-
PDF
- Split View
-
Views
-
Cite
Cite
Jafar H Ghithan, Jennifer M Noel, Thomas J Roussel, Maureen A McCall, Bruce W Alphenaar, Sergio B Mendes, Photobleaching reduction in modulated super-resolution microscopy, Microscopy, Volume 70, Issue 3, June 2021, Pages 278–288, https://doi.org/10.1093/jmicro/dfaa062
Close - Share Icon Share
Abstract
Important breakthroughs in far-field imaging techniques have been made since the first demonstrations of stimulated emission depletion (STED) microscopy. To date, the most straightforward and widespread deployment of STED microscopy has used continuous wave (CW) laser beams for both the excitation and depletion of fluorescence emission. A major drawback of the CW STED imaging technique has been photobleaching effects due to the high optical power needed in the depletion beam to reach sub-diffraction resolution. To overcome this hurdle, we have applied a synchronous detection approach based on modulating the excitation laser beam, while keeping the depletion beam at CW operation, and frequency filtering the collected signal with a lock-in amplifier to record solely the super-resolved fluorescence emission. We demonstrate here that such approach allows an important reduction in the optical power of both laser beams that leads to measurable decreases in photobleaching effects in STED microscopy. We report super-resolution images with relatively low powers for both the excitation and depletion beams. In addition, typical unwanted scattering effects and background signal generated from the depletion beam, which invariably arises from mismatches in refractive index in the material composing the sample, are largely reduced by using the modulated STED approach. The capability of acquiring super-resolution images with relatively low power is quite relevant for studying a variety of samples, but particularly important for biological species as exemplified in this work.
Introduction
In far-field optical microscopy, the maximum resolution due to diffraction is on the order of one-half of the light wavelength, or ∼200 nm for visible light [1–4]. A series of innovative strategies have recently been devised to overcome the diffraction limit in far-field imaging [5]. One of those innovative techniques is stimulated emission depletion (STED) microscopy, which was developed by Stefan Hell (Nobel prize winner in chemistry in 2014) and co-workers [6, 7] and has been widely adopted specially for optical imaging in life science investigations [8–14]. The STED imaging technique relies on the reversible excitation and depletion by two overlapping laser beams of an optically active state in a sub-population of species at the nanometer scale. The Airy pattern of an excitation beam is combined with a doughnut-shaped depletion beam that is red-shifted and out of the spectral region of detection. Due to stimulated emission by the depletion beam on the periphery of the Airy pattern, the fluorescence emission becomes constricted to the region around the zero intensity of the doughnut profile. A super-resolved image is then constructed by scanning the sample underneath the beams and sequentially collecting fluorescence signal on a pixel-by-pixel basis.
Implementation of STED microscopy was challenging in the past [15], and several strategies have been developed to facilitate its applications [16, 17]. The most straightforward realization of the STED technique uses continuous-wave (CW) laser beams for the excitation and depletion [18, 19] of the optically active species. In principle, there is no fundamental limit to the spatial resolution obtainable by STED microscopy, which is essentially determined by the extent that the intensity of the depletion beam can be varied over a short distance [20]. For reaching a high spatial resolution (i.e. a tightly confined point spread function), a strong depletion beam is invariably required, which brings several practical difficulties. One of the crucial drawbacks of a strong depletion beam in STED imaging is photobleaching. In addition, a relatively high excitation laser power is generally desirable to maximize the fluorescence emission and improve the image quality, which also increases photobleaching effects. Several approaches have been proposed to reduce photobleaching effects in STED microscopy, such as decreasing the dwell time in the scans, increasing the field of view in STED imaging [21–24] or applying a time-gated approach. In time-gated STED imaging, the reduction of the saturating intensity has led to acquire STED images with higher resolution at lower depletion power [25–27]. Another technical problem that arises from a strong depletion beam is due to its probability to excite other optically active species. This undesirable effect can be reduced by detuning the depletion wavelength far from the excitation band and by using a narrow-band pass filter centered at the fluorescence emission; however, the effect may still be non-negligible for strong depletion beams. Another important point that should be taken into account is the preparation of samples for STED microscopy such as using an embedding medium that matches precisely the refractive index of the supporting substrate because any small mismatch may result in unwanted back-reflected background from the depletion laser beam. A strong depletion power has also been reported to induce optical trapping [20]. Typically, in STED microscopy, only a small number of dyes can be studied by a certain pair of excitation and depletion wavelengths. To allow for the possibility of using a single laser wavelength in the depletion beam to work with multiple fluorophores of different emission spectra, a general concept named modulated STED was proposed by Ronzitti and colleagues [28]. The methodology is based on an intensity modulation of the excitation laser beam combined with synchronous detection of the fluorescence signal with a lock-in amplifier while using a CW depletion beam for reaching super-resolution in far-field imaging. They have demonstrated the ability of the modulated STED methodology to work with a broader range of dyes while deploying the same wavelength for the depletion laser.
In this work, we apply the modulated STED approach to reduce photobleaching effects in STED microscopy by decreasing the undesirable background signal that invariably comes from the application of the depletion beam and by lowering the average power of the applied excitation and depletion laser beams. We report unambiguous experimental data to demonstrate that applying a modulated STED technique can indeed reduce photobleaching effects. In addition, we experimentally determined the point spread function of the modulated STED technique at different power levels and showed its ability to reach super-resolved images at low power levels for both the excitation and depletion laser beams We also showed the ability of the modulated STED to acquire super-resolution images working in a scenario where an embedding medium does not match the index of refraction of a conventional cover glass.
