-
PDF
- Split View
-
Views
-
Cite
Cite
S. Fowler, A. Webber, B. S. Cooper, A. Phimister, K. Price, Y. Carter, C. C. Kibbler, A. J. H. Simpson, S. P. Stone, Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series, Journal of Antimicrobial Chemotherapy, Volume 59, Issue 5, May 2007, Pages 990–995, https://doi.org/10.1093/jac/dkm014
Close - Share Icon Share
Abstract
To investigate the effect of reinforcing a narrow-spectrum antibiotic policy on antibiotic prescription and Clostridium difficile infection (CDI) rates by feedback of antibiotic use to doctors, as part of a departmental audit and feedback programme.
A prospective controlled interrupted time-series (ITS) study, with pre-defined pre- and post-intervention periods, each of 21 months.
Three acute medical wards for elderly people in a teaching hospital.
Six thousand one hundred and twenty-nine consecutive unselected acute medical admissions aged ≥80 years.
A ‘narrow-spectrum’ antibiotic policy (reinforced by an established programme of audit and feedback of antibiotic usage and CDI rates) was introduced, following an unplanned rise in amoxicillin/clavulanate (Augmentin) use. It targeted broad-spectrum antibiotics for reduction (cephalosporins and amoxicillin/clavulanate) and narrow-spectrum antibiotics for increase (benzyl penicillin, amoxicillin and trimethoprim). Changes in the use of targeted antibiotics (intervention group) were compared with those of untargeted antibiotics (control group) using segmented regression analysis. Changes in CDI rates were examined by the Poisson regression model. Methicillin-resistant Staphylococcus aureus (MRSA) acquisition rates acted as an additional control.
There was a reduction in the use of all targeted broad-spectrum antibiotics and an increase in all targeted narrow-spectrum antibiotics, statistically significant for sudden change and/or linear trend. All other antibiotic use remained unchanged. CDI rates fell with incidence rate ratios of 0.35 (0.17, 0.73) (P = 0.009). MRSA incidence did not change [0.79 (0.49, 1.28); P = 0.32].
This is the first controlled prospective ITS study to use feedback to reinforce antibiotic policy and reduce CDI. Multicentre ITS or cluster randomized trials of this and other methods need to be undertaken to establish the most effective means of optimizing antibiotic use and reducing CDI.
Introduction
Clostridium difficile infection (CDI) is an increasingly common healthcare-associated infection, affecting primarily elderly patients, and is subject to mandatory surveillance in the UK.1–3 It is a consequence of rising broad-spectrum antibiotic use, such as third-generation cephalosporins and amoxicillin/clavulanate.1 National guidelines recommend narrow-spectrum antibiotic policies1–3 and suggest a variety of methods to ensure these are implemented, commenting that ‘despite the apparent increased awareness of CDI and its link with antibiotic use, levels of infection are still rising and prescribing behaviour needs to be addressed’.1 The Health Care Commission surveyed all NHS Trusts in 2005 and reported that 38% did not restrict broad-spectrum antibiotic use.4 Systematic review of interventions to improve prescribing,5 including those that examine the effect on CDI6 showed that nearly all were poor-quality unplanned studies with no control groups or outcomes and inadequate statistical analysis. Well-designed interrupted time-series (ITS) studies with control outcomes would provide much stronger evidence of the effectiveness of interventions, facilitate synthesis of evidence from different studies and help prioritize interventions for definitive multicentre randomized controlled trials (RCTs).7,8 Systematic reviews9,10 suggest feedback may change healthcare workers' implementation of evidence-based guidelines, although feedback is not mentioned in national guidelines as a way of ‘addressing prescribing behaviour’.1 We have previously described the use of feedback in this context11 and now report a subsequent prospective controlled interrupted time-series study, arising from our department's clinical audit programme, which investigated the effect of reinforcing a narrow-spectrum antibiotic policy by feeding back the use of antibiotics and CDI rates to doctors working on acute-care medical wards for the elderly where CDI was endemic.
