-
PDF
- Split View
-
Views
-
Cite
Cite
Satoshi Hirose, Maki Touma, Rieka Go, Yoshinori Katsuragi, Yoshiyuki Sakuraba, Yoichi Gondo, Manabu Abe, Kenji Sakimura, Yukio Mishima, Ryo Kominami, Bcl11b prevents the intrathymic development of innate CD8 T cells in a cell intrinsic manner, International Immunology, Volume 27, Issue 4, April 2015, Pages 205–215, https://doi.org/10.1093/intimm/dxu104
Close - Share Icon Share
Abstract
If Bcl11b activity is compromised, CD4+CD8+ double-positive (DP) thymocytes produce a greatly increased fraction of innate CD8+ single-positive (SP) cells highly producing IFN-γ, which are also increased in mice deficient of genes such as Itk, Id3 and NF-κB1 that affect TCR signaling. Of interest, the increase in the former two is due to the bystander effect of IL-4 that is secreted by promyelocytic leukemia zinc finger-expressing NKT and γδT cells whereas the increase in the latter is cell intrinsic. Bcl11b zinc-finger proteins play key roles in T cell development and T cell-mediated immune response likely through TCR signaling. We examined thymocytes at and after the DP stage in Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre and Bcl11b+/S826G mice, carrying the allele that substituted serine for glycine at the position of 826. Here we show that Bcl11b impairment leads to an increase in the population of TCRαβhighCD44highCD122high innate CD8SP thymocytes, together with two different developmental abnormalities: impaired positive and negative selection accompanying a reduction in the number of CD8SP cells, and developmental arrest of NKT cells at multiple steps. The innate CD8SP thymocytes express Eomes and secrete IFN-γ after stimulation with PMA and ionomycin, and in this case their increase is not due to a bystander effect of IL-4 but cell intrinsic. Those results indicate that Bcl11b regulates development of different thymocyte subsets at multiple stages and prevents an excess of innate CD8SP thymocytes.
Introduction
TCRs consisting of TCRαβ heterodimers are expressed on CD4+CD8+ double-positive (DP) thymocytes after assembly of TCR genes via V(D)J recombination. Subsequent differentiation depends on selection steps that are influenced by the strength and duration of TCR signals (1). The few cells bearing TCRαβ with appropriate reactivity to peptide-MHC complexes on thymic epithelial cells undergo positive selection, whereas those with self-reactivity are eliminated by apoptotic processes known as negative selection (2). Surviving thymocytes differentiate into mature CD4+CD8− or CD4−CD8+ single-positive (SP) cells, which emigrate to the periphery (3). The TCRαβ DP cells also produce differentiated other thymocyte subsets that include innate T lymphocytes such as CD1d-dependent invariant NKT cells, the development of which is controlled by the promyelocytic leukemia zinc finger (PLZF) transcription factor (4). Those subsets exhibit activated phenotypes even before antigen stimulation, thereby being enabled to respond quickly upon antigen stimulation.
Recently, it was reported that the PLZF-expressing NKT and γδ T cells promote another subset of the T-cell lineage from DP cells, CD8SP thymocytes with memory- and innate-like characteristics that highly produce IFN-γ. An increase of the innate CD8SP thymocytes has been reported in mice deficient of genes such as IL-2 inducible T cell kinase [ITK (5, 6)], Kruppel-like factor 2 [KLF2 (7)], CREB-binding protein [CBP (8)] and inhibitor of DNA binding 3 [Id3 (9)]. Of interest, the increase is due to the bystander effect of IL-4 that is produced and secreted by PLZF-expressing cells (7, 9, 10). One exception is the increase in NF-κB1-deficient mice that do not require IL-4 for producing the innate CD8 T cells. Hence, the loss of NF-κB1 acts in a cell-intrinsic manner (11).
The Bcl11b/Ctip2 gene encodes zinc-finger proteins that play key roles in T cell development and the T cell-mediated immune response [(12–14), reviewed in refs. (15, 16)]. Bcl11b was first isolated as a tumor suppressor gene in mouse thymic lymphomas, a model for human acute T-cell lymphoblastic leukemia (T-ALL), and mutations or deletion was reported in T-ALL (17–21). Loss of Bcl11b resulted in cessation of development at the CD4−CD8− double-negative (DN) stage (14, 22) and conversion of T cell precursors to NK-like cells (23–25). Induced inactivation of Bcl11b in mature CD8SP cells in vitro resulted in a conversion of CD8SP cells into NK-like cells (25). Conditional deletion of Bcl11b at the DP stage showed severe developmental arrest at the DP stage due to impaired positive selection causing the precursors to fail to produce CD4SP and CD8SP cells (26) and NKT cells (27, 28).