Methods
Setup
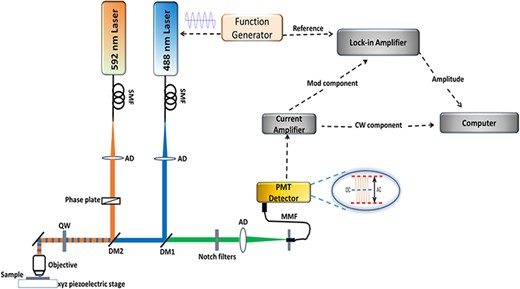
The STED microscope built for this work is shown schematically in Fig. 1 and follows the major features of a previously reported configuration [18]. The excitation laser at 488 nm was delivered by a single spatial mode OBIS laser with a maximum power of 60 mW (488-60 LS, Coherent, Santa Clara, CA, USA). The depletion beam at 592 nm was provided by a CW single spatial mode fiber laser with a maximum power of 1.5 W (MPB Communications, Montreal, Canada). In order to further spatially filter the excitation and depletion beams, each beam was coupled to a polarization-maintaining single-mode fiber (P1-488PM-FC-5, Thorlabs, Newton, NJ, USA) using a 40× microscope objective with a numerical aperture of 0.65 (Newport, Irvine, CA, USA) mounted on a single-mode fiber launch unit (MAX350D, Thorlabs, Newton, NJ, USA). The output of each fiber port was mounted on a four-axis stage, which provided the necessary degrees of freedom to collimate and reach co-linearity among the optical beams. Each beam was collimated using an aberration-corrected lens (PAF-X-18-PC-C, Thorlabs, Newton, NJ, USA) with a focal length of 18.4 mm that was mounted on a five-axis stage. The depletion beam passed through a vortex phase plate (VPP-1, RPC Photonic Inc., Rochester, NY, USA), which was used to generate the characteristic doughnut pattern. The excitation beam was directed toward the microscope objective with a 100× magnification and a numerical aperture of 1.35 (RMS100X-PFOD Olympus, Thorlabs, Newton, NJ, USA) mounted on the optical microscope (BX51M, Olympus, Center Valley, PA, USA) using a dichroic mirror DM1 (ZT488rdc-UF2, Chroma, McHenry, IL, USA). The depletion beam was also directed toward the microscope objective using another dichroic mirror DM2 (z590sp-rdc, Chroma, McHenry, IL, USA).
Experimental setup with the 488- and 592-nm laser lines used for the excitation and depletion sources, respectively. The laser beams were injected into polarization-maintaining single-mode fibers with focusing lenses. The excitation beam was modulated using a function generator. The depletion laser was passed through a vortex phase plate to generate the doughnut shape. Both beams were circularly polarized by a quarter-wave plate. The depletion beam was collinearly aligned with the excitation laser coupled into the microscope imaging path. Laser focusing and fluorescence collection were done through an objective lens (Olympus, 100X, 1.35 NA). Sample was scanned using an XYZ piezoelectrically driven stage. A photomultiplier tube was used for light detection. The detected signal was synchronized to the excitation laser modulation using a lock-in amplifier connected to a computer for data acquisition.
Before being directed to the objective, both the excitation and depletion beams were sent through a quarter-wave plate (AQWP025M-600, Thorlabs, Newton, NJ, USA), which was used to generate symmetrically and circularly polarized beams at the objective back focal plane. In order to ensure the circularity of the polarization, a broad-band metallic mirror has been used to reflect the beams through the objective (21010, Chroma, McHenry, IL, USA). The fluorescence emitted from the sample was collected by the same objective lens and passed through the dichroic mirrors DM1 and DM2. The signals were then spectrally cleaned via notch filters (NF03-488E-25 and NF03-594E-25, Semrock, Rochester, NY, USA) and coupled using an achromatic doublet (AC254-030-A, Thorlabs, Newton, NJ, USA) into a multi-mode optical fiber (M31L02, Thorlabs, Newton, NJ, USA). The opening diameter of the multi-mode optical fiber is ∼80% of the back-projected Airy disk, which served as the confocal aperture.
A photomultiplier tube (H5783, Hamamatsu, Hamamatsu, Japan) that was connected to a low-noise current preamplifier (SR570, Stanford Research Systems, Sunnyvale, CA, USA) was used to detect the photons emitted from the fluorescent sample. For experiments under intensity-modulated excitation, |$\left( {{{{I_{max}} - {I_{min}}} \over {{I_{max}} + {I_{min}}}} = 1} \right)$|, the 488-nm laser was directly modulated by a sinusoidal waveform from a function generator and the detected fluorescence signal was sent to a lock-in amplifier (SR830 DSP, Stanford Research Systems, Sunnyvale, CA, USA) that was tuned to the fundamental modulation frequency of the excitation beam. The amplitude of the modulated signal was sent to a personal computer using a programmable gate array board (BNC 21010, National Instruments, Austin, TX, USA). The images were acquired using a scanning piezoelectric stage (Nano-PDQ350, Mad City Labs Inc., Madison, WI, USA). A home-built LabView code was used to control the stage motion, record the optical signal and build the images. Data were acquired while the stage was set in linear motion, where the dwell time corresponds to the time duration of laser radiation over one single pixel, which is the smallest spatial unit deployed in the image formation.