Materials and methods
Setting
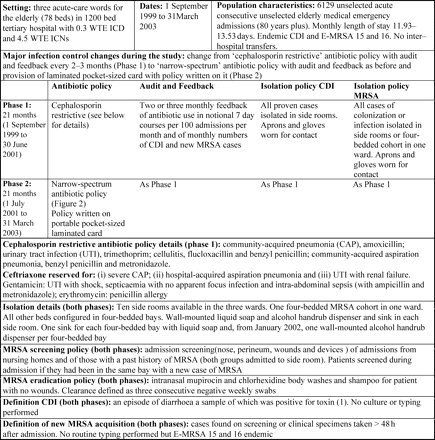
Three acute-care wards for the elderly (78 beds), admitting consecutive, unselected general medical emergency admissions over the age of 80 years, where a cephalosporin restrictive antibiotic policy with audit and feedback of antibiotic use and CDI rates was in place to reduce levels of CDI.11
Population, infection control policies and resources and case definitions
Figure 1 gives details of these throughout the study, during which only the antibiotic policies (Figure 2) changed.
Population, clinical setting, nature and timing of antibiotic prescribing and infection control interventions. ICD, infection control doctor; ICN, infection control nurse; WTE, whole time equivalent.
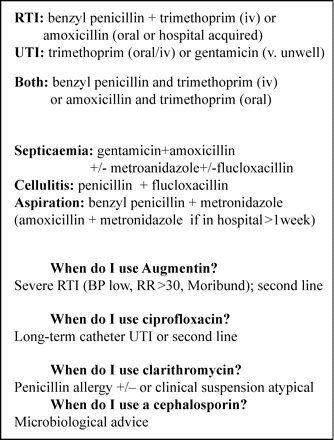
Narrow-spectrum antibiotic policy pocket card text. These details were given to all doctors on a laminated card, the first side of which gave the indications for treatment of different antibiotics and the reverse side gave doses. iv, intravenous.
Rationale for the study
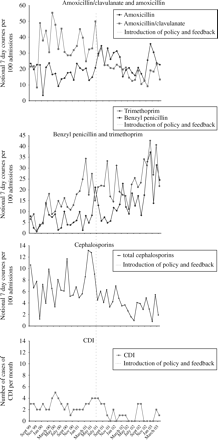
From September 1999, use of amoxicillin/clavulanate (an antibiotic not in the department's antibiotic policy) had begun and became commonplace (Figure 3). This was an unplanned change in prescribing practice. The CDI rate had not increased, but in order to prevent a future rise, it was decided, in April 2001, upon routine review of the data in the audit programme, to introduce a new ‘narrow-spectrum’ antibiotic policy starting in July 2001, without knowing the data from April, May and June 2001, by which time 21 months of data would have been collected, and to evaluate its effect at the end of March 2003, after an equal period of time (21 months) had elapsed.
Monthly antibiotic use (notional 7 day courses per 100 admissions) and total monthly. CDI infections before and after the intervention (July 2001).
Intervention
The new policy (Figure 2) recommended less use of amoxicillin/clavulanate, increased use of benzyl penicillin, trimethoprim and amoxicillin and further restricting cephalosporin use. There were also prompts for the use of metronidazole, ciprofloxacin and clarithromycin, but these were not targeted for any particular change, nor were flucloxacillin, gentamicin or teicoplanin. Doctors were given a laminated pocket version of the policy to carry and continued to receive feedback, every 8–12 weeks, of individual antibiotic usage (the number of notional 7 day courses per 100 admissions per month) together with CDI rates (cases per month) and methicillin-resistant Staphylococcus aureus (MRSA) cases (per month). Antibiotic usage was fed back in this format, rather than in defined daily doses per 1000 bed days, to help doctors visualize the percentage of patients treated with individual antibiotics.
Outcomes
The primary outcome was changes in the level and linear trend of prescribing of targeted antibiotics. Changes in untargeted antibiotic use acted as an additional control, strengthening the experimental design.7,8 The secondary outcome was monthly counts of CDI, adjusted for numbers of admissions. MRSA count data (new cases) acted as an additional control, as it was thought unlikely that this would change due to the intervention.
Adverse effects
Monthly crude, but not infection specific, mortality was recorded.
Potential confounders
Data were collected on the length of stay, alcohol hand-rub use and numbers of admissions, but not on bed-occupancy, staffing levels, agency rates, nor on numbers of inter-ward transfers, admissions from nursing homes or patients admitted with CDI. Case mix and laboratory methods for processing specimens were unaltered. In November 2000, a ward was reopened, explaining the rise in admissions.
