenhas AF.
Hong SW, Jon JH, Kwak JM, Nam HG.
James EK, Olivares FL.
James EK, Reis VM, Olivares FL, Baldani JI, Dobereiner J.
Kanvinde L, Sastry GRK.
Kistner C, Parniske M.
Krusell L, Madsen LH, Sato S, et al.
Kumar S, Tamura K, Jakobsen IB, Nei M.
Kyte J, Doolittle RF.
Lange J, Xie Z, Broughton WJ, Vögeli-Lange R, Boller T.
Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P.
Li J, Lease KA, Tax FE, Walker JC.
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC.
Logeman J, Schell J, Willmitzer L.
Matsubayashi Y, Ogawa M, Morita A, Sakagami Y.
Matsubayashi Y.
Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ.
Nishimura R, Hayashi M, Wu GJ, et al.
Nogueira EM, Vinagre F, Masuda HP, Vargas C, Pádua VLM, Silva FR, Santos RV, Baldani JI, Ferreira PCG, Hemerly AS.
Olivares FL, James EK, Baldani JI, Döbereiner J.
Pastuglia M, Swarup R, Rocher A, Saindrenan P, Roby D, Dumas C, Cock JM.
Purdy LH, Liu LJ, Dean JL.
Radutoiu S, Madsen LH, Madsen EB, et al.
Reinhold-Hurek B, Hurek T.
Reis VM, Olivares FL, Döbereiner J.
Reis VM, de los Santos PE, Tenorio-Salgado S, et al.
Ricaud C, Ryan CC.
Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM.
Sevilla M, Burris RH, Gunapala N, Kennedy C.
Shiu SH, Bleecker AB.
Shiu SH, Bleecker AB.
Shiu SH, Karlowski WM, Pan R, Tzeng Y, Mayer KFX, Li W.
Sikorski MM, Biesiadka J, Kasperska AE, Kopcinska J, Lotocka B, Golinowski W, Legocki AB.
Stracke S, Kistner C, Yoshida S, et al.
Tatusova TA, Madden TL.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG.
Urquiaga S, Cruz HS, Boddey RM.
Vargas C, Pádua VLM, Nogueira EM, Vinagre F, Masuda HP, Silva FR, Baldani JI, Ferreira PCG, Hemerly AS.
Wang Z-Y, Seto H, Fujioka S, Yoshida S, Chory J.
Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, De Bruijn FJ, Stougaard J, Szczyglowski K.




Comments