-
PDF
- Split View
-
Views
-
Cite
Cite
Juri Karjagin, Rein Pähkla, Tõnis Karki, Joel Starkopf, Distribution of metronidazole in muscle tissue of patients with septic shock and its efficacy against Bacteroides fragilis in vitro, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 3, March 2005, Pages 341–346, https://doi.org/10.1093/jac/dkh544
Close - Share Icon Share
Abstract
Objectives: Studies investigating the target site concentration of antibiotics, such as
Materials and methods: Six patients admitted to the ICU of Tartu University Clinics with a diagnosis of septic shock were studied. Patients receiving metronidazole treatment within 48 h before the study or with a BMI > 35 were excluded. Metronidazole muscle tissue concentration was assessed by a microdialysis technique. Based on the microdialysis data, similar kinetics were simulated in in vitro experiments using Bacillus fragilis strains with MIC90s of 0.125 mg/L (BF125) and 1.0 mg/L (BF1).
Results: Metronidazole concentrations in plasma achieved a mean (s.d.) value of 11.4±2.0 mg/L at 30 min after administration of a single 500 mg intravenous dose, while in the muscle tissue, maximum concentrations of 8.2±4.5 mg/L were achieved at 140±92.3 min after the dose. When this metronidazole time course was simulated in vitro, the time to 99.9% kill ranged from 1.0 to 1.4 h for BF125 and from 1.8 to 3.5 h for BF1, while the eradication time ranged from 1.7 to 2.5 h and from 3.4 to 6.5 h, respectively. No regrowth was detected.
Conclusion: Pharmacokinetic–pharmacodynamic simulation of metronidazole interstitial concentrations shows a high efficacy of the drug in septic patients.
Introduction
Sepsis and septic shock are major causes of mortality in intensive care units (ICUs) and aggressive fluid resuscitation, adequate antimicrobial therapy and local surgical treatment are the cornerstones of sepsis management.1–3 In patients with severe sepsis, the interstitial space often becomes the major site for bacterial infection, and, consequently, the target site for antimicrobial drugs. The target site concentration of protein-free antibiotics in patients with sepsis or septic shock has been evaluated in several studies. These studies have shown that interstitial concentrations of both β-lactams and fluoroquinolones differ between healthy volunteers and septic patients.4–6
Pharmacokinetic–pharmacodynamic (PK–PD) approaches to the study of antimicrobial action are gaining in popularity and possibly could become a new standard for drug development,7,8 as they reflect drug–microorganism relations in a time-dependent manner. Protein-free drug concentrations in tissues can be measured using a microdialysis technique3 and based on these data, the pharmacokinetics of the drug can be evaluated and simulated in vitro in the presence of microorganisms for pharmacodynamic study.6,9
Metronidazole is a well established antimicrobial agent, which is widely used for the prophylaxis and treatment of anaerobic infections.10 The pharmacokinetic studies that led to the current dosage of metronidazole had several disadvantages, as plasma or total tissue concentrations were measured instead of target-tissue concentration11,12 and were also conducted on healthy volunteers.11–13 Metronidazole has a clinical efficacy profile in the surgical population. However, in patients with microcirculatory impairment, such as severe sepsis or septic shock, the pharmacokinetics and, consequently, the target-site concentration of the drug might be severely altered.
The aims of this study were (i) to describe pharmacokinetics of metronidazole using a muscle tissue microdialysis technique in patients with septic shock and (ii) to test the activity of metronidazole in an in vitro pharmacodynamic model at different single doses.
Materials and methods
The ethics committee of the University of Tartu approved the study. Because the patients were unconscious, their relatives received a detailed description of the study protocol and written informed consent was obtained. The study was carried out in accordance with the Declaration of Helsinki.
Patients
Six male patients admitted to the General ICU of Tartu University Clinics with a diagnosis of septic shock according to the American College of Chest Physicians and the Society of Critical Care Medicine consensus conference criteria were included.14 Overweight patients with a body mass index (BMI) over 35 and patients that had received metronidazole treatment within 48 h before the study were excluded. Mean age of the patients was 58.2 years (range 32.0–69.0 years), mean weight 77.5 kg (range 70.0–95.0 kg) and mean BMI 25.8 (range 22.9–31.0). Causes of septic shock were two cases of bronchopneumonia, two cases of peritonitis from the purulent cholecystitis and colonic perforation, one case of urosepsis and one case of phlegmon of the shoulder and arm. Metronidazole was not included in the treatment regimen and was administered once as a dose of 500 mg intravenously (iv).
Microdialysis
Microdialysis catheters (CMA 60 catheters with 30 mm long polyamide membrane with cut-off of 20 kDa) were placed into the m. vastus lateralis of the right thigh just above the knee. Microdialysis was carried out using a CMA 107 microdialysis pump and CMA T1 perfusion fluid containing Na+ 147 mmol/L, K+ 4.0 mmol/L, Ca2+ 2.3 mmol/L and Cl− 156 mmol/L, which was perfused at a rate of 2 μL/min. Baseline sampling (approximately 60 μL) was carried out during the first 30 min after insertion of the probe. During the next 30 min, another sample of approximately 60 μL was collected during retrodialysis with a metronidazole 5 mg/L solution to assess in vivo recovery of the drug.15 A 15 min washout period was then allowed. Thereafter, 500 mg of metronidazole (Metronidazol Nycomed; Nycomed Austria GmbH, Linz, Austria) was given intravenously by infusion over 10 min. Individual microdialysate samples were collected between 0 and 0.5 h, 0.5 and 1.0 h, 1.0 and 1.5 h, 1.5 and 2.0 h, 2.0 and 2.5 h, 2.5 and 3.0 h, 3.0 and 4.0 h, 4.0 and 5.0 h, 5.0 and 6.0 h, 6.0 and 7.0 h, 7.0 and 8.0 h, 8.0 and 9.0 h, and 9.0 and 10.0 h. Samples were immediately frozen and stored at −20°C until further analysis.