Fluorophore sample preparation
For validation of the proposed approach, we used samples of carboxylate-modified fluorosphere beads with a typical size of 20 nm, and the beads are labeled with a BODIPY dye. The beads are swollen in organic solvents with a sufficient amount of dye to fill the space around the polystyrene chains. The solvents are then removed and the hydrophobic dye is trapped inside the beads (F8787, Thermo Fisher Scientific, Waltham, MA, USA). The fluorosphere beads feature an absorption peak at 505 nm and a maximum fluorescence emission at 515 nm. Samples with the beads of 20-nm size were prepared according to the following protocol: first the acquired fluorescent solution was sonicated for 15 min, then diluted (∼1:105) in ethanol (SHBD20324, Sigma, Saint Louis, MO, USA). The diluted sample was sonicated again for another 15 min. The fluorescent species were immobilized on microscope glass coverslips (VWR 24 × 60 mm, VWR, Radnor, PA, USA). The coverslip was initially cleaned with ethanol and coated with poly-L-lysine (P8920, Sigma, Saint Louis, MO, USA) by incubating the whole sample for 10 min inside the poly-L-lysine; poly-L-lysine was used as a linker. The coverslip was washed off with deionized water and blown-dried with nitrogen gas. A drop (∼20 μL) of the diluted fluorescent solution was pipetted onto the coverslip and allowed to immobilize onto the surface for about ∼10 min, before washing with deionized water and drying with nitrogen gas. Finally, a drop (∼20 μL) of 2,2`-thiodiethanol (TDE) (88 559, Sigma, Saint Louis, MO, USA), which has an index of refraction of ∼1.521, was added to the sample by drop-casting as an embedding medium. To obtain an embedding medium of TDE that has an index of refraction of ∼1.51, TDE was mixed with phosphate-buffered saline (PBS) with a ratio of 97% (v/v) TDE and then adjusted to a pH of ∼7.5 with HCL/NaOH solutions [29]. The coverslip was then pressed against a microscope slide and sealed with nail polish. To test the ability of the modulated STED technique to obtain super-resolution images even under conditions of substantial mismatch in index of refraction between the cover slip and the embedding medium, a sample of the fluorescent beads was prepared under the same protocol mentioned above, but instead of using TDE we used Vectashield (H-1000-10, Vectorslabs, Burlingame, CA, USA) with an index of refraction of 1.45 as the embedding medium.
Biological sample preparation
Our STED system was tested using retinal slices with fluorescently labeled structures as a biological sample. All experimental procedures involving animals were approved by the University of Louisville Animal Care and Use Committee. Transgenic mice with green fluorescent protein (GFP)-labeled bipolar cells [30] were euthanized using anesthetic overdose, and eyes were removed and retinas dissected in 0.01 M PBS. The retina was fixed by immersion in 4% paraformaldehyde for 20 min and then incubated for an hour each in a series of sucrose solutions in 0.1 M phosphate buffer of increasing concentrations (5%, 10%, 15% and 20%). The retina was then incubated for 1 h in Tissue Tek OCT tissue freezing media (4583, Ted Pella, Inc., Redding, CA, USA) and 20% sucrose (2:1) and flash frozen using liquid nitrogen in the same solution. Frozen blocks were sliced using a cryostat to yield vertical sections of the retina in which the three nuclear layers were all visible (bipolar cells reside in the middle nuclear layer). Cryoslices were dried on coated slides for 30 to 60 min and then incubated in 0.01 M PBS for 10 min followed by incubation in blocking solution (10% normal donkey serum in 0.5% triton X in PBS) for 1 h at room temperature. Slides were incubated in primary antibody diluted 1:1000 in blocking solution overnight at 4°C. Primary antibodies included chicken anti-GFP (Invitrogen, Carlsbad, CA, USA) to label the GFP-positive bipolar cells and mouse anti-C-terminal binding protein 2 (Chemicon, Shinagawa-Ku, Japan), a marker for the ribbon synapse. Slides were washed in PBS (4 × 10 min) and then incubated for 1.5 h at room temperature in secondary antibody diluted 1:1000 in PBS. Secondary antibodies included donkey anti-chicken and donkey anti-mouse conjugated Alexa 488. Slides then were washed in PBS (4 × 10 min) and embedded in TDE medium; to prevent cellular structure damaging due to osmotic shock [29], the exchange of water with TDE was done slowly by incubating the labeled samples in a series of diluted TDE solution with PBS: 10% (v/v) TDE, 25% (v/v) TDE, 50% (v/v) TDE and 97% (v/v) TDE. The samples were incubated for 5 min in each diluted solution of TDE from low to high concentration, and then, the samples were mounted in 97% (v/v) TDE. Finally, slides were covered with glass coverslips (thickness of 170 µm) then sealed with nail polish.
Results and discussion
In this work, all power measurements were taken at the entrance aperture of the microscope objective and the quoted values are averages over time in either the CW or alternating current (AC)-modulated laser beams.
Effects of the excitation beam on the photobleaching rate
We first used our experimental setup under the conventional confocal configuration (thus, no depletion beam in these measurements) and studied how several parameters of the excitation beam affect the photobleaching process. Specifically, we tested the effects of the average excitation power under CW and AC operation, the modulation frequency under AC modulation, and the dwell time of laser radiation.