Data collection
Data collection was coordinated by the department's registrars, using routinely collected pharmacy, microbiology, trust, supplies and departmental death audit data, as part of their routine audit responsibility.
Analysis
The effect of the intervention was assessed using a segmented regression analysis, as described elsewhere,5,7,12,13 to estimate changes between the pre-intervention (September 1999 to June 2001) and intervention phases (July 2001 to March 2003), accounting for both sudden step-wise changes in the level of antibiotic usage and changes in linear trends of antibiotic usage. Residuals of the fitted models indicated substantial heteroskedasticity but no autocorrelation. To obtain robust variance estimates, we therefore used the Huber–White sandwich estimator. The time series of count data corresponding to CDI and new MRSA acquisitions were analysed by the Poisson regression model to estimate the incidence rate ratio (IRR) associated with the intervention, adjusting for changing numbers of admissions [if λj is the expected number of acquisitions in month j, and ln(λj) = β0+β1x1+ln(Aj), where x1 is an the indicator variable taking the value of 1 in the second phase and zero in the first, Aj represents the exposure given by the number of admissions in month j and β1 are the estimated coefficients of the regression model, then the IRR associated with the intervention is given by exp(β1)]. Exploratory analysis of the count data with models including exponential trends was also performed. To account for the autocorrelation seen in these time series, we used weighted empirical adaptive variance estimators.14 We considered P values ≤0.05 to be statistically significant. Analysis was performed in Stata 815 and R 1.7.1.16
Ethical considerations
This was an audit study and was part of the department's clinical governance programme. It used anonymized confidential routinely collected data, and the new antibiotic policy was discussed with colleagues in the Public Health Laboratory Service and the Royal Free NHS Trust's Infection Control Committee. The policy of our institution is that ethical approval is not required for audit projects.
Results
Table 1 shows a reduction in the use of antibiotics targeted for reduction (amoxicillin/clavulanate and cephalosporins), significant for both sudden change in level and continuing long-term linear trend for decreased use. In those targeted for increased use, there was a significant increase in the long-term trend for benzyl penicillin and in sudden change for amoxicillin, which was not reversed long term. For trimethoprim there was no evidence of change, although there was a marked trend for increased use before the intervention, which continued after the intervention. Non-targeted antibiotics acted as a control and showed no evidence of changes in either trend or level post-intervention.
Changes in antibiotic use after the intervention assessed using a statistical model allowing for both linear trend and level to change after the intervention antibiotic