Plasma samples
Blood samples (6 mL) were taken at the end of each microdialysis collection from the arterial line located either in the radial or the brachial artery. The blood was drawn into the Vacutainer test tubes with lithium heparin. The samples were centrifuged immediately at 2.8g for 10 min, and plasma was separated and stored in Eppendorf tubes at −20°C until further analysis.
Drug assay
The metronidazole assay was described in detail in our previous paper.13 Briefly, the metronidazole concentration was analysed by an HPLC method. Samples were prepared by treatment with acetonitrile. The chromatographic system consisted of a Lichrosorb RP-18 pre-column, Lichrosorb RP-18, 5 μm, 250×3.2 mm column, and an ultraviolet detector measuring at 318 nm. The mobile phase consisted of acetonitrile/0.01 M aqueous phosphate solution (NaH2PO4), 15:85 (v/v), with a flow rate of 0.7 mL/min and a column temperature of 23–25°C (room temperature).
Pharmacokinetic data
Individually obtained values were used for the calculation of in vivo recovery of metronidazole and the concentration of the drug in muscle tissue according to the following equations:
Recovery (%)=100−(concentrationdialysate/concentrationperfusate) ×100
Tissue concentration (mg/L)=(concentrationdialysate×100)/ in vivo recovery value
where the perfusate is a solution which was used for perfusion of microdialysis probe and the dialysate is a solution which was obtained from the microdialysis vial.
Pharmacokinetic profile: Vss, t1/2, CL and AUC0–10 values were obtained from a two-compartmental model, calculated using Kinetica 2000 (version 3.0 demo; InnaPhase Corporation, USA). t1/2 in muscle tissue was calculated using non-compartmental analysis. The AUC0–10 muscle/AUC0–10 plasma ratio was used as a measure of metronidazole penetration into the muscle tissue.
Pharmacodynamic model
Microorganisms. MIC90 and time–kill studies were carried out with two clinical strains of Bacteroides fragilis BF1 and BF125. Two replicates of each strain were used and the arithmetical mean is presented in the results.
Media. Growth and time–kill assays were carried out in pre-reduced and cation-adjusted Wilkins-Chalgren broth (Oxoid, Basingstoke, UK), containing vitamin K and haemin. Wilkins-Chalgren agar (Oxoid) enriched with 5% sheep blood was used for plating experimental samples for colony number determination. All media were pre-reduced in an anaerobic chamber before inoculation.
Susceptibility testing. The MIC90 of metronidazole for both isolates used was determined by Etest (AB Biodisk, Solna, Sweden) as proposed by the manufacturer. Pre-reduced blood agar plates were used for susceptibility testing. Inoculated plates were incubated at 35°C inside an anaerobic chamber (Bactron, Sheldon Manufacturing, Portland, OR, USA) at 5% CO2, 5% H2 and 90% N2 for 48 h. Both B. fragilis strains were fully susceptible to metronidazole according to published breakpoints. The MICs of metronidazole for B. fragilis BF1 and BF125 were 1.0 and 0.125 mg/L, respectively.
Inoculum. Both organisms were placed on Wilkins-Chalgren agar plates and incubated overnight at 35°C in an anaerobic chamber. The microorganisms were diluted with pre-reduced sterile saline until the turbidity of the suspension matched that of a 0.5 McFarland standard (∼1×108 cfu/mL). The suspension (0.5 mL) was then used to inoculate into test flasks with Wilkins-Chalgren broth. This yielded a starting inoculum of approximately 1–5×106 cfu/mL. The actual size of each inoculum was also determined via colony counts.
Time–kill assay. Based on the microdialysis-derived mean time–concentration curve from the muscle tissue after 500 mg of iv metronidazole administration, we simulated a similar curve in an in vitro model. We also carried out similar experiments for tissue concentration–time profiles following 250 mg and 1000 mg doses of metronidazole by multiplying the 500 mg profile by factors of 0.5 and 2, respectively, as a linear relationship exists between dose and plasma concentration for doses of 200–2000 mg.16 The bacterial suspension was added to a test flask with 50 mL of Wilkins-Chalgren broth. Thereafter, metronidazole was added to achieve a step by step increase in the drug concentration up to the level equivalent to the peak level in the interstitial space fluid. Commercially available metronidazole solution, (Metronidazol Nycomed; Nycomed Austria GmbH), with a concentration of 5 mg/mL was used in in vitro experiments. Adding an appropriate amount of broth medium to the inoculated broth at 30 min intervals simulated decreasing metronidazole concentration. Altogether, six flasks containing no antimicrobial agent were used as a growth control and for the construction of the growth curve without metronidazole. After homogenization, all flasks were incubated at 35°C in an anaerobic chamber. At pre-determined time points during each experimental run (0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9 and 10 h), samples (50 μL) were removed from the inoculated flask, serially diluted in sterile saline and plated (50 μL) on Wilkins-Chalgren agar palates. Samples were also taken after 24 h to check for possible regrowth. Dilution were utilized to increase the accuracy of viable counts and to minimize antibiotic carryover. The lowest limit of detection was 1 cfu per 50 μL of Wilkins-Chalgren broth or 1.3 log cfu. Inoculated plates were incubated anaerobically at 35°C for 48 h and colony counts were carried out visually. A resazurin indicator was used to ensure that conditions remained anaerobic throughout the experiments. Results were assessed by plotting log10 cfu against time. The killing rate over time was determined as bactericidal if a reduction of 3 log10 cfu/mL ( ≥ 99.9% reduction cfu/mL) could be achieved. No corrections were made for dilutional effects and the metronidazole concentration/time profile was not confirmed by assay.