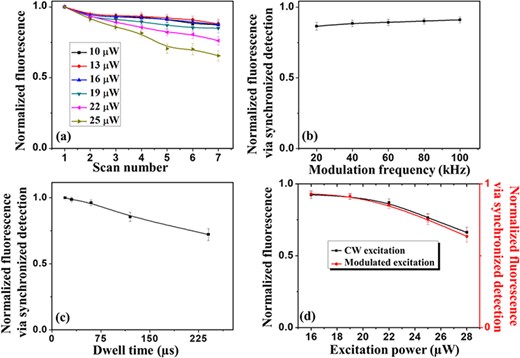
Data shown in Fig. 2(a) describe the influence of the CW excitation power on the photobleaching process on the 20-nm carboxylate beads sample under a dwell time of 75 μs and a pixel size of 15 nm. We found that an excitation power of ∼16 μW and below is sufficiently low to avoid significant photobleaching over several scans (after 7 scans the normalized fluorescence intensity is still above 87% of its initial value at the first scan). However, we also noticed that for excitation powers <16 μW, the images were significantly faded, an observation that will be detailed later in this section. High photobleaching effects were in fact measured when imaging with excitation powers that were significantly higher than 16 μW. One can observe that those results set tight constraints for efficiently operating with a CW excitation beam.
(a) Normalized fluorescence signal as a function of the number of scans for different CW excitation powers in the confocal imaging configuration. Each data point represents the average of normalized intensities of five different beads; for each bead, the normalized intensity was calculated as I_i⁄I_1, where I_1 represents the intensity of a particular bead at the first confocal image and I_i refers to its intensity at the i-th image. For all images at a particular excitation power, the dwell time (75 μs), the pixel size (15 nm) and the scan area were kept constant. For each different excitation power, a different scan spot was adopted. (b) Normalized fluorescent signal in modulated confocal imaging as a function of the modulation frequency at an average power of 16 μW, a dwell time of 100 μs and a pixel size of 15 nm. For each modulation frequency, a different scan spot was adopted. (c) Normalized fluorescent signal in modulated confocal imaging as a function of the dwell time at an average power of 16 μW, a pixel size of 15 nm and a modulation frequency of 95 kHz. For each dwell time, a different scan spot was adopted. (d) Normalized fluorescent signal in both CW confocal and AC-modulated confocal (95 kHz) as a function of the average excitation power at a dwell time of 100 μs and a pixel size of 15 nm. For each average excitation power, a different scan spot was adopted. In (b), (c) and (d), each data point represents the average of normalized intensities of five different fluorescent beads, and for each bead, the normalized fluorescence intensity was calculated as I_5⁄I_1, where I_1 is the intensity measured in the first scan and I_5 is the intensity after the same area was scanned five times.
To test the effects on the photobleaching rate of AC modulation in the excitation beam at different frequencies, an average excitation power of 16 μW was chosen and a bandwidth pass filter was deployed in the current preamplifier to help (the lock-in amplifier) removing any DC signal component present in the collected fluorescent signal. The integration time of the modulated fluorescence signal (set in the lock-in amplifier) and the pixel dwell time (defined through the LabView code) were matched to avoid aliasing effects. The values of the integration time (100 μs), the pixel dwell time (100 μs) and the range in the band pass filter on the current preamplifier (10–300 kHz) were set in accordance to the lowest frequency of modulation deployed for the laser, which was 20 kHz. The experimental results summarized in Fig. 2(b) indicate that changing the modulation frequency while maintaining the average power and dwell time fixed has little impact on the photobleaching rate.
However, these results should not obscure the fact that higher modulation frequencies can afford shorter integration times, which can then decrease the photobleaching rate. These observations are confirmed by the data shown in Fig. 2(c) where the modulation frequency (95 kHz) and average power (16 μW) were fixed, but the dwell time (and the matched lock-in amplifier integration time) was varied. Therefore, it is certainly not surprising that higher frequencies of modulation combined with shorter dwell times tend to minimize photobleaching effects.
Figure 2(d) shows how the average power (for both the CW and AC-modulated beams) affects the photobleaching process while the dwell time (and lock-in amplifier integration time) is kept constant. It can be noticed that there is only a negligible difference on the photobleaching rate of the CW or AC-modulated beams if they have the same average power.
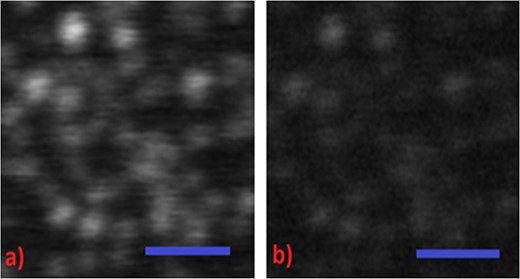
As mentioned previously, the confocal images constructed under CW excitation with power lower than 16 μW (at a dwell time of 75 μs) were faded due to a low signal-to-noise ratio (SNR). To exemplify this point, Fig. 3 compares side-by-side images acquired under CW and AC-modulated laser excitation at an average power of ∼7 μW and a dwell time of 30 μs. As one can clearly observe the modulated excitation laser beam combined with synchronous detection of a lock-in amplifier provides a fluorescence signal that is frequency filtered and amplified resulting in an image of increased SNR compared with the corresponding CW image.
A side-by-side comparison of carboxylate fluorescent beads of 20 nm recorded in the (a) modulated confocal and (b) CW confocal configurations at an average excitation power of 7 μW. Bead images are not well recorded in the CW confocal image, but recorded with significantly better SNR in the modulated confocal recordings. Scale bar in both images is 1 μm. In both images, pixel size and dwell time correspond to 15 nm and 30 μs, respectively.