The expression of Bcl11b exists in DP, CD4SP and CD8SP cells, but its effect on maturation of these cells and differentiation of other T subsets after the DP stage remains open because of severe inhibition of positive selection in Bcl11bF/FCD4-cre mice as described above. We thus generated the mice carrying a hypomorphic Bcl11bS826G allele that impairs Bcl11b function without eliminating it, by the aid of the RIKEN large scale ENU Mutagenesis Program (29). Better than in complete Bcl11b knockouts, DP thymocytes in Bcl11bKO/S826G and Bcl11b+/S826G mice survive within the thymus, which allows us to address this issue. Here, we demonstrate that Bcl11b impairment leads to the production of innate CD8SP thymocytes highly producing IFN-γ in a cell-intrinsic manner, whereas the impairment inhibits NKT differentiation at multiple steps. We also show that Bcl11b regulates thymocyte development after the DP stage at multiple processes in addition to the development before the DP stage.
Methods
Mice
C57BL/6 mice harboring a floxed allele of Bcl11b were generated by Katsuragi et al. (30). A pair of loxP sequences was introduced into the Bcl11b locus by gene targeting, which allows exon 2 deletion mediated by Cre recombinase (Supplementary Figure 1 is available at International Immunology Online). Bcl11bF/+CD4cre thymocytes showed efficient deletion of the flox allele by Cre protein expressed by the CD4cre transgene (31). Mice harboring the Bcl11bS826G mutant allele were generated in the ENU mutagenesis program directed by Gondo (29) and backcrossed to C57BL/6 background for more than 10 generations. Details of Bcl11bS826G allele genotyping are available on request. C57BL/6-Ly5.1 congenic mice were obtained from the RIKEN Bioresource Center (Tsukuba, Japan). Experiments were approved by the Niigata University Animal Ethics and Experiment Committee.
Flow cytometric analysis
Flow cytometric (FCM) analysis was performed as previously described (14). In brief, single cell suspensions of thymocytes or splenocytes were prepared from thymus or spleen and 1–2×106 cells were incubated with antibodies in phosphate-buffered saline containing 2% fetal calf serum and 0.2% NaN3 for 20min at 4°C. The monoclonal antibodies (mAbs) used were: anti-CD4-PerCP-Cy5.5, -PE-Cy7 or -APC (RM4-5), anti-CD8α-PE, -APC or -APC-Cy7 (53–6.7), anti-CD8β-PE (YTS156.7.7), anti-CD24-PE (M1/69), anti-CD44-FITC or -APC (IM7), anti-CD69-PE (H1.2F3), anti-CD122-PE (TM-β1), anti-Ly5.1-APC (A20), anti-NK1.1-Biotin (PK136), anti-γδTCR-PE (eBioGL3), anti-mouse H-Y TCR-FITC (T3.70), anti-TCRβ-FITC, PE or APC, BV421 (H57-597). For detection of biotinylated antibodies, streptavidin-PerCP was used. They were purchased from BioLegend (San Diego, CA, USA), BD Biosciences (San Jose, CA, USA) or eBioscience (San Diego, CA, USA). For NKT cell detection, conjugated CD1d tetramers loaded with αGalCer (ProImmune, Oxford, UK) were used. To prevent non-specific binding of mAbs, we added CD16⁄32 (93; eBioscience) before staining with labeled mAbs. Dead cells and debris were excluded from the analysis by appropriate gating of forward scatter (FSC) and side scatter (SSC). When needed, propidium iodide was added (final concentration, 1 µg ml−1) to eliminate dead cells. Intracellular staining of Eomes was done as described previously (7). Cell surface markers were stained with antibodies, then cells were fixed with the Foxp3 Staining Buffer Set (eBioscience). Permeabilized cells were incubated for 1h with Alexa Fluor 647-anti-Eomes (Dan11mag; eBioscience). Cells were analyzed by a FACScan, FACS Calibur or FACS Aria (BD Biosciences) flow cytometer, and data were analyzed using the Flow-Jo (Tree-Star, Ashland, OR, USA) or FACSDiva (BD Biosciences) software.