Statistical analysis. The change in colony counts over time was determined by linear regression analysis of the time–kill plots. The time to ≥ 99.9% reduction in cfu/mL, the time to total eradication, and the rate of reduction of cfu/mL were determined by linear regression. The rate of killing was defined as the slope of the killing curve from the start of the experiment to the time of the detection limit. The reduction in viable counts and figures were calculated using Graph Pad Prism (San Diego, CA, USA) software.
The data are presented as arithmetical means ± standard deviation (s.d.), if not otherwise stated.
Results
Patient demographic data are shown in Table 1.
Pharmacokinetics
Recovery of metronidazole from the muscle tissue during retrodialysis ranged from 24.7% to 82.0% (mean value 54.9 ± 20.0%).
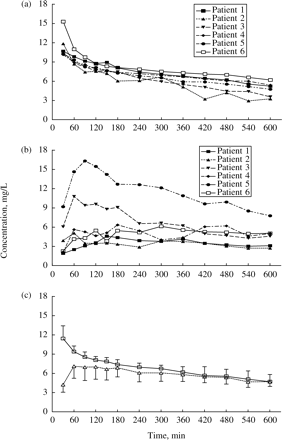
The individual and mean concentration versus time curves of metronidazole in plasma and muscle tissue after a single dose of 500 mg are shown in Figure 1. The AUC0–10 muscle/AUC0–10 plasma ratio of 0.88 ± 0.47 indicates a good penetration of metronidazole into the muscle tissue in patients with septic shock. Other pharmacokinetic parameters, calculated from plasma and muscle tissue concentrations of the drug, are shown in Table 2.
Pharmacodynamic model
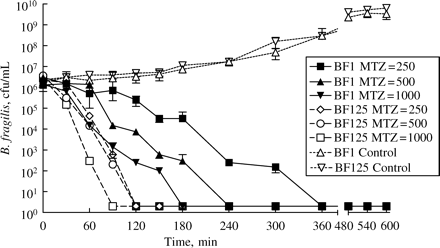
The pharmacodynamics of metronidazole against two strains of B. fragilis (BF1 and BF125) are shown in Figure 2 and Table 3. All three simulated metronidazole doses were rapidly bactericidal against both strains of B. fragilis, with viable counts falling 5–6 logs to undetectable levels and remaining below this limit for the following 10 h. The control regimens (with no drug) exhibited approximately a 3 log increase in cfu/mL by 10 h test time. However, no regrowth on the control plates was detected after 24 h.
Discussion
The pharmacokinetics of metronidazole have been assessed in several studies, using plasma concentrations,10,12 biopsies11 or skin blisters.17 However, few studies have been carried out on critically ill patients, where disturbances of macro- and microcirculation could significantly alter the pharmacokinetics and pharmacodynamics of the drug.12
In this study, protein-free interstitial concentrations of metronidazole have been measured for the first time in patients with septic shock. Using a microdialysis technique, we have shown that the maximum concentration of the drug in muscle tissue was 8.2 ± 4.5 mg/L in these patients. The important advantage of the microdialysis technique is that it allows us to assess the protein-free concentrations of the drug in tissue, i.e. to study the fraction of the drug which exerts an effect. We have used a similar technique in healthy gynaecological patients and found the mean maximal concentration of the drug in muscle tissue was comparable to this study.13 Metronidazole penetration into the muscle tissue, measured as the AUC0–10 muscle/AUC0–10 plasma ratio, therefore appears to be similar between septic patients and healthy volunteers, 0.88 ± 0.47 and 0.91 ± 0.19,13 respectively. The Cmax in plasma appears to be slightly lower in septic patients than in healthy volunteers, 11.4 ± 2.0 and 16.5 ± 4.6 mg/L,13 respectively. Increased Vss due to capillary leakage and interstitial oedema as well as differences in haemodynamic profile and use of haemodialysis could most likely explain these findings. These two factors (capillary leakage and interstitial oedema) might also explain the wide inter-subject variation of data seen in this study where the maximum concentration of metronidazole in the muscle tissue of septic patients differs over a factor of two-fold between the individuals (Figure 1b).
Metronidazole penetration into tissues has also been studied by Bielecka-Grzela & Klimowicz,18 who used cutaneous microdialysis in healthy volunteers. They assessed the AUC0–8 skin/AUC0–8 plasma ratio as a measure of tissue penetration, finding a value of 0.672 ± 0.196. Thus, our data indicate that the penetration of metronidazole into the peripheral tissues of septic patients is at least as good as in healthy patients.
The results of Cmax, Vss, CL and AUC, calculated from the plasma measurements of metronidazole in this study, were quite similar to our previous observations in a relatively healthy population,13 and as described in the review by Lamp et al.10 The results of the study are in accordance with low metronidazole protein binding (< 20%).10 As can be seen from Figure 1(c), the difference between the total plasma concentration and the protein-free muscle tissue concentration after equilibration is small. On the basis of our findings, it could be concluded that septic shock has a minimal influence on the distribution of metronidazole in plasma and muscle tissue.