In summary, as one might expect, confocal imaging with a modulated excitation beam at high modulation frequency, short dwell time and low excitation power can lead to a reduction in the photobleaching rate. In the contrary, CW confocal imaging aimed to minimize photobleaching by decreasing the dwell time and the excitation power tend to diminish the overall fluorescence signal, which typically becomes problematic to discriminate features of low contrast and leads to poorer images.
Effects of applying simultaneously excitation and depletion beams on the photobleaching rate
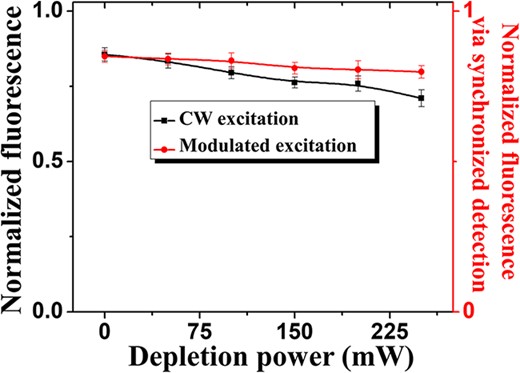
We then investigated how the simultaneous application of the excitation and depletion beams affects the photobleaching rate; this analysis was done for both a CW and an AC-modulated excitation laser beam while the depletion beam was maintained at CW operation. During these measurements, the phase plate, which is responsible for the characteristic doughnut-shaped beam profile in STED microscopy, was intentionally not present in the optical path, and therefore, we had in these measurements the overlap of two TEM00 Gaussian beams (excitation/depletion) at the back focal plane of the microscope objective. The fluorescence intensity from the 20-nm beads was measured as a function of the depletion beam power. Following the results presented in Fig. 2, an average optical power of 16 μW for the 488-nm excitation laser beam was adopted for both the CW and AC-modulated measurements. Two images were collected when only the excitation beam was illuminating the sample. In between the two collected images, a scan that included both the excitation and depletion beams was done without acquiring any imaging data at this step. The data presented in Fig. 4 represent the ratio of the second image (image after the sample had been exposed to the simultaneous illumination by the excitation/depletion beams) to the first image (image before the sample had been exposed to the simultaneous illumination by the excitation/depletion beams). For each bead, the fluorescence signal measured in the second image was normalized with respect to the fluorescence signal obtained in the first image. Fig. 4 shows the fluorescence intensity as a function of the depletion power, where each data point corresponds to the average of the normalized fluorescence signal of five different beads. As shown in Fig. 4, the AC-modulated excitation data shows less photobleaching effects compared with the CW excitation and as expected the photobleaching increases as the depletion power rises. As seen previously in Fig. 2(d), without the presence of the depletion beam, only a negligible difference in photobleaching is observed between the CW and AC-modulated configurations, regardless of the power in the excitation beam. When a depletion beam with a Gaussian profile is overlaid on the excitation beam, photobleaching becomes more severe especially when the excitation laser is at CW operation and the optical power in the depletion beam is high. It has been reported [31] that the population of the excited triplet state drastically changes with the modulation of the excitation beam as it allows for spontaneous relaxation of the excited fluorophore between two excitation pulses and this lead to a reduction in the triplet state population. A higher population in the triplet state enhances the probability of photobleaching [32], especially when a depletion beam of high intensity is applied on an excited fluorophore [33]. Therefore, modulating the excitation light source can be beneficial to mitigate the pile up of the triplet state population and its undesired excitation to further reactive states when a high depletion beam power is applied. Thus, in the case of an AC-modulated excitation, the population in the triplet state affected by the depletion beam is less severe than in the case of using a CW operation in the excitation laser.
Normalized fluorescence intensity under illumination with the excitation beam only, but after the sample had been exposed to two simultaneously overlapping Gaussian beams: one for the excitation and one for the depletion; black curve corresponds to a CW excitation beam, and red curve corresponds to an AC-modulated excitation beam. Two images were collected when only the excitation beam was illuminating the sample. In between the two collected images, a scan that included both the excitation and depletion beams was done without acquiring any imaging data at this step. The normalized fluorescence in the y-axis represents the ratio of the second image (image after the sample had been exposed to the simultaneous illumination by the excitation/depletion beams) to the first image (image before the sample had been exposed to the simultaneous illumination by the excitation/depletion beams). The x-axis describes the power used in the depletion beam applied in between the collected images. Different scan areas were adopted for the CW and modulated excitation approaches. Also, each specific intensity in the depletion beam was performed at a different scan area. For all data points, the dwell time (100 μs)and the pixel size (15 nm) were kept constant.