BrdU intracellular staining
For BrdU incorporation experiments, 100 μl of BrdU solution (10mg ml−1) was injected intra-peritoneally to mice. Thymuses were harvested 1h after BrdU administration. Thymocytes were fixed with cytofix/Cytoperm (BD Bioscience) and analyzed with the use of the BD Bioscience BrdU Flow Kit according to the manufacturer’s instruction. In brief, cells were suspended at a concentration of 1–2×106 cells ml−1, fixed, permeabilized and incubated with DNaseI (300 μg ml−1) for 60min at 37°C. After washing, cells were incubated with FITC conjugated anti-BrdU antibodies (3D4) for 20min at room temperature. Cells were resuspended in staining buffer and analyzed by a FACSCalibur flow cytometer.
Bone marrow chimeric mice
Bone marrow (BM) cells were prepared from the tibia and femur of 2–3 month-old C57BL/6-Ly5.1 or Bcl11bF/S826GCD4cre (Ly5.2) mice and were depleted of red blood cells using ammonium-chloride-potassium lysis buffer. Then, 1×107 mixed BM cells were intravenously injected into irradiated (8 Gy) B6-Ly5.1 mice. Six weeks after the injection, recipient mice were sacrificed, and donor-derived thymocytes and splenocytes were analyzed by FCM.
Gene array hybridization and data analysis
TCRβhighCD4−CD8+ thymocytes were sorted from Bcl11bF/S826GCD4cre and control wild-type mice with FACS AriaII (BD). Total RNA was prepared with RNAiso plus (TAKARA Bio, Shiga, Japan), analyzed for quality control with Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and quantified with NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cRNA was synthesized with a Low Input Quick Amp Labeling Kit, One-Color (Agilent Technologies) and hybridized on an Agilent SurePrint G3 Mouse GE 8x60K Microarray, using a standard Agilent protocol. Signal was detected by a DNA microarray Scanner G2565CA (Agilent Technologies) and analyzed by Feature Extraction (Agilent Technologies) software. Raw data were normalized with the robust multiarray average algorithm, which provides expression values. Pairwise analyses were performed with R software to obtain probes with a >2-fold change.
Functional gene-set enrichment analysis was done with software from the DAVID bioinformatics database [Database for Annotation, Visualization and Integrated Discovery; Version 6.7; National Institute for Allergy and Infectious Diseases of the US National Institutes of Health (32)]. P values calculated with DAVID represent a Fisher’s exact test.
In vitro cytokine production
For stimulation with PMA and ionomycin, cells were isolated and then plated at a density of 1×106 cells per ml in RPMI medium plus 10% (vol/vol) FCS. Cells were incubated for 5h with PMA (50ng ml−1) (Sigma–Aldrich) and ionomycin (1.5 µM) (Sigma–Aldrich) and for 3h with GolgiStop (0.67 µl ml−1) (BD Bioscience) before harvest. Cell surface markers were stained, then a Cytofix/Cytoperm kit (BD Bioscience) was used for fixation and permeabilization. PE-anti-IFN-γ (XMG1.2; eBioscience) was used for intracellular staining.
Statistical analysis
P values were calculated with Student’s t-test. P values of <0.05 were considered significant.