The pharmacokinetics of iv metronidazole in critically ill patients has been studied by Plaisance et al.12 They found that in patients with renal or liver disease there is a prolongation of the clearance and half-life of the drug (half-life ranged from 7.98 to 42.4 h). Our results did not address these findings, as none of our patients had liver function impairment.
The half-life calculated by fitting the plasma concentrations to a two-compartment model was longer than in the healthy volunteers described by Lamp et al.,10 13.2 ± 5.3 versus 6–10 h, respectively. However, in our previous study, we found the half-life of metronidazole was 12.9 ± 4.9 h in relatively healthy female patients.13 Therefore, it is difficult to conclude whether the half-life of metronidazole is prolonged or not in patients with septic shock.
It has been suggested that metronidazole exhibits a concentration-dependent killing effect against anaerobic pathogens.10 As the MIC90 of metronidazole for the B. fragilis group ranges from < 0.25 to 8 mg/L,19 there is a possibility that a sufficient target concentration of the drug may not be achieved. Therefore we carried out a PK–PD analysis with two different B. fragilis strains, isolated in the microbiology laboratory of our hospital. The model was based on the interstitial concentrations of metronidazole, measured in the muscle tissue of the septic patients, and this confirmed the high activity of the drug against B. fragilis strains. Eradication time for the B. fragilis with the highest MIC (1 mg/L) was only 6.49 ± 0.05 h for the lowest dose studied and no regrowth was detected within the next 24 h.
An important limitation of the present study is that the metronidazole concentrations in the test flasks were not controlled during the in vitro experiments. However, to the best of our knowledge, metronidazole is chemically stable and not metabolized by B. fragilis in amounts which could affect the final concentration of the drug in the test flasks. Another factor which theoretically could influence the bacterial killing rate, is the dilution effect caused by adding extra broth in the in vitro experiments. At 3 h, the volume of broth in the test flask had increased by 15%, whereas the count of bacteria had decreased at least by 10 times (Figure 2); at 6 h, the respective numbers were 30% and 106 times. Based on these data, we believe that the effect of dilution on bacterial killing rate is minimal. The third limitation concerns sampling. Plasma samples were taken at the end of microdialysate collection and plasma time points do not exactly match microdialysis time points, which produces a difficulty in interpretation of the AUC muscle/AUC plasma ratio. But, as the decline in plasma and microdialysate concentrations was not steep, such inaccuracy has minimal influence on the AUC muscle/AUC plasma ratio.
Taken together, our clinical and experimental data indicate that metronidazole could be administered twice or even once daily in patients with septic shock. However, such a change in clinical practice needs studies with multiple dosing regimes, like those carried out by Lewis et al.20 They used a PK–PD model of oral administration of metronidazole to determine whether the newer extended release oral preparation, twice or once daily, had the same activity compared with immediate release oral preparations given three times a day. Similar to our findings, the authors observed rapid bactericidal activity ( ≥ 99.9% reduction) by 12 h with both regimens and no regrowth during the next 48 h.20
In conclusion, the present data demonstrate that the distribution of metronidazole is not affected in patients with septic shock. After a single iv dose of 500 mg metronidazole, effective concentrations against B. fragilis are achieved in muscle tissue of these patients. Further studies are needed to clarify whether once or twice daily administration of metronidazole is appropriate for clinical use of the drug.
Deceased.
Time course of metronidazole concentrations in plasma and muscle tissue in septic shock patients after a single iv dose of 500 mg. The individual time course of metronidazole in plasma (a) and in muscle tissue (b); patients are represented by individual lines: filled squares and solid line for patient one; filled triangles and dotted line for patient two; filled inverted triangles and dashed line for patient three; filled diamonds and dashed-dotted line for patient four; filled circles and dashed double-dotted line for patient five and open squares and solid line for patient six. (c) Mean time course in plasma, open squares and solid line; and mean time course in muscle tissue, open triangles and dotted line; vertical solid lines represent standard deviations (s.d.).
Time–killing curves of B. fragilis after in vitro simulation of concentration versus time profile of metronidazole in the muscle tissue of patients with septic shock. Time–killing curves are represented as follows: solid line and filled squares for BF1 and metronidazole (MTZ) dose of 250 mg; solid line and filled triangles for BF1 and metronidazole dose of 500 mg; solid line and filled inverted triangles for BF1 and metronidazole dose of 1000 mg; dashed line and open diamonds for BF125 and metronidazole dose of 250 mg; dashed line and open circles for BF125 and metronidazole dose of 500 mg; dashed line and open squares for BF125 and metronidazole dose of 1000 mg. Dotted line and open triangles for BF1 control growth without metronidazole and dotted line and open inverted triangles for BF125 control growth.