Efficiency of depleting the fluorescence state with CW and AC-modulated excitation beams
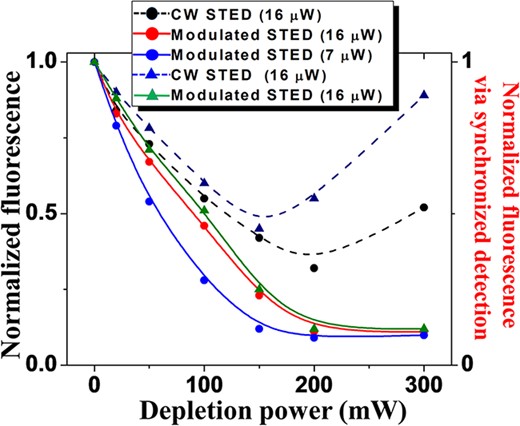
A crucial characteristic for good performance in STED microscopy is the efficiency of the depletion beam to reduce the fluorescent signal created by the excitation laser without generating spurious noise. The fluorescence reduction for different depletion intensities measured on the 20-nm carboxylate-modified beads for the CW and AC-modulated excitation beams were investigated here. In these measurements, samples of diluted beads embedded in either TDE or Vectashield were used. Again, these measurements were done by overlapping in the image plane two (excitation/depletion) TEM00 Gaussian beams and the depletion beam was kept at CW operation. For the AC-modulated excitation, a frequency of 95 kHz was deployed, and a bandwidth pass filter of 30 to 100 kHz was set in the current preamplifier. The fluorescence signal of a single bead irradiated by the excitation light fixed at a particular average power (either 7 or 16 μW) was recorded as the intensity of the depletion beam was varied from low (zero) to high values. Then, the detected fluorescence signal at each depletion power was normalized by the signal obtained when the bead was irradiated with only the excitation beam (zero intensity in the depletion beam). Fig. 5 shows that under CW excitation a substantial background signal was present at high depletion powers, and this background noise becomes even larger when the beads are embedded in a medium (Vectashield) whose refractive index that does not match the one from the cover glass. On the other hand, also shown in Fig. 5, when using the AC-modulated excitation scheme to image beads embedded in both TDE and Vectashield media, the background generated by the depletion beam was largely eliminated and, as desired, the fluorescence signal decreased as we increased the power of the depletion beam. It is worth mentioning that under CW excitation at lower power (∼7 μW), we could not properly record a fluorescence signal (data/image not shown here). However, in the AC-modulated excitation with an average power of 7 μW, the fluorescence signal was well recorded, and it featured the characteristic monotonic signal reduction as the depletion beam was increased. The results for the AC-modulated excitation in Fig. 5 show that at an average excitation power of 7 μW requires a depletion beam of ∼150 mW to quench the fluorescence signal down to 12% of its initial value, while an average excitation power of 16 μW needs a depletion beam of ∼300 mW for the same level of quenching. It is well known that the depletion beam is more effective at lower excitation powers and the AC modulation in the excitation beam allows us to acquire images with lower power in both the excitation and depletion beams, which will synergistically contribute to reduce photobleaching effects.
Fluorescence reduction for different intensities of the depletion beam measured on the 20-nm carboxylate-modified bead samples with both the AC-modulated excitation (solid) and CW excitation (dashed) configurations at two different average excitation powers (7 μW and 16 μW) and two different embedding media: TDE (circles) and Vectashield (triangles).
Super-resolution imaging with CW STED and AC-modulated STED configurations
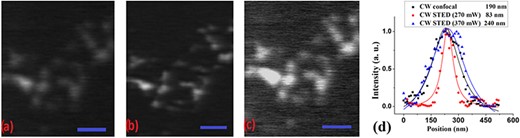
For the measurements discussed below, we had inserted the vortex phase plate in the optical path of the depletion beam for creating the characteristic doughnut-shaped profile. First, we compared our setup operating under the conventional CW confocal configuration (where only a CW excitation beam is present) and the CW STED configuration (where a CW excitation beam is combined with a CW depletion beam). We utilized the 20-nm carboxylate-modified fluorospheres samples embedded in TDE. The excitation wavelength for both configurations was at 488 nm. For the CW STED configuration, a depletion beam at the wavelength of 592 nm was deployed. The dwell time was set to 30 μs, and the pixel size was 15 nm. The power measured at the entrance aperture of the microscope objective lens was 16 μW for the excitation beam and 270 mW for the depletion beam. Image Pro Plus and Origin software were used for handling the acquired images. Resolution estimation was performed based on statistical measurements of the full-width at half-maximum of a Lorentzian fitted curve of the intensity profile for more than five different single particles of each image. The CW confocal image is shown in Fig. 6(a), which is to be compared with the CW STED image shown in Fig. 6(b). As shown in Fig. 6(d), the CW confocal imaging showed an estimated resolution of ∼190 nm (within the diffraction limit, as expected). Same statistical resolution estimation was carried out for the CW STED measurements, and the estimated average size of the imaged single beads was 83 nm. Undesirable background effects (as previously discussed) became relevant when we increased the depletion power toward higher levels in an attempt to improve (reduce) the point spread function as shown in Fig. 6(c) for a depletion power of 370 mW.
A side-by-side comparison of carboxylate-modified fluorescent beads recorded in the (a) CW confocal and (b) CW STED configurations. Closely spaced beads are not well resolved in the CW confocal image, but are better discernible in the CW STED recordings. (c) As previously observed background effects appeared in the CW STED configuration when we increased the depletion power to 370 mW. Scale bar in all images is 1 μm. The normalized intensity profiles in (d) show that the spatial resolution measured approximately 190 nm for the CW confocal image, and for the CW STED image, it was determined to be |$\sim$|83 nm at depletion power of 270 mW and 240 nm at depletion power 370 m.