Results
CD8SP cells with reduced Bcl11b acquired an innate phenotype
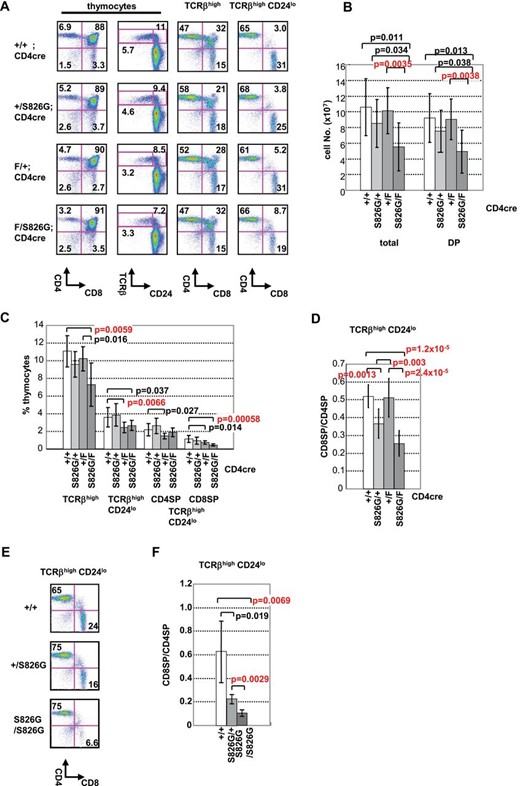
Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre and Bcl11b+/S826G mice of the C57BL/6 (B6) background were generated by mating Bcl11b-floxed mice, CD4cre mice and the Bcl11b+/S826G mice carrying the amino-acid substitution of S at the position of 826 for G. Figure 1(A) shows patterns of FCM analysis with CD4, CD8, CD24 and TCRβ expression, and Fig. 1(B) summarizes the cell number of total and DP thymocytes. Bcl11bF/S826GCD4cre mice reduced the thymocyte number to a half of wild-type control mice and this reduction was due to the decrease of DP cells, whereas Bcl11b+/S826GCD4cre or Bcl11bF/+CD4cre mice did not much differ in the number from wild-type controls. Figure 1(C) summarizes the percentages of different thymocytes. TCRβhighCD24low mature thymocytes after the DP stage were significantly reduced in Bcl11bF/S826GCD4cre and Bcl11bF/+CD4cre mice. Consistently, a significant reduction of CD8+ T cells was observed in the spleen of those mice (Supplementary Figure 2 is available at International Immunology Online). The reduction of the TCRβhighCD24low cell number was not observed in Bcl11b+/S826G mice, suggesting that loss of one Bcl11b allele, but not the presence of the Bcl11bS826G allele, impairs positive selection. In contrast, Bcl11b+/S826G mice showed the decrease of CD8SP cells more than CD4SP cells (Fig. 1D). Moreover, Bcl11bS826G/S826G mice showed severe impairment of CD8SP maturation (Fig. 1E and F). These suggest that the presence of the Bcl11bS862G allele adversely affects the CD8SP maturation.
Impaired positive selection and CD8 maturation in Bcl11bF/S826GCD4cre mice. FCM profiles of CD4, CD8, CD24 and TCRβ expression are shown in thymocytes from Bcl11bF/S862GCD4cre, Bcl11bF/+CD4cre, Bcl11b+/S862GCD4cre and Bcl11b+/+CD4cre mice. (A) Left two panels are CD4 versus CD8 and TCRβ versus CD24 in thymocytes, respectively. Right two panels are CD4 versus CD8 in TCRβhigh thymocytes and in mature TCRβhighCD24low thymocytes as indicated above. The percentage is shown in quadrants or gates. (B) The cell number of total and DP thymocytes was analyzed in Bcl11bF/S826GCD4cre (n = 8), Bcl11bF/+CD4cre (n = 10), Bcl11b+/S826GCD4cre (n = 10), and Bcl11b+/+CD4cre (n = 6) mice. (C) The average percentage of cells in quadrants is shown of TCRβhigh thymocytes, TCRβhighCD24low thymocytes, TCRβhighCD24low CD4SP thymocytes and TCRβhighCD24low CD8SP thymocytes. (D) The ratio of CD8SP per CD4SP cells is shown in TCRβhighCD24low thymocytes. Bcl11bF/S826GCD4cre (n = 8), Bcl11bF/+CD4cre (n = 10), Bcl11b+/S826GCD4cre (n = 10) and control Bcl11b+/+CD4cre (n = 6) mice were analyzed. (E) FCM profile of CD4 and CD8 expression in mature TCRβhighCD24low thymocytes from Bcl11bS826G/S826G, Bcl11b+/S826G and control Bcl11b+/+ mice. The percentage in indicated quadrants is shown. (F) The ratio of CD8SP per CD4SP cells in TCRβhighCD24low thymocytes is shown. Bcl11bS826G/S826G (n = 4), Bcl11b+/S826G (n = 4) and control Bcl11b+/+ (n = 4) mice were analyzed. Error bars indicate the standard deviation (SD). Statistical differences were tested by the Student’s t-test, and P values are shown (B–D, F).