Patients' data
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 66 | 32 | 63 | 65 | 69 | 54 | ||||||
| APACHE II (at admission) | 16 | 5 | 15 | 22 | 44 | 24 | ||||||
| Vasopressors during the study | Dopa/Nor/Dobu | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | ||||||
| Volume replacement during 12 h, mL | 1625 | 2475 | 2095 | 2170 | 3500 | 2280 | ||||||
| Urine output during12 h, mL | 540 | CVVHD | 1950 | 1350 | 900 | 1350 | ||||||
| Leucocytes count, at baseline, mm−3 | 15.5 | 5.53 | 17.3 | 15.7 | 18.2 | 29.5 | ||||||
| CRV, mmol/L | 178 | 255 | 251 | 101 | 292 | 289 | ||||||
| Haemodynamic profile at baseline | ||||||||||||
| heart rate, min | 142 | 86 | 102 | 78 | 112 | 84 | ||||||
| MAP, mmHg | 99 | 86 | 110 | 103 | 84 | 89 | ||||||
| mean PAP, mmHg | 46 | 33 | 43 | 30 | 29 | 40 | ||||||
| PAOP, mmHg | 36 | 28 | 29 | 15 | 12 | 21 | ||||||
| CI, L/min/m2 | 4.54 | 3.12 | 3.28 | 3.69 | 2.67 | 3.75 | ||||||
| SVRI, dynes·s·cm−5/m2 | 1444 | 1894 | 2144 | 1974 | 2159 | 2049 | ||||||
| PVRI, dynes·s·cm−5/m2 | 176 | 128 | 341 | 325 | 510 | 406 | ||||||
| Oxygen profile at baseline | ||||||||||||
| DO2, mL/min/m2 | 557 | 442 | 471 | 490 | 559 | 624 | ||||||
| VO2, mL/min/m2 | 152 | 193 | 138 | 164 | 180 | 132 | ||||||
| Qs/Qt, % | 22.6 | 13.2 | 9.9 | 6.8 | 20.6 | 13.6 | ||||||
| FiO2, % | 50.0 | 40.0 | 45.0 | 45.0 | 50.0 | 40.0 | ||||||
| PaO2, mmHg | 86.0 | 80.0 | 176 | 155 | 180 | 114 | ||||||
| SvO2, % | 71.0 | 55.0 | 72.0 | 68.0 | 65.0 | 79.0 | ||||||
| pH | 7.37 | 7.37 | 7.44 | 7.42 | 7.29 | 7.43 | ||||||
| BE, mmol/L | −0.2 | −3.3 | 2.5 | −5.4 | −9.0 | 3.4 | ||||||
| lactate, mmol/L | 3.8 | 6.0 | 1.4 | 2.8 | 5.1 | 1.2 | ||||||
| Hgb, g/L | 108 | 107 | 104 | 124 | 162 | 123 | ||||||
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 66 | 32 | 63 | 65 | 69 | 54 | ||||||
| APACHE II (at admission) | 16 | 5 | 15 | 22 | 44 | 24 | ||||||
| Vasopressors during the study | Dopa/Nor/Dobu | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | ||||||
| Volume replacement during 12 h, mL | 1625 | 2475 | 2095 | 2170 | 3500 | 2280 | ||||||
| Urine output during12 h, mL | 540 | CVVHD | 1950 | 1350 | 900 | 1350 | ||||||
| Leucocytes count, at baseline, mm−3 | 15.5 | 5.53 | 17.3 | 15.7 | 18.2 | 29.5 | ||||||
| CRV, mmol/L | 178 | 255 | 251 | 101 | 292 | 289 | ||||||
| Haemodynamic profile at baseline | ||||||||||||
| heart rate, min | 142 | 86 | 102 | 78 | 112 | 84 | ||||||
| MAP, mmHg | 99 | 86 | 110 | 103 | 84 | 89 | ||||||
| mean PAP, mmHg | 46 | 33 | 43 | 30 | 29 | 40 | ||||||
| PAOP, mmHg | 36 | 28 | 29 | 15 | 12 | 21 | ||||||
| CI, L/min/m2 | 4.54 | 3.12 | 3.28 | 3.69 | 2.67 | 3.75 | ||||||
| SVRI, dynes·s·cm−5/m2 | 1444 | 1894 | 2144 | 1974 | 2159 | 2049 | ||||||
| PVRI, dynes·s·cm−5/m2 | 176 | 128 | 341 | 325 | 510 | 406 | ||||||
| Oxygen profile at baseline | ||||||||||||
| DO2, mL/min/m2 | 557 | 442 | 471 | 490 | 559 | 624 | ||||||
| VO2, mL/min/m2 | 152 | 193 | 138 | 164 | 180 | 132 | ||||||
| Qs/Qt, % | 22.6 | 13.2 | 9.9 | 6.8 | 20.6 | 13.6 | ||||||
| FiO2, % | 50.0 | 40.0 | 45.0 | 45.0 | 50.0 | 40.0 | ||||||
| PaO2, mmHg | 86.0 | 80.0 | 176 | 155 | 180 | 114 | ||||||
| SvO2, % | 71.0 | 55.0 | 72.0 | 68.0 | 65.0 | 79.0 | ||||||
| pH | 7.37 | 7.37 | 7.44 | 7.42 | 7.29 | 7.43 | ||||||
| BE, mmol/L | −0.2 | −3.3 | 2.5 | −5.4 | −9.0 | 3.4 | ||||||
| lactate, mmol/L | 3.8 | 6.0 | 1.4 | 2.8 | 5.1 | 1.2 | ||||||
| Hgb, g/L | 108 | 107 | 104 | 124 | 162 | 123 | ||||||
APACHE II, Acute Physiology And Chronic Health Evaluation; Dopa, dopamine; Nor, noradrenaline; Dobu, dobutamine; MAP, mean arterial pressure; PAP, pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; CI, cardiac index; SVRI, systemic vascular resistance index; PVRI, pulmonary vascular resistance index; DO2, oxygen delivery; VO2, oxygen consumption; Qs/Qt, shunt fraction; FiO2, fraction of oxygen; PaO2, oxygen partial pressure; SvO2, mixed venous blood saturation; BE, base excess; Hgb, concentration of haemoglobin; CRV, C-reactive protein concentration.