| Antibiotic . | Baseline levela . | Initial trendb . | P value . | Change in level after the interventiona . | P value . | Change in trend after the interventionb . | P value . |
|---|---|---|---|---|---|---|---|
| Antibiotics targeted for decreased use | |||||||
| cephalosporins | 5.63 (2.70, 8.55) | 0.15 (−0.08, 0.37) | 0.193 | −3.61 (−6.47, −0.76) | 0.015 | −0.28 (−0.52, −0.03) | 0.030 |
| amoxicillin/clavulanate | 31.03 (18.57, 43.48) | 0.36 (−0.47, 1.18) | 0.384 | −13.10 (−21.87,−4.32) | 0.004 | −0.98 (−1.89, −0.07) | 0.035 |
| Antibiotics targeted for increased use | |||||||
| benzyl penicillin | 4.00 (−0.01, 8.00) | 0.22 (−0.16, 0.60) | 0.243 | −4.75 (−11.66, 2.17) | 0.173 | 0.83 (0.19, 1.47) | 0.012 |
| trimethoprim | 3.47 (−0.86, 7.80) | 0.92 (0.51, 1.34) | <0.001 | −5.85 (−14.49, 2.78) | 0.178 | −0.32 (−1.03, 0.38) | 0.361 |
| amoxicillin | 18.61 (13.20, 24.02) | −0.08 (−0.43, 0.26) | 0.632 | 11.21 (5.21, 17.20) | 0.001 | −0.24 (−0.82, 0.33) | 0.397 |
| Other (untargeted) antibiotics | 44.55 (35.66, 53.44) | 0.45 (−0.23, 1.14) | 0.188 | −3.08 (−16.13, 9.97) | 0.636 | −0.43 (−1.46, 0.60) | 0.405 |
| Antibiotic . | Baseline levela . | Initial trendb . | P value . | Change in level after the interventiona . | P value . | Change in trend after the interventionb . | P value . |
|---|---|---|---|---|---|---|---|
| Antibiotics targeted for decreased use | |||||||
| cephalosporins | 5.63 (2.70, 8.55) | 0.15 (−0.08, 0.37) | 0.193 | −3.61 (−6.47, −0.76) | 0.015 | −0.28 (−0.52, −0.03) | 0.030 |
| amoxicillin/clavulanate | 31.03 (18.57, 43.48) | 0.36 (−0.47, 1.18) | 0.384 | −13.10 (−21.87,−4.32) | 0.004 | −0.98 (−1.89, −0.07) | 0.035 |
| Antibiotics targeted for increased use | |||||||
| benzyl penicillin | 4.00 (−0.01, 8.00) | 0.22 (−0.16, 0.60) | 0.243 | −4.75 (−11.66, 2.17) | 0.173 | 0.83 (0.19, 1.47) | 0.012 |
| trimethoprim | 3.47 (−0.86, 7.80) | 0.92 (0.51, 1.34) | <0.001 | −5.85 (−14.49, 2.78) | 0.178 | −0.32 (−1.03, 0.38) | 0.361 |
| amoxicillin | 18.61 (13.20, 24.02) | −0.08 (−0.43, 0.26) | 0.632 | 11.21 (5.21, 17.20) | 0.001 | −0.24 (−0.82, 0.33) | 0.397 |
| Other (untargeted) antibiotics | 44.55 (35.66, 53.44) | 0.45 (−0.23, 1.14) | 0.188 | −3.08 (−16.13, 9.97) | 0.636 | −0.43 (−1.46, 0.60) | 0.405 |
95% confidence intervals are given in parentheses.
aIn units of 7 day courses per 100 admissions.
bIn units of 7 day courses per 100 admissions per month.
Changes in antibiotic use after the intervention assessed using a statistical model allowing for both linear trend and level to change after the intervention antibiotic
| Antibiotic . | Baseline levela . | Initial trendb . | P value . | Change in level after the interventiona . | P value . | Change in trend after the interventionb . | P value . |
|---|---|---|---|---|---|---|---|
| Antibiotics targeted for decreased use | |||||||
| cephalosporins | 5.63 (2.70, 8.55) | 0.15 (−0.08, 0.37) | 0.193 | −3.61 (−6.47, −0.76) | 0.015 | −0.28 (−0.52, −0.03) | 0.030 |
| amoxicillin/clavulanate | 31.03 (18.57, 43.48) | 0.36 (−0.47, 1.18) | 0.384 | −13.10 (−21.87,−4.32) | 0.004 | −0.98 (−1.89, −0.07) | 0.035 |