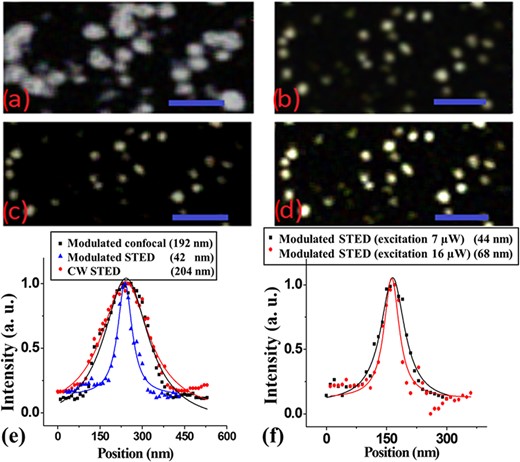
Next, a modulated STED technique (where an AC-modulated excitation beam is combined with a CW depletion beam) was applied to similar samples of the 20-nm carboxylate-modified fluorescent beads embedded in TDE medium. The average power for the excitation beam was kept at 16 μW while the depletion beam power was increased to 410 mW (as opposed to the previously 270 and 370 mW). The excitation beam was modulated at 95 kHz while the depletion beam was kept at CW operation. A bandwidth pass filter of 30 to 100 kHz was set in the current preamplifier to remove any DC signal component from the collected fluorescent signal before being sent to the lock-in amplifier. As previously mentioned in Effects of the excitation beam on the photobleaching rate section, the integration time in the lock-in amplifier was set to be longer than the period of the modulation frequency, while the lock-in amplifier integration time and the pixel dwell time were matched. As before, the dwell time was set to 30 μs with a pixel size of 15 nm. For comparison, a confocal image obtained under modulation of the excitation beam (without the presence of the depletion beam) was collected and shown in Fig. 7(a) as modulated confocal image. Fig. 7(b) shows image of the modulated STED configuration with imaging parameters as described above. The average intensity profile of five different beads showed an approximate spatial resolution for the modulated confocal of 192 nm as shown in Fig. 7(e), which is similar and consistent with the diffraction-limited results described in Fig. 6(d). However, the modulated STED image (Fig. 7(b)) showed a point spread function of ∼42 nm (Fig. 7(e)), which represents a 2-fold reduction in the point spread function when compared to the best CW STED data (Fig. 6(d)) and an approximately 5-fold improvement when confronted with either the CW confocal (Fig. 6(d)) or modulated confocal (Fig. 7(e)) techniques. It is worth reiterating that when operating the depletion beam at higher power in the CW STED technique (e.g. 410 mW instead of 270 mW) the point spread function actually increased and became worst, as noticed in Fig. 6(c) compared with Fig. 6(b), which was caused by fluorophore excitation with the depletion beam or even back-scattered light of the depletion beam from the sample under investigation. Because in modulated STED those detrimental effects from the CW depletion beam are eliminated through a frequency filtering process a higher spatial resolution could be achieved. Also, as noticed in the images shown in Fig. 3, by using synchronous detection in the modulated configuration the SNR can be improved, which allows for imaging at relatively low excitation power. A lower excitation power was tested under the modulated STED imaging. Fig. 7(c) shows the modulated STED image for the same sample of carboxylate beads, except that the average excitation beam power was set to be 7 μW (instead of the previously 16 μW) and the depletion beam power was lowered to 310 mW (lower than the 410-mW previously used). The lower power in the modulated STED scan shows a statistical measured point spread function of 44 nm, which is approximately the same size as that observed in Fig. 7(b) using higher excitation and depletion beam powers. In addition, our results in Fig. 5 showed that the efficiency of the fluorescence depletion is higher under modulated STED configuration with relatively lower excitation power. Fig. 7(f) shows that while imaging under the modulated STED configuration at lower excitation power (Fig. 7(c)), the estimated spatial resolution is lower than the modulated STED imaging at higher excitation power (Fig. 7(d)). Clearly, the modulated STED technique allows high resolution images to be achieved using lower power levels as compared to the CW STED methodology; those results are consistent with the conclusions and results presented in Fig. 5. As described earlier, the ability to acquire super-resolution images at low powers of the excitation and depletion beams has a direct consequence in decreasing the photobleaching effects in STED imaging.
Images for (a) modulated confocal at an excitation power of 16 μW, (b) modulated STED at an average excitation power of 16 μW and depletion power of 410 mW, (c) modulated STED at an average excitation power of 7 μW and depletion power of 310 mW and (d) modulated STED image at an excitation power of 16 μW and depletion power of 310 mW. The sample consisted of carboxylate-modified 20-nm beads, and the same sample region was imaged by the different approaches. Scale bar is 500 nm for all images. (e) Normalized intensity profiles of the same bead in three imaging configurations described in (a), (b) and for CW STED (image is not shown). The CW STED at a high power level shows degradation in the spatial resolution compared with the results of the confocal image. On the other hand, the spatial resolution measured for the modulated STED image at (b) was approximately 42 nm and that for the modulated confocal was |$\sim$|192 nm, at excitation power of 16 μW and depletion power of 410 mW. (f) Normalized intensity profiles of the same bead in the two images in (c) and (d). The spatial resolution measured for the modulated STED image at excitation power of 7 μW and STED power of 310 mW was approximately 44 nm and that for the modulated STED image at excitation power of 16 μW and STED power of 310 mW was approximately 68 nm. The full-width at half-maximum shown in the profiles was estimated based on a fitted Lorentzian curve.