Patients' data
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 66 | 32 | 63 | 65 | 69 | 54 | ||||||
| APACHE II (at admission) | 16 | 5 | 15 | 22 | 44 | 24 | ||||||
| Vasopressors during the study | Dopa/Nor/Dobu | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | ||||||
| Volume replacement during 12 h, mL | 1625 | 2475 | 2095 | 2170 | 3500 | 2280 | ||||||
| Urine output during12 h, mL | 540 | CVVHD | 1950 | 1350 | 900 | 1350 | ||||||
| Leucocytes count, at baseline, mm−3 | 15.5 | 5.53 | 17.3 | 15.7 | 18.2 | 29.5 | ||||||
| CRV, mmol/L | 178 | 255 | 251 | 101 | 292 | 289 | ||||||
| Haemodynamic profile at baseline | ||||||||||||
| heart rate, min | 142 | 86 | 102 | 78 | 112 | 84 | ||||||
| MAP, mmHg | 99 | 86 | 110 | 103 | 84 | 89 | ||||||
| mean PAP, mmHg | 46 | 33 | 43 | 30 | 29 | 40 | ||||||
| PAOP, mmHg | 36 | 28 | 29 | 15 | 12 | 21 | ||||||
| CI, L/min/m2 | 4.54 | 3.12 | 3.28 | 3.69 | 2.67 | 3.75 | ||||||
| SVRI, dynes·s·cm−5/m2 | 1444 | 1894 | 2144 | 1974 | 2159 | 2049 | ||||||
| PVRI, dynes·s·cm−5/m2 | 176 | 128 | 341 | 325 | 510 | 406 | ||||||
| Oxygen profile at baseline | ||||||||||||
| DO2, mL/min/m2 | 557 | 442 | 471 | 490 | 559 | 624 | ||||||
| VO2, mL/min/m2 | 152 | 193 | 138 | 164 | 180 | 132 | ||||||
| Qs/Qt, % | 22.6 | 13.2 | 9.9 | 6.8 | 20.6 | 13.6 | ||||||
| FiO2, % | 50.0 | 40.0 | 45.0 | 45.0 | 50.0 | 40.0 | ||||||
| PaO2, mmHg | 86.0 | 80.0 | 176 | 155 | 180 | 114 | ||||||
| SvO2, % | 71.0 | 55.0 | 72.0 | 68.0 | 65.0 | 79.0 | ||||||
| pH | 7.37 | 7.37 | 7.44 | 7.42 | 7.29 | 7.43 | ||||||
| BE, mmol/L | −0.2 | −3.3 | 2.5 | −5.4 | −9.0 | 3.4 | ||||||
| lactate, mmol/L | 3.8 | 6.0 | 1.4 | 2.8 | 5.1 | 1.2 | ||||||
| Hgb, g/L | 108 | 107 | 104 | 124 | 162 | 123 | ||||||
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 66 | 32 | 63 | 65 | 69 | 54 | ||||||
| APACHE II (at admission) | 16 | 5 | 15 | 22 | 44 | 24 | ||||||
| Vasopressors during the study | Dopa/Nor/Dobu | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | Dopa/Nor | ||||||
| Volume replacement during 12 h, mL | 1625 | 2475 | 2095 | 2170 | 3500 | 2280 | ||||||
| Urine output during12 h, mL | 540 | CVVHD | 1950 | 1350 | 900 | 1350 | ||||||
| Leucocytes count, at baseline, mm−3 | 15.5 | 5.53 | 17.3 | 15.7 | 18.2 | 29.5 | ||||||
| CRV, mmol/L | 178 | 255 | 251 | 101 | 292 | 289 | ||||||
| Haemodynamic profile at baseline | ||||||||||||
| heart rate, min | 142 | 86 | 102 | 78 | 112 | 84 | ||||||
| MAP, mmHg | 99 | 86 | 110 | 103 | 84 | 89 | ||||||
| mean PAP, mmHg | 46 | 33 | 43 | 30 | 29 | 40 | ||||||
| PAOP, mmHg | 36 | 28 | 29 | 15 | 12 | 21 | ||||||
| CI, L/min/m2 | 4.54 | 3.12 | 3.28 | 3.69 | 2.67 | 3.75 | ||||||
| SVRI, dynes·s·cm−5/m2 | 1444 | 1894 | 2144 | 1974 | 2159 | 2049 | ||||||
| PVRI, dynes·s·cm−5/m2 | 176 | 128 | 341 | 325 | 510 | 406 | ||||||
| Oxygen profile at baseline | ||||||||||||
| DO2, mL/min/m2 | 557 | 442 | 471 | 490 | 559 | 624 | ||||||
| VO2, mL/min/m2 | 152 | 193 | 138 | 164 | 180 | 132 | ||||||
| Qs/Qt, % | 22.6 | 13.2 | 9.9 | 6.8 | 20.6 | 13.6 | ||||||
| FiO2, % | 50.0 | 40.0 | 45.0 | 45.0 | 50.0 | 40.0 | ||||||
| PaO2, mmHg | 86.0 | 80.0 | 176 | 155 | 180 | 114 | ||||||
| SvO2, % | 71.0 | 55.0 | 72.0 | 68.0 | 65.0 | 79.0 | ||||||
| pH | 7.37 | 7.37 | 7.44 | 7.42 | 7.29 | 7.43 | ||||||
| BE, mmol/L | −0.2 | −3.3 | 2.5 | −5.4 | −9.0 | 3.4 | ||||||
| lactate, mmol/L | 3.8 | 6.0 | 1.4 | 2.8 | 5.1 | 1.2 | ||||||
| Hgb, g/L | 108 | 107 | 104 | 124 | 162 | 123 | ||||||
APACHE II, Acute Physiology And Chronic Health Evaluation; Dopa, dopamine; Nor, noradrenaline; Dobu, dobutamine; MAP, mean arterial pressure; PAP, pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; CI, cardiac index; SVRI, systemic vascular resistance index; PVRI, pulmonary vascular resistance index; DO2, oxygen delivery; VO2, oxygen consumption; Qs/Qt, shunt fraction; FiO2, fraction of oxygen; PaO2, oxygen partial pressure; SvO2, mixed venous blood saturation; BE, base excess; Hgb, concentration of haemoglobin; CRV, C-reactive protein concentration.