| Antibiotics targeted for increased use | |||||||
| benzyl penicillin | 4.00 (−0.01, 8.00) | 0.22 (−0.16, 0.60) | 0.243 | −4.75 (−11.66, 2.17) | 0.173 | 0.83 (0.19, 1.47) | 0.012 |
| trimethoprim | 3.47 (−0.86, 7.80) | 0.92 (0.51, 1.34) | <0.001 | −5.85 (−14.49, 2.78) | 0.178 | −0.32 (−1.03, 0.38) | 0.361 |
| amoxicillin | 18.61 (13.20, 24.02) | −0.08 (−0.43, 0.26) | 0.632 | 11.21 (5.21, 17.20) | 0.001 | −0.24 (−0.82, 0.33) | 0.397 |
| Other (untargeted) antibiotics | 44.55 (35.66, 53.44) | 0.45 (−0.23, 1.14) | 0.188 | −3.08 (−16.13, 9.97) | 0.636 | −0.43 (−1.46, 0.60) | 0.405 |
| Antibiotic . | Baseline levela . | Initial trendb . | P value . | Change in level after the interventiona . | P value . | Change in trend after the interventionb . | P value . |
|---|---|---|---|---|---|---|---|
| Antibiotics targeted for decreased use | |||||||
| cephalosporins | 5.63 (2.70, 8.55) | 0.15 (−0.08, 0.37) | 0.193 | −3.61 (−6.47, −0.76) | 0.015 | −0.28 (−0.52, −0.03) | 0.030 |
| amoxicillin/clavulanate | 31.03 (18.57, 43.48) | 0.36 (−0.47, 1.18) | 0.384 | −13.10 (−21.87,−4.32) | 0.004 | −0.98 (−1.89, −0.07) | 0.035 |
| Antibiotics targeted for increased use | |||||||
| benzyl penicillin | 4.00 (−0.01, 8.00) | 0.22 (−0.16, 0.60) | 0.243 | −4.75 (−11.66, 2.17) | 0.173 | 0.83 (0.19, 1.47) | 0.012 |
| trimethoprim | 3.47 (−0.86, 7.80) | 0.92 (0.51, 1.34) | <0.001 | −5.85 (−14.49, 2.78) | 0.178 | −0.32 (−1.03, 0.38) | 0.361 |
| amoxicillin | 18.61 (13.20, 24.02) | −0.08 (−0.43, 0.26) | 0.632 | 11.21 (5.21, 17.20) | 0.001 | −0.24 (−0.82, 0.33) | 0.397 |
| Other (untargeted) antibiotics | 44.55 (35.66, 53.44) | 0.45 (−0.23, 1.14) | 0.188 | −3.08 (−16.13, 9.97) | 0.636 | −0.43 (−1.46, 0.60) | 0.405 |
95% confidence intervals are given in parentheses.
aIn units of 7 day courses per 100 admissions.
bIn units of 7 day courses per 100 admissions per month.
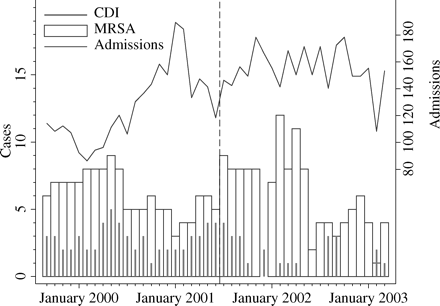
The Poisson regression showed a significant fall in CDI associated with the intervention [IRR 0.35 (0.17, 0.73), P = 0.009], but not in MRSA (control outcome) [0.79 (0.49, 1.28); P = 0.32] (Figures 3 and 4). Further modelling, which allowed for exponential trends in the MRSA and CDI incidence, again showed a significant effect on CDI (the best fitting model showed a significant decreasing exponential trend in CDI following the intervention, but no trend before). In contrast, there was no decrease in level or trend for MRSA following the intervention (the best fitting model showed a significant decreasing exponential trend throughout the study, with a sudden step-wise increase immediately following the intervention).
Monthly count data for CDI, new cases of MRSA and numbers of admissions pre- and post-intervention (July 2001).
Crude mortality was unaltered, fluctuating randomly between 4.7% and 21% each month, with a pre- and post-intervention mean (median) of 14.6% (14.75%) and 13.6% (14.3%) each month. The length of stay fluctuated randomly between 11.93 and 13.53 days each month.