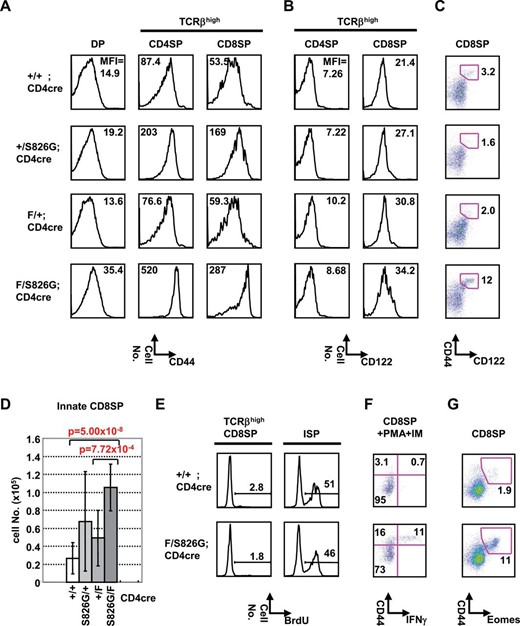
Another key finding was high expression of CD44 in Bcl11bF/S826GCD4cre DP, CD4SP and CD8SP cells (Fig. 2A). This increased expression was also detected in the subsets of Bcl11bKO/S826G mice (Supplementary Figure 3 is available at International Immunology Online) and in those of Bcl11b+/S826G mice though the level of increase was much lower. Expression of CD122 was not markedly increased in total TCRβhigh thymocytes in either Bcl11bF/S826GCD4cre or Bcl11b+/S826G mice (Fig. 2B); however, a population expressing both CD44 and CD122 at higher levels was expanded to 12% of CD8SP (CD4−CD8+) cells in Bcl11bF/S826GCD4cre mice (Fig. 2C), in contrast to 1.6–3.2% in the other genotypes. Bcl11bF/S826GCD4cre CD4SP cells did not express CD122 (Fig. 2B). Thus, there was a certain fraction of innate-like CD8SP thymocytes with elevated expression of both CD44 and CD122 in Bcl11bF/S826GCD4cre mice. This is not because of preferred survival of the innate-like population among CD8SPs, because the cell number of innate-like CD8SP increased in Bcl11bF/S826GCD4cre mice (Fig. 2D). The increase of CD44highCD122+CD8+ T cells was also observed in Bcl11bF/S826GCD4cre mice at 2 weeks of age (Supplementary Figure 4 is available at International Immunology Online). Therefore, those innate-like CD8SP cells did not arise from re-entered circulating memory-like T cells that had been originally formed in peripheral lymphoid tissues (33). Supplementary Figure 5, available at International Immunology Online, shows that Bcl11bF/S826GCD4cre αβTCR thymocytes expressed CD8β at the same level as CD8α on the cell surface, indicating that the CD8SP cells in Bcl11bF/S826GCD4cre mice are not due to expansion of the CD8αα T cells, cells of another innate CD8 T cell lineage (34).
CD8SP thymocytes with innate characteristics in Bcl11bF/S826GCD4cre mice. FCM analysis of thymocytes from Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre, Bcl11b+/S826GCD4cre and Bcl11b+/+CD4cre mice. (A) CD44 expression is shown in DP thymocytes (left panel), in TCRβhigh CD4 thymocytes (middle) and TCRβhigh CD8 thymocytes (right). (B) CD122 expression is shown in TCRβhigh CD4 thymocytes (left) and TCRβhigh CD8 thymocytes (right). (C) Expression of CD44 and CD122 is shown in CD8 thymocytes. CD44highCD122high thymocytes are marked by rectangles. (D) The cell number of CD44highCD122+CD8+ T cells was analyzed in Bcl11bF/S826GCD4cre (n = 8), Bcl11bF/+CD4cre (n = 10), Bcl11b+/S826GCD4cre (n = 10), and Bcl11b+/+CD4cre (n = 6) mice. Error bars indicate the SD. Statistical differences are tested by the Student’s t-test, and P values are shown. (E) BrdU incorporation in TCRβhigh CD8 thymocytes. FCM analysis was carried out for thymocytes obtained from mice that were BrdU incorporated for 1h. The BrdU incorporation of ISP cells is shown as a positive control. (F) IFN-γ expression is shown in CD8SP thymocytes of mice after 5h of stimulation of PMA plus ionomycin in vitro. IFN-γ+ fraction is marked by quadrants. (G) Expression of CD44 and Eomes is shown in CD8SP thymocytes. CD44highEomeshigh thymocytes are marked by rectangles.
Assay for cell proliferation by BrdU incorporation did not show a high incorporation in CD8SP thymocytes but in immature single positive (ISP) cells (Fig. 2E). This indicated that proliferating cells were not included in the CD8SP population, suggesting that the CD44high CD8SP thymocytes are not a result of accumulation due to increased cell proliferation in the thymus. To determine whether the cells were functionally innate-like, we next assessed IFN-γ production in Bcl11bF/S826GCD4cre CD8SP thymocytes, since a hallmark of innate- or memory-like T cells is the ability to rapidly secrete effector cytokines following activation (Fig. 2F). Stimulation with PMA plus ionomycin in vitro provided the production of IFN-γ in 1% of wild-type CD8SP thymocytes but rapid secretion of this cytokine >15-fold in Bcl11bF/S826GCD4cre CD8SP (CD4-CD8+) cells. The T-box transcription factor Eomes, which is associated with expression of CD122 and secretion of IFN-γ in memory CD8+ T cells (35, 36), was also highly expressed in these CD44highCD8SP thymocytes (Fig. 2G). In addition, splenic CD8+ T cells showed elevated CD44 expression in Bcl11bF/S826GCD4cre mice, accompanying decreased CD62L expression (Supplementary Figure 6 is available at International Immunology Online). Collectively, those results indicate an increase of phenotypically and functionally innate-like CD8SP thymocytes in Bcl11bF/S826GCD4cre mice.
Characterizing Bcl11bF/S826G CD4cre CD8SP thymocytes
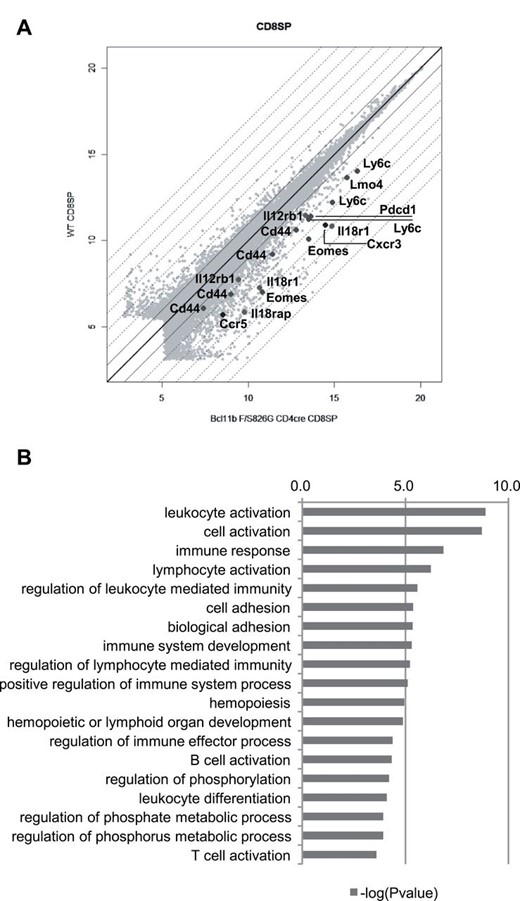
RNA expression of CD8SP thymocytes was compared between Bcl11bF/S826GCD4cre and wild-type mice by microarray analysis. It showed that 1021 and 447 genes were >2-fold up-regulated and down-regulated, respectively (data not shown). The up-regulated genes included CD44, Eomes, Pdcd1 (PD-1), Ly6c1 and Lmo4 (Fig. 3A), and the former two were those detected in this study and the others were genes previously detected in Bcl11b-deficient DP cells (26, 27). A key finding from ontology analysis was elevated expressions of cytokine receptors (Il12rb1, Il18r1 and Il18rap) and chemokine receptors (Cxcr3 and Ccr5), which belong to immune response genes (Fig. 3B and Supplementary Figure 7 is available at International Immunology Online). The up-regulation of those genes is implicated in the activation phenotype, a characteristic of the innate phenotype, that facilitates prompt response of immune cells upon stimulation (37, 38). It may be noteworthy that no marked change was observed for components of NF-κB signaling such as RelA and RelB (data not shown), though NF-κB1-deficient mice produce the innate CD8+ cells as described in the Introduction.
Transcriptional profile of the innate CD8SP cells. (A) Comparison of gene expressions of CD8SP thymocytes between Bcl11bF/S826GCD4cre mice and wild-type mice. Indicated are genes selected among genes that were expressed more than twice in Bcl11bF/S826GCD4cre CD8SP cells. (B) Selected terms of gene ontology (GO) functional categories are shown for upregulated genes in Bcl11bF/S826GCD4cre CD8SP cells.
Impaired NKT cell development in Bcl11b mutant mice