Main pharmacokinetic parameters calculated for the study population
| . | AUC0–10 (mg/L × h) . | t1/2 (h) . | Cmax (mg/L) . | Tmax (min) . | CL (mL/min) . | Vss (L) . |
|---|---|---|---|---|---|---|
| Plasma | 66 ± 8.3 | 13.2 ± 5.3 | 11.4 ± 2.0 | 30a | 56.2 ± 26.9 | 53.5 ± 4.0 |
| Muscle | 57.9 ± 29.9 | 27.3 ± 23.4b | 8.2 ± 4.5 | 140 ± 92.3 |
| . | AUC0–10 (mg/L × h) . | t1/2 (h) . | Cmax (mg/L) . | Tmax (min) . | CL (mL/min) . | Vss (L) . |
|---|---|---|---|---|---|---|
| Plasma | 66 ± 8.3 | 13.2 ± 5.3 | 11.4 ± 2.0 | 30a | 56.2 ± 26.9 | 53.5 ± 4.0 |
| Muscle | 57.9 ± 29.9 | 27.3 ± 23.4b | 8.2 ± 4.5 | 140 ± 92.3 |
Results presented as arithmetical means ± s.d.
Time of the first plasma sample.
Calculated using non-compartmental analysis.
Main pharmacokinetic parameters calculated for the study population
| . | AUC0–10 (mg/L × h) . | t1/2 (h) . | Cmax (mg/L) . | Tmax (min) . | CL (mL/min) . | Vss (L) . |
|---|---|---|---|---|---|---|
| Plasma | 66 ± 8.3 | 13.2 ± 5.3 | 11.4 ± 2.0 | 30a | 56.2 ± 26.9 | 53.5 ± 4.0 |
| Muscle | 57.9 ± 29.9 | 27.3 ± 23.4b | 8.2 ± 4.5 | 140 ± 92.3 |
| . | AUC0–10 (mg/L × h) . | t1/2 (h) . | Cmax (mg/L) . | Tmax (min) . | CL (mL/min) . | Vss (L) . |
|---|---|---|---|---|---|---|
| Plasma | 66 ± 8.3 | 13.2 ± 5.3 | 11.4 ± 2.0 | 30a | 56.2 ± 26.9 | 53.5 ± 4.0 |
| Muscle | 57.9 ± 29.9 | 27.3 ± 23.4b | 8.2 ± 4.5 | 140 ± 92.3 |
Results presented as arithmetical means ± s.d.
Time of the first plasma sample.
Calculated using non-compartmental analysis.
Pharmacodynamic data obtained from the in vitro experiment
| Strain, metronidazole dose . | Cmax/MIC90 . | AUC0–10/MIC90 . | Time to kill 99.9%,a h . | Time to eradication,b h . | Slope of the kill curve (kill rate), h−1 . | r2 (kill curve) . |
|---|---|---|---|---|---|---|
| B. fragilis 0.125–250 mg | 28.4 | 231 | 1.41 ± 0.01 | 2.53 ± 0.01 | −2.39 ± 0.30 | 0.942 |
| B. fragilis 0.125–500 mg | 56.8 | 463 | 1.29 ± 0.08 | 2.27 ± 0.08 | −2.75 ± 0.15 | 0.991 |
| B. fragilis 0.125–1000 mg | 113 | 926 | 0.95 ± 0.03 | 1.69 ± 0.02 | −3.63 ± 0.42 | 0.974 |
| B. fragilis 1.0–250 mg | 3.55 | 29.0 | 3.47 ± 0.05 | 6.49 ± 0.05 | −0.90 ± 0.08 | 0.945 |
| B. fragilis 1.0–500 mg | 7.10 | 57.9 | 2.43 ± 0.12 | 4.33 ± 0.08 | −1.42 ± 0.13 | 0.955 |
| B. fragilis 1.0–1000 mg | 14.2 | 115 | 1.84 ± 0.00 | 3.42 ± 0.00 | −1.71 ± 0.14 | 0.966 |
| Strain, metronidazole dose . | Cmax/MIC90 . | AUC0–10/MIC90 . | Time to kill 99.9%,a h . | Time to eradication,b h . | Slope of the kill curve (kill rate), h−1 . | r2 (kill curve) . |
|---|---|---|---|---|---|---|
| B. fragilis 0.125–250 mg | 28.4 | 231 | 1.41 ± 0.01 | 2.53 ± 0.01 | −2.39 ± 0.30 | 0.942 |
| B. fragilis 0.125–500 mg | 56.8 | 463 | 1.29 ± 0.08 | 2.27 ± 0.08 | −2.75 ± 0.15 | 0.991 |
| B. fragilis 0.125–1000 mg | 113 | 926 | 0.95 ± 0.03 | 1.69 ± 0.02 | −3.63 ± 0.42 | 0.974 |
| B. fragilis 1.0–250 mg | 3.55 | 29.0 | 3.47 ± 0.05 | 6.49 ± 0.05 | −0.90 ± 0.08 | 0.945 |
| B. fragilis 1.0–500 mg | 7.10 | 57.9 | 2.43 ± 0.12 | 4.33 ± 0.08 | −1.42 ± 0.13 | 0.955 |
| B. fragilis 1.0–1000 mg | 14.2 | 115 | 1.84 ± 0.00 | 3.42 ± 0.00 | −1.71 ± 0.14 | 0.966 |
Results presented as means ± s.d.
Time required to achieve a 99.9% kill of the inoculum.
Time required to decrease viable counts below the limit of detection.
Pharmacodynamic data obtained from the in vitro experiment
| Strain, metronidazole dose . | Cmax/MIC90 . | AUC0–10/MIC90 . | Time to kill 99.9%,a h . | Time to eradication,b h . | Slope of the kill curve (kill rate), h−1 . | r2 (kill curve) . |
|---|---|---|---|---|---|---|
| B. fragilis 0.125–250 mg | 28.4 | 231 | 1.41 ± 0.01 | 2.53 ± 0.01 | −2.39 ± 0.30 | 0.942 |
| B. fragilis 0.125–500 mg | 56.8 | 463 | 1.29 ± 0.08 | 2.27 ± 0.08 | −2.75 ± 0.15 | 0.991 |
| B. fragilis 0.125–1000 mg | 113 | 926 | 0.95 ± 0.03 | 1.69 ± 0.02 | −3.63 ± 0.42 | 0.974 |
| B. fragilis 1.0–250 mg | 3.55 | 29.0 | 3.47 ± 0.05 | 6.49 ± 0.05 | −0.90 ± 0.08 | 0.945 |
| B. fragilis 1.0–500 mg | 7.10 | 57.9 | 2.43 ± 0.12 | 4.33 ± 0.08 | −1.42 ± 0.13 | 0.955 |
| B. fragilis 1.0–1000 mg | 14.2 | 115 | 1.84 ± 0.00 | 3.42 ± 0.00 | −1.71 ± 0.14 | 0.966 |
| Strain, metronidazole dose . | Cmax/MIC90 . | AUC0–10/MIC90 . | Time to kill 99.9%,a h . | Time to eradication,b h . | Slope of the kill curve (kill rate), h−1 . | r2 (kill curve) . |
|---|---|---|---|---|---|---|
| B. fragilis 0.125–250 mg | 28.4 | 231 | 1.41 ± 0.01 | 2.53 ± 0.01 | −2.39 ± 0.30 | 0.942 |
| B. fragilis 0.125–500 mg | 56.8 | 463 | 1.29 ± 0.08 | 2.27 ± 0.08 | −2.75 ± 0.15 | 0.991 |
| B. fragilis 0.125–1000 mg | 113 | 926 | 0.95 ± 0.03 | 1.69 ± 0.02 | −3.63 ± 0.42 | 0.974 |
| B. fragilis 1.0–250 mg | 3.55 | 29.0 | 3.47 ± 0.05 | 6.49 ± 0.05 | −0.90 ± 0.08 | 0.945 |
| B. fragilis 1.0–500 mg | 7.10 | 57.9 | 2.43 ± 0.12 | 4.33 ± 0.08 | −1.42 ± 0.13 | 0.955 |
| B. fragilis 1.0–1000 mg | 14.2 | 115 | 1.84 ± 0.00 | 3.42 ± 0.00 | −1.71 ± 0.14 | 0.966 |
Results presented as means ± s.d.
Time required to achieve a 99.9% kill of the inoculum.
Time required to decrease viable counts below the limit of detection.
We dedicate this article to the memory of our co-author, colleague and friend, Dr Rein Pähkla, who died on 5 October 2004. We are grateful to Dr Dmitri Avlassevitch for his help and valuable comments in preparing this work. This work was supported by Estonian Science Foundation grant nos. 5304, 4619 and 4428.
References
Friedman, G., Silva, E. & Vincent, J. L. (
Rivers, E., Nguyen, B., Havstad, S. et al. (
Müller, M., Haag, O., Burgdorff, T. et al. (
Joukhadar, C., Frossard, M., Mayer, B. X. et al. (
Joukhadar, C., Klein, N., Mayer, B. X. et al. (
Zeitlinger, M. A., Dehghanyar, P., Mayer, B. X. et al. (
FDA-CDER (1997). Guidance for Industry—Evaluating Clinical Studies of Antimicrobials in the Division of Anti-Infective Drug Products. U.S. Food and Drug Administration, Rockville, MD, USA.
Liu, P., Müller, M. & Derendorf, H. (
Sauermann, R., Zeitlinger, M., Erovic, B. M. et al. (
Lamp, K., Freeman, C. D., Klutman, N. E. et al. (
Kling, P. & Burman, L. G. (
Plaisance, K. I., Quintiliani, R. & Nightingale, C. H. (
Karjagin, J., Pähkla, R. & Starkopf, J. (
Bone, R. C., Sibbald, W. J., Cerra, F. B. et al. (
Ståhle, L., Segersvärd, S. & Ungerstedt, U. (
Hardman, J. G. & Limbird, L. E. (
Klimowicz, A., Nowak, A. & Bielecka-Grzela, S. (
Bielecka-Grzela, S. & Klimowicz, A. (
Mandell, G. L., Douglas, R. G. & Bennet, J. E. (
Author notes
1Anaesthesiology and Intensive Care Clinic, University of Tartu, 8 L. Puusepa Street; Departments of 2Pharmacology and 3Microbiology, University of Tartu, Tartu, Estonia