-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Hogardt, Sabine Schmoldt, Monika Götzfried, Kristin Adler, Jürgen Heesemann, Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients, Journal of Antimicrobial Chemotherapy, Volume 54, Issue 6, December 2004, Pages 1057–1061, https://doi.org/10.1093/jac/dkh470
Close - Share Icon Share
Abstract
Objectives and methods: With their potent activity against Gram-negative bacteria, the polymyxins are important alternative antibiotics for cystic fibrosis (CF) patients. A retrospective evaluation of polymyxin activity against 6001 Pseudomonas aeruginosa, 150 Achromobacter xylosoxidans and 506 Stenotrophomonas maltophilia CF isolates was initiated. In addition, we looked at how polymyxin susceptibility testing was affected by the testing method (agar dilution versus microdilution), the agent (polymyxin E versus polymyxin B), incubation time (24 h versus 48 h) and by different interpretative criteria (German DIN, French FSM, British BSAC).
Results: Polymyxin B exhibited reasonable activity against P. aeruginosa (MIC90≤2 mg/L), whereas it was less active against A. xylosoxidans (MIC90≤16 mg/L) and S. maltophilia (MIC90≤16 mg/L). During 2000–2002, polymyxin B resistance in P. aeruginosa, S. maltophilia and A. xylosoxidans was found to be 6.7%, 17.0% and 29.9% (corresponding to 12.4%, 20.7% and 35.4% of infected patients), respectively. When the agar dilution method was used, polymyxin E exhibited higher MICs than polymyxin B. The microdilution method produced lower polymyxin MICs than the agar dilution method. Therefore, the microdilution MICs after prolonged incubation (48 h) and the agar dilution MICs of polymyxin B correlated best (AUC of 0.93, r2 of 0.44 and s of 0.83).
Conclusions: Polymyxin resistance among common CF pathogens is not rare, thus underlining the necessity of accurate susceptibility testing. When compared with the agar dilution method, it was found that the microdilution method is a valid, rapid and cost effective alternative for the determination of polymyxin activity. The performance of the microdilution method was most reliable after prolonged incubation (48 h) at a susceptibility breakpoint of ≤4 mg/L according to the BSAC guidelines (specificity 91%, sensitivity 89%, 1.5% very major errors).
Introduction
Pseudomonas aeruginosa is the predominant respiratory pathogen among cystic fibrosis (CF) patients, causing chronic pulmonary infection and progressive deterioration in lung function. P. aeruginosa as well as other CF pathogens, such as Stenotrophomonas maltophilia and Achromobacter xylosoxidans, are resistant to most of the commonly used antibiotics. In addition, recurrent antibiotic treatment results in an increase in selection of multiresistant subpopulations necessitating alternative antibiotic agents, such as the polymyxins, which show potent activity against Gram-negative bacteria. Colistin, often termed polymyxin E because of its main component, was first introduced in 1959, but was later abandoned because less toxic antimicrobial agents (aminoglycosides, β-lactams) were available. The potent antibacterial activity of polymyxin favours its use in the treatment of infections with multidrug-resistant bacteria. There is, therefore, a resurgence in the use of polymyxins for the treatment of CF lung infection.