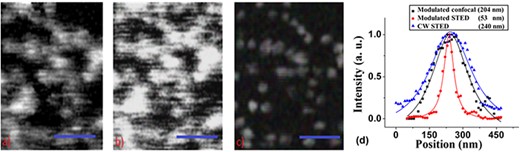
Figure 8 show images for comparison of modulated confocal, CW and AC-modulated STED images of the 20-nm carboxylate-modified fluorospheres when embedded in a medium (Vectashield) that creates a mismatch in refractive index against the sample under investigation. All parameters (pixel size, dwell time, modulation frequency) were the same as previously described. The average excitation power and the depletion power were 16 μW and 350 mW, respectively. Super-resolution STED imaging on this type of samples is extremely difficulty in the CW STED configuration, as illustrated in Fig. 8(b). On the other hand, as shown in Fig. 8(c), our results show that even under such adverse conditions, the AC-modulated STED is still able to overcome those effects that are present due to mismatch in the refractive index of the sample and the embedding medium. It is important to note that under modulated STED condition the back scattered light is still present and non-negligible, but the synchronous detection approach is able to filter out the undesirable background and only record the desired signal.
Comparison of the (a) AC-modulated confocal and (b) CW STED images for the 20-nm carboxylate-modified fluorospheres embedded in a medium (Vectashield) that creates a significant refractive-index contrast. (c) Modulated STED image for the same sample and area as in (a) and (b). Scale bar is 500 nm in all images. (d) The normalized intensity profiles of the same particles in the modulated STED image, CW STED and its modulated confocal counterpart. The estimated average spatial resolution measured was |$\sim$|190 nm for the modulated confocal image, |$\sim$|53 nm for the modulated STED image and 240 nm in CW STED due to background effects.
Super-resolution imaging of biological samples with modulated STED
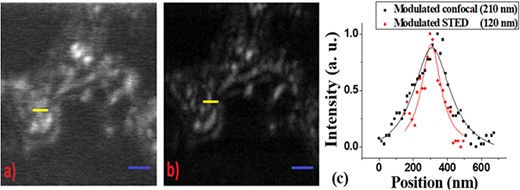
To demonstrate the benefits of modulated STED for imaging biological samples, we present in Fig. 9 a side-by-side comparison of modulated confocal and modulated STED pictures of retinal slices that were fluorescently labeled with Alexa 488. The samples were embedded in TDE medium. For these results the average power measured in the entrance aperture of the objective lens was between 2.0 and 2.5 µW for the excitation beam at 488 nm and 95 mW for the depletion beam at 592 nm. The pixel dwell-time was 30 µs, the pixel size was 15 nm, and a modulation frequency of 85 kHz was deployed for the excitation beam. The data show a clear improvement in optical resolution and in signal-to-background ratio using the modulated STED technique. Intensity line profiles of the smallest features in modulated STED images showed a full-width at half-maximum of ∼120 nm, as compared to the diffraction-limit resolution of ∼210 nm for the modulated confocal. Antibody labeling those biological samples does not fill the cells completely and results in splotchy labels [34]; however, those spots are clearly resolved in modulated STED image Fig. 9(b).
Imaging of retina bipolar cells (Gus-GFP-PNA) labeled with Alexa 488; the images show the bipolar cell bodies. Image from modulated confocal approach is shown in (a) and that from modulated STED is shown in (b). Spatial resolution of the smallest features is presented in (c), where the black trace corresponds to the modulated confocal image and the red trace for the modulated STED. The modulated STED image demonstrates distinguishable particles that cannot be resolved by the modulated confocal imaging. Excitation power used was |$\sim$|2.0 to 2.5 μW, and depletion power was 95 mW. Pixel dwell time was set at 30 μs. Scale bars in both images are 1 μm.
Conclusions
An important embodiment of STED microscopy is based on applying a strong CW optical depletion beam to shrink the point spread function created by the Airy pattern of a CW excitation beam. To reach super-resolved images in this CW STED imaging technique, a strong power in the depletion beam is required. Such high power can cause undesirable background signals and damaging effects on many fluorophores in general and especially on biological samples. We demonstrated here that by applying an AC-modulated excitation beam in the STED technique, the fluorescence signal of interest, which is filtered in the frequency domain by means of synchronous detection, is free of many undesirable background effects and can be detected with high fidelity at much lower levels. A direct consequence of such approach is that the average power in both the AC-modulated excitation beam and the CW depletion beam can be substantially diminished without negative impact on the image quality. We demonstrated here that spatial resolutions consistent with conventional STED microscope [19] can be achieved by deploying relatively lower power levels for both the excitation and depletion beams. Those lower power levels under the AC-modulated STED technique have a direct benefit in reducing photobleaching effects. We experimentally showed that the photobleaching rate in STED imaging is decreased by modulating the excitation beam, and this photobleaching reduction in the AC-modulated STED configuration compared with the CW STED technique was shown to be substantial at higher levels of depletion irradiance. In addition, we have demonstrated the usefulness of the modulated STED technique in reducing the applied laser power for imaging sensitive biological samples. Typically, biological samples may feature unwanted back-scattered background from a strong depletion beam under conventional CW STED imaging due to local mismatches in refractive index. The modulated STED technique was shown to be helpful in overcoming those difficulties as well. Despite rapid advances and availability of high-power lasers, their costs are still high. Therefore, the ability to reach high spatial resolutions with relatively low power levels is certainly of great interest. The lower laser intensity in modulated STED opens the possibility for new STED microscopy implementations based on a modulated laser source that reduces complexity and costs.
Funding
This work was supported by National Science Foundation under grant award NSF MRI 1126279.
Disclosures
Authors declare no financial interest in the manuscript.