Discussion
The main findings of this study were that introduction of a narrow-spectrum antibiotic policy, reinforced by feedback, was associated with significant changes in targeted antibiotics and a significant reduction in CDI. This is the first planned prospective controlled ITS to evaluate an intervention to change antibiotic prescribing and the first to use feedback. It had pre-specified outcomes, a pre-defined endpoint, sufficient time points to allow trend analysis, appropriate statistical analyses and consideration of common potential confounders. In particular, the use of control outcomes unlikely to be affected by the intervention, in addition to the pre-intervention ‘control’ phase, increases the strength of the evidence by protecting the study against the major threats to the validity of uncontrolled ITS designs.13,17,18 This is because most other changes (such as length of stay, case mix, seasonality, bed-occupancy, staffing levels or use of agency staff), apart from the intervention, which could have caused observed changes in the outcome measures, would also have been expected to affect the control outcomes. This controlled ITS design is therefore considered a strong quasi-experimental design,6–8,13,17,18 especially for evaluating interventions to change antibiotic use.13 The statistical methods used and the fact that the intervention was part of a planned study and not a response to unusually high infection or antibiotic usage rates protect the study against regression to mean effects, common in ITS studies.6,8,17–19 The study design overcomes most weaknesses described in systematic reviews of interventions to improve antibiotic usage4 or to reduce levels of nosocomial infection.6,8,19–21 Its reporting is compliant with, and intended to be an exemplar of, the consensus-agreed CONSORT equivalent for infection control studies, the ORION statement (Guidelines for Transparent Reporting of Outbreak Reports and Intervention Studies of Nosocomial Infection).21
Although the use of additional controls excludes most potential confounders as plausible alternative explanations of the fall in CDI, it does not exclude others, in particular, the numbers of patients admitted with CDI from the community. This could not be assessed, as routine microbiological data collection did not allow differentiation of community- and hospital-acquired CDI. However, CDI is considered largely nosocomial,1 and we were unaware of cases admitted with CDI unattributable to a previous admission. It was always possible to isolate cases of CDI, so an excess of unisolated cases in the pre-intervention cannot provide an alternative explanation for its fall in the post-intervention phase. Although we had access to alcohol hand-rub usage, which fluctuated at 4.5–6 mL per patient-bed-day throughout the study, we had no data on liquid soap usage, which would be more likely to affect CDI rates, as handwashing with soap and water removes C. difficile spores more effectively.22 However we do not think it likely that soap use would have risen sufficiently to effect such a change in CDI rates. The study only examined crude mortality, because of the lack of resources to examine infection-specific mortality such as that due to respiratory and urinary infections. Blinded assessment of this from notes review is hard to organize and depends on the quality of documentation. However, departmental monthly death audits, which independently audited the case notes of all patients who died, did not suggest a rise in deaths from either infection, and we think it is unlikely that this was obscured by a fall in directly attributable deaths from CDI, as the rate of CDI itself was low. The study is also limited by the absence of economic data, as it did not intend to examine cost-effectiveness.
Although we are aware of one other planned prospective ITS study12 that adequately assesses an antibiotic intervention (ward pharmacists successfully reviewing and modifying doctors' prescriptions each day), it did not use an additional control outcome and did not examine microbiological outcomes. Although national guidelines unequivocally report numerous examples of the success of restrictive antibiotic policies in reducing CDI, systematic reviews5,6 show nearly all to be methodologically flawed, with only five studies of sufficient quality for inclusion in the Cochrane systematic review of antibiotic interventions and their effect on microbiological outcomes.5,6 Only one of these showed a reduction in CDI associated with a significant decrease in broad-spectrum antibiotic use, including third-generation cephalosporins, although no specific data are provided on their use. The current study is therefore the best evidence to date that cephalosporin and amoxicillin/clavulanate restriction significantly reduced the use of each and the rate of CDI.
No study has adequately examined the use of feedback to change antibiotic prescribing. Our report is consistent with recent systematic reviews showing feedback helps healthcare workers adhere to clinical guidelines,9,10 a view consistent with current national recommendations,3,4,23 but it is not possible to tell the relative contributions of the laminated card and the feedback, although their combined use was highly effective and may provide a replicable simple intervention for acute care of the elderly units to reduce CDI rates. Given the national priority accorded to surveillance and reduction of CDI, a more virulent form of which has recently been reported,24 the findings are important.
The antibiotics chosen for the policy reflected local antibiotic susceptibilities for the main causative organisms. The use of cephalosporins in the pre-intervention phase was low compared with many hospitals, but the intervention was highly successful and might therefore be even more so in units with higher cephalosporin use.
The impact of interventions may also be dependent on the enthusiasm of local clinicians and, in this respect, the ‘pharmacist review’ method described earlier12 may be of more general use. Generalizability can only be addressed by further research. The current study design is a strong one, potentially feasible and replicable in many hospitals, which would allow synthesis of results, confirm the reproducibility of the intervention and suggest interventions worth assessing in definitive controlled trials.
Acknowledgements
We thank Alexandra Wu, Dan Lee and Jacqueline Morris, Consultant Physicians, Department of Health Services for Elderly People, Royal Free NHS Trust, Pond Street, London NW3 2PG, for supporting and implementing the policy.
Transparency declarations
None to declare.