The polymyxins (polymyxin E, polymyxin B) are amphipathic polypeptide antibiotics. Their basic structure comprises a cyclic peptide; the d-leucine in polymyxin E is substituted by d-phenylalanine in polymyxin B. The polycationic peptide ring interacts with the lipid A of lipopolysaccharide (LPS), allowing penetration through the outer membrane by displacing Ca2+ and Mg2+. Insertion between the phospholipids of the cytoplasmic membrane leads to a loss of membrane integrity and ultimately to bacterial cell death.1 Nowadays, colistin is administered by inhalation, and with increasing frequency intravenously, with a level of adverse effects that is lower than that previously reported.2,3
The in vitro susceptibility testing of polymyxins is hampered by different factors. Up-to-date studies concerning the reliability of testing procedures are limited. The accuracy of disc diffusion assays is unsatisfactory because polymyxins diffuse poorly into agar and consequently no reliable correlation of zone diameters and MICs has been found.4 The interpretative criteria for quantitative in vitro testing are not applied uniformly because of differences in current national guidelines. The breakpoints (for systemic use only) of the German DIN (S ≤0.5 mg/L, I=1–2 mg/L, R ≥4 mg/L), the British BSAC (S ≤4 mg/L, R ≥8 mg/L) and the French FSM (S ≤2 mg/L, R >2 mg/L) vary considerably. Updated NCCLS breakpoints are currently not available. Consequently, the reported rates of polymyxin resistance among P. aeruginosa have varied a great deal, from 3% to 15.3%.5,6
Only the negatively charged colistin sulphomethate (a mixture of different sulphomethyl substituted polymyxins) is approved for systemic clinical administration due to its lower toxicity. In vitro, colistin sulphomethate exhibits 4–8-fold lower activity than the sulphate salt. In vivo, the intravenous formulation hydrolyses to the active, positively charged colistin base at an unknown rate. Therefore, colistin sulphomethate supposedly retains the activity of polymyxin E (accounting for nearly 85% of colistin). For susceptibility testing in the microbiological laboratory, the sulphate salts of polymyxin E or B are used, assuming that both agents are equally active. So far only a few studies addressing the pharmacokinetics and the clinical efficacy of colistin sulphomethate are available.7,8 To verify the in vitro breakpoints, the dosing regimens and the efficacy of polymyxin administration among CF patients, further studies are essential to address in vivo hydrolysis, polymyxin sputum accumulation and binding to sputum components.
In this work, we provide an evaluation of the in vitro susceptibility testing procedures for polymyxin. Another aim of this study was to evaluate the polymyxin resistance rates against the predominant CF pathogens, such as P. aeruginosa, S. maltophilia and A. xylosoxidans isolated during 2000–2002.
Materials and methods
Bacterial strains
Four hundred and one P. aeruginosa (220 non-mucoid, 181 mucoid), 50 A. xylosoxidans, 50 S. maltophilia CF and 100 P. aeruginosa non-CF isolates were assessed for polymyxin susceptibility. All isolates were recovered from CF respiratory secretions or from non-CF patients (intensive care unit: 50 blood culture isolates, 50 isolates from tracheal aspirates) and stored in glycerol stocks at −70°C until further use.
Data analysis
MIC results were analysed by linear regression (slope s) and by determination of the coefficient that displayed the shared variation between two variates (r2; calculated from the Spearman coefficient). The reproducibility was defined as the percentage of MICs found within the ± 1–log2 range, whereas differences ≥2–log2 steps were defined as discordant results. For the statistical analysis, off-scale results were included. Categorical agreement was defined as results within the same susceptibility category. Errors were ranked as follows: very major error (vmj), false-susceptible; major error (mj), false-resistant. ROC (receiver operating characteristic) analysis was performed using ACOMED software (Leipzig).
MIC determination
Polymyxin B sulphate and polymyxin E sulphate were obtained from Sigma-Aldrich. The agar dilution method was performed according to the NCCLS recommendations.9 Microdilution plates containing polymyxin E (Merlin Diagnostika, Bornheim-Hersel, Germany) were inoculated with 5 × 105 cfu/mL using H-Medium supplied for fastidious bacteria and were examined for growth after 24 and 48 h of incubation. Polymyxin was tested at various concentrations, namely: 0.5, 1, 2, 4, 8 and 16 mg/L. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains.
Results
Evaluation of broth microdilution versus agar dilution for the antimicrobial susceptibility testing of polymyxin
The polymyxin susceptibility of 401 P. aeruginosa CF isolates was determined by the microdilution method (polymyxin E) and the agar dilution method (polymyxin E, B). In general, the agar dilution method generated higher MICs (this was more pronounced for polymyxin E; Table 1) than the microdilution method. Microdilution MICs tend to increase with prolonged incubation time (data not shown). The best correlation was found for polymyxin B agar dilution MICs when compared with 48 h microdilution MICs (24 h MICs: s = 0.63, r2=0.14; 48 h MICs: s = 0.82, r2=0.44). For polymyxin E, the data are as follows: 24 h MICs: s = 0.56, r2=0.21; 48 h MICs: s = 0.76, r2=0.24. By testing 100 P. aeruginosa isolates in duplicate, the agar dilution method exhibited a 100% reproducibility for both polymyxin B (s = 0.99, r2=0.94) and polymyxin E (s = 0.97, r2=0.93). Microdilution polymyxin E MICs after 24 and 48 h were reproducible in 92% (s = 0.89, r2=0.12) and 86% (s = 0.45, r2=0.28), respectively (data not shown). This confirms that the s and r2 values have to be interpreted critically.
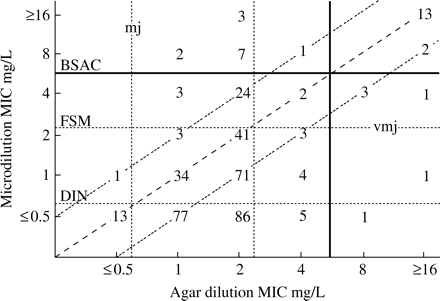
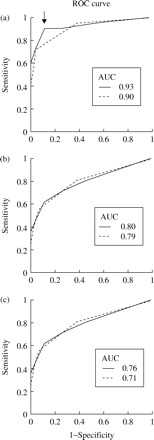
Moreover, the categorical assessment of polymyxin activity using different available interpretative criteria varied widely. The distribution of 48 h microdilution and agar dilution MICs is shown in Figure 1. Depending on applied breakpoints, the rate of unequivocally susceptible strains was in the range 3.5%–82% up to 91.5% (DIN, FSM and BSAC, Figure 1). Using the BSAC breakpoint, vmj and mj for 24 h MICs occurred for polymyxin B in 2.5% and 1.5% (48 h MICs, 1.5% and 3.2%) and for polymyxin E in 4.7% and 1% (48 h MICs, 3.2% and 3.2%), respectively. To assess the ability of the microdilution method to discriminate between resistant isolates, ROC curves were calculated from polymyxin B data. The test accuracy with respect to different cut-off values was measured by the area under the ROC curve (AUC). AUC was determined for different agar dilution breakpoints (Figure 2a–). The best overall performance for the microdilution method was found for 48 h MICs at a breakpoint of ≤4 mg/L for both methods (AUC 0.93, specificity 91%, sensitivity 89%). In summary, bearing in mind the appropriate breakpoint and incubation time, the microdilution method would appear to be a reliable method with which to determine polymyxin susceptibility.
Antimicrobial activity of polymyxin against P. aeruginosa, S. maltophilia and A. xylosoxidans
As shown in Table 1, polymyxin B exhibited reasonable activity against P. aeruginosa (MIC90 ≤ 2 mg/L), since it was less active against A. xylosoxidans (MIC90 ≤ 6 mg/L) and S. maltophilia (MIC90 ≤16 mg/L). Polymyxin E was slightly less active against all three species. The MIC distribution of non-CF P. aeruginosa isolates (MIC90 ≤ 2 mg/L) does not differ significantly from that of CF isolates, whereas no MICs ≥8 mg/L were detected. To perform a more representative analysis of polymyxin resistance among P. aeruginosa, A. xylosoxidans and S. maltophilia isolates, we analysed microbiological resistance data obtained during the routine microbiological testing of CF specimens collected during 2000–2002 (based on polymyxin B agar dilution and ≥8 mg/L as the breakpoint for resistance). In summary, 15.6%–18.7% of S. maltophilia isolates, 27.9%–30.9% of A. xylosoxidans isolates and 6.5%–6.8% of P. aeruginosa isolates were polymyxin resistant (Table 2.). Since several P. aeruginosa phenotypes per patient were commonly reported, e.g. mucoid (mu) and non-mucoid (nm) phenotypes, we additionally determined the percentage of infected patients with a resistant isolate. The average rate of patients with resistant isolates was 12.4% for P. aeruginosa, 20.7% for S. maltophilia and 35.4% for A. xylosoxidans, respectively.
Discussion and conclusions
The polymyxins are characterized purportedly by a rare development of resistance, which favours their use in the treatment of multiresistant Gram-negative bacteria. Among CF patients, who are regularly challenged by polymyxin inhalation, resistance may occur more frequently. Here, we show that polymyxin B exhibits reasonable activity against P. aeruginosa, but is less active against A. xylosoxidans and S. maltophilia. This emphasizes that the development of P. aeruginosa polymyxin resistance among CF patients is not rare. By comparison, no resistance was found for non-CF P. aeruginosa isolates (Table 1). Usually, in a non-CF setting, challenge with polymyxins does not occur. Therefore, we can assume that the selection of polymyxin-resistant phenotypes is unlikely. Nevertheless, polymyxins are an indispensable option in the antibacterial therapy of CF lung infection, including mixed infections with multidrug-resistant pathogens.10 Hence, polymyxin susceptibility testing should be performed whenever the drug is considered for systemic therapy, and the reliable testing of polymyxin activity in the microbiological laboratory is crucial.
We compared the microdilution method with the ‘gold standard’ agar dilution susceptibility test method to determine polymyxin activity. Using the agar dilution method with polymyxin E as the test agent yielded higher MICs predicting a higher polymyxin resistance frequency. With a maximum shared variation of 44%, the correlation between both methods appears to be unsatisfactory. However, the evidence of the statistical analysis is limited because most P. aeruginosa polymyxin MICs were within two dilution steps. Therefore, there is an overbalance of the few high polymyxin MICs. The application of 4 mg/L as the cut-off value caused only 1.5% of unacceptable vmjs. Additionally, ROC curve analysis confirmed that MIC determination after 48 h at a breakpoint of ≤4 mg/L results in the best overall test performance. Therefore, with the advantages of cost effectiveness and rapidity the microdilution method would appear to be a reliable method to determine the in vitro susceptibility of polymyxin. However, revision of polymyxin breakpoints by the German authorities is advised. Because of lower inter-method variability, polymyxin B should be recommended as the reference agent for the more stringent agar dilution method. However, by using 4 mg/L as the breakpoint for categorical assessment by the agar dilution method, only a marginal variation was observed between polymyxin B and polymyxin E.
Scattergram results of the broth microdilution 48 h polymyxin E MICs and agar dilution polymyxin B MICs obtained for 401 P. aeruginosa CF isolates. The numbers represent the occurrences observed at each point. The diagonal dashed line represents complete agreement, whereas the dotted lines represent MIC variation within ± 1–log2. The horizontal and vertical dotted lines indicate the susceptibility breakpoints of different authorities (BSAC ≤4 mg/L; FSM ≤2 mg/L; DIN ≤0.5 mg/L). The area for vmj and mj using breakpoints according to BSAC is indicated (black solid line).
ROC curves calculated for polymyxin microdilution assay concerning different agar dilution breakpoints: (a) ≤4 mg/L BSAC; (b) ≤2 mg/L FSM; (c) ≤0.5 mg/L DIN. AUC for 24 h (grey dotted lines) and 48 h (black solid lines) microdilution MICs is shown. The point with the highest specificity (91%), sensitivity (89%) and the predicted cut-off value (4 mg/L) for microdilution is indicated by an arrow.
MIC distribution of polymyxin B and E for P. aeruginosa, A. xylosoxidans and S. maltophilia determined by the reference agar dilution method
| . | . | . | MIC (mg/L) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism (number, origin) . | Polymyxin . | . | ≤ 0.5 . | 1 . | 2 . | 4 . | 8 . | ≥16 . | |||||
| P. aeruginosa (401, CF) 220 nm/181 mua | BE | n RP CP n RP CP | 14 3.5% 3.5% 1 0.2% 0.2% | 119 29.7% 33.2% 12 3.0% 3.2% | 232 57.9% 91.1% 213 53.1% 56.3% | 15 3.7% 94.8% 145 36.2% 92.5% | 4 1% 95.8% 10 2.5% 95.0% | 17 4.2% 100% 20 5.0% 100% | |||||
| P. aeruginosa (100, nCF) | BE | n RP CP n RP CP | 2 2% 2% 0 0% 0% | 26 26% 28% 3 3% 3% | 65 65% 93% 55 55% 58% | 7 7% 100% 38 38% 93% | 0 0% 100% 4 4% 100% | 0 0% 100% 0 0% 100% | |||||
| A. xylosoxidans (50, CF) | BE | n RP CP n RP CP | 2 4% 4% 3 6% 6% | 2 4% 8% 2 4% 10% | 14 28% 36% 7 14% 24% | 16 32% 68% 15 30% 54% | 7 14% 82% 7 14% 68% | 9 18% 100% 16 32% 100% | |||||
| S. maltophilia (50, CF) | BE | n RP CP n RP CP | 4 8% 8% 3 6% 6% | 4 8% 16% 3 6% 12% | 14 28% 44% 1 2% 14% | 10 20% 64% 11 22% 36% | 9 18% 82% 10 20% 56% | 9 18% 100% 22 44% 100% | |||||
| . | . | . | MIC (mg/L) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism (number, origin) . | Polymyxin . | . | ≤ 0.5 . | 1 . | 2 . | 4 . | 8 . | ≥16 . | |||||
| P. aeruginosa (401, CF) 220 nm/181 mua | BE | n RP CP n RP CP | 14 3.5% 3.5% 1 0.2% 0.2% | 119 29.7% 33.2% 12 3.0% 3.2% | 232 57.9% 91.1% 213 53.1% 56.3% | 15 3.7% 94.8% 145 36.2% 92.5% | 4 1% 95.8% 10 2.5% 95.0% | 17 4.2% 100% 20 5.0% 100% | |||||
| P. aeruginosa (100, nCF) | BE | n RP CP n RP CP | 2 2% 2% 0 0% 0% | 26 26% 28% 3 3% 3% | 65 65% 93% 55 55% 58% | 7 7% 100% 38 38% 93% | 0 0% 100% 4 4% 100% | 0 0% 100% 0 0% 100% | |||||
| A. xylosoxidans (50, CF) | BE | n RP CP n RP CP | 2 4% 4% 3 6% 6% | 2 4% 8% 2 4% 10% | 14 28% 36% 7 14% 24% | 16 32% 68% 15 30% 54% | 7 14% 82% 7 14% 68% | 9 18% 100% 16 32% 100% | |||||
| S. maltophilia (50, CF) | BE | n RP CP n RP CP | 4 8% 8% 3 6% 6% | 4 8% 16% 3 6% 12% | 14 28% 44% 1 2% 14% | 10 20% 64% 11 22% 36% | 9 18% 82% 10 20% 56% | 9 18% 100% 22 44% 100% | |||||
CF, cystic fibrosis; nm, non-mucoid; mu, mucoid; nCF, non-CF (50 tracheal aspirate isolates; 50 blood culture isolates); n, number of isolates per tested MIC; RP, relative MIC distribution (%); CP, cumulative MIC distribution (%).
Significantly more nm than mu P. aeruginosa CF isolates were found (0.8% against 4.5%; P ≤ 0.01; shown by χ2).
MIC distribution of polymyxin B and E for P. aeruginosa, A. xylosoxidans and S. maltophilia determined by the reference agar dilution method
| . | . | . | MIC (mg/L) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism (number, origin) . | Polymyxin . | . | ≤ 0.5 . | 1 . | 2 . | 4 . | 8 . | ≥16 . | |||||
| P. aeruginosa (401, CF) 220 nm/181 mua | BE | n RP CP n RP CP | 14 3.5% 3.5% 1 0.2% 0.2% | 119 29.7% 33.2% 12 3.0% 3.2% | 232 57.9% 91.1% 213 53.1% 56.3% | 15 3.7% 94.8% 145 36.2% 92.5% | 4 1% 95.8% 10 2.5% 95.0% | 17 4.2% 100% 20 5.0% 100% | |||||
| P. aeruginosa (100, nCF) | BE | n RP CP n RP CP | 2 2% 2% 0 0% 0% | 26 26% 28% 3 3% 3% | 65 65% 93% 55 55% 58% | 7 7% 100% 38 38% 93% | 0 0% 100% 4 4% 100% | 0 0% 100% 0 0% 100% | |||||
| A. xylosoxidans (50, CF) | BE | n RP CP n RP CP | 2 4% 4% 3 6% 6% | 2 4% 8% 2 4% 10% | 14 28% 36% 7 14% 24% | 16 32% 68% 15 30% 54% | 7 14% 82% 7 14% 68% | 9 18% 100% 16 32% 100% | |||||
| S. maltophilia (50, CF) | BE | n RP CP n RP CP | 4 8% 8% 3 6% 6% | 4 8% 16% 3 6% 12% | 14 28% 44% 1 2% 14% | 10 20% 64% 11 22% 36% | 9 18% 82% 10 20% 56% | 9 18% 100% 22 44% 100% | |||||
| . | . | . | MIC (mg/L) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism (number, origin) . | Polymyxin . | . | ≤ 0.5 . | 1 . | 2 . | 4 . | 8 . | ≥16 . | |||||
| P. aeruginosa (401, CF) 220 nm/181 mua | BE | n RP CP n RP CP | 14 3.5% 3.5% 1 0.2% 0.2% | 119 29.7% 33.2% 12 3.0% 3.2% | 232 57.9% 91.1% 213 53.1% 56.3% | 15 3.7% 94.8% 145 36.2% 92.5% | 4 1% 95.8% 10 2.5% 95.0% | 17 4.2% 100% 20 5.0% 100% | |||||
| P. aeruginosa (100, nCF) | BE | n RP CP n RP CP | 2 2% 2% 0 0% 0% | 26 26% 28% 3 3% 3% | 65 65% 93% 55 55% 58% | 7 7% 100% 38 38% 93% | 0 0% 100% 4 4% 100% | 0 0% 100% 0 0% 100% | |||||
| A. xylosoxidans (50, CF) | BE | n RP CP n RP CP | 2 4% 4% 3 6% 6% | 2 4% 8% 2 4% 10% | 14 28% 36% 7 14% 24% | 16 32% 68% 15 30% 54% | 7 14% 82% 7 14% 68% | 9 18% 100% 16 32% 100% | |||||
| S. maltophilia (50, CF) | BE | n RP CP n RP CP | 4 8% 8% 3 6% 6% | 4 8% 16% 3 6% 12% | 14 28% 44% 1 2% 14% | 10 20% 64% 11 22% 36% | 9 18% 82% 10 20% 56% | 9 18% 100% 22 44% 100% | |||||
CF, cystic fibrosis; nm, non-mucoid; mu, mucoid; nCF, non-CF (50 tracheal aspirate isolates; 50 blood culture isolates); n, number of isolates per tested MIC; RP, relative MIC distribution (%); CP, cumulative MIC distribution (%).
Significantly more nm than mu P. aeruginosa CF isolates were found (0.8% against 4.5%; P ≤ 0.01; shown by χ2).
Prevalence of polymyxin-resistant (polymyxin B MIC ≥8 mg/L) P. aeruginosa, A. xylosoxidans and S. maltophilia CF isolates observed over a 3 year period
| Year: . | . | 2000 . | 2001 . | 2002 . |
|---|---|---|---|---|
| Total patient number: . | . | 528 . | 490 . | 491 . |
| Average visits/patient: . | Polymyxin B . | 3.0 . | 3.0 . | 2.4 . |
| P. aeruginosa | S R PR | 2143 149/(6.5) 12.3% | 1996 145/(6.8) 11.5% | 1462 106/(6.8) 13.5% |
| A. xylosoxidans | S R PR | 31 12/(27.9) 35.5% | 38 17/(30.9) 31.8% | 36 16/(30.8) 38.9% |
| S. maltophilia | S R PR | 146 29/(16.6) 19.2% | 135 25/(15.6) 20.0% | 139 32/(18.7) 22.8% |
| Year: . | . | 2000 . | 2001 . | 2002 . |
|---|---|---|---|---|
| Total patient number: . | . | 528 . | 490 . | 491 . |
| Average visits/patient: . | Polymyxin B . | 3.0 . | 3.0 . | 2.4 . |
| P. aeruginosa | S R PR | 2143 149/(6.5) 12.3% | 1996 145/(6.8) 11.5% | 1462 106/(6.8) 13.5% |
| A. xylosoxidans | S R PR | 31 12/(27.9) 35.5% | 38 17/(30.9) 31.8% | 36 16/(30.8) 38.9% |
| S. maltophilia | S R PR | 146 29/(16.6) 19.2% | 135 25/(15.6) 20.0% | 139 32/(18.7) 22.8% |
Age distribution of patients (2–44 years). On average, 1395 × 1 Mega Colistin parenteral (Grünenthal) per year was supplied to CF clinicians. Tested isolates per year (S, number of susceptible isolates; R, number of resistant isolates/% resistant isolates), PR patients harbouring a polymyxin-resistant isolate in % of P. aeruginosa, A. xylosoxidans or S. maltophilia culture-positive (infected) patients.
Prevalence of polymyxin-resistant (polymyxin B MIC ≥8 mg/L) P. aeruginosa, A. xylosoxidans and S. maltophilia CF isolates observed over a 3 year period
| Year: . | . | 2000 . | 2001 . | 2002 . |
|---|---|---|---|---|
| Total patient number: . | . | 528 . | 490 . | 491 . |
| Average visits/patient: . | Polymyxin B . | 3.0 . | 3.0 . | 2.4 . |
| P. aeruginosa | S R PR | 2143 149/(6.5) 12.3% | 1996 145/(6.8) 11.5% | 1462 106/(6.8) 13.5% |
| A. xylosoxidans | S R PR | 31 12/(27.9) 35.5% | 38 17/(30.9) 31.8% | 36 16/(30.8) 38.9% |
| S. maltophilia | S R PR | 146 29/(16.6) 19.2% | 135 25/(15.6) 20.0% | 139 32/(18.7) 22.8% |
| Year: . | . | 2000 . | 2001 . | 2002 . |
|---|---|---|---|---|
| Total patient number: . | . | 528 . | 490 . | 491 . |
| Average visits/patient: . | Polymyxin B . | 3.0 . | 3.0 . | 2.4 . |
| P. aeruginosa | S R PR | 2143 149/(6.5) 12.3% | 1996 145/(6.8) 11.5% | 1462 106/(6.8) 13.5% |
| A. xylosoxidans | S R PR | 31 12/(27.9) 35.5% | 38 17/(30.9) 31.8% | 36 16/(30.8) 38.9% |
| S. maltophilia | S R PR | 146 29/(16.6) 19.2% | 135 25/(15.6) 20.0% | 139 32/(18.7) 22.8% |
Age distribution of patients (2–44 years). On average, 1395 × 1 Mega Colistin parenteral (Grünenthal) per year was supplied to CF clinicians. Tested isolates per year (S, number of susceptible isolates; R, number of resistant isolates/% resistant isolates), PR patients harbouring a polymyxin-resistant isolate in % of P. aeruginosa, A. xylosoxidans or S. maltophilia culture-positive (infected) patients.
This work was funded by the German BMBF (Bundesministerium für Bildung und Forschung).
References
Evans, M. E., Feola, J. F. & Rapp, R. P. (
Ledson, M. J., Gallagher, M. J., Cowperthwaite, C. et al. (
Bosso, J. A., Liptak, C. A., Seilheimer, D. K. et al. (
Matsen, J. M., Koepcke, M. J. & Quie, P. G. (
Pitt, T. L., Sparrow, M., Warner, M. et al. (
Schulin, T. (
Li, J., Coulthard, K., Milne, R. et al. (
Li, J., Turnidge, J., Milne, R. et al. (
National Committee for Clinical Laboratory Standards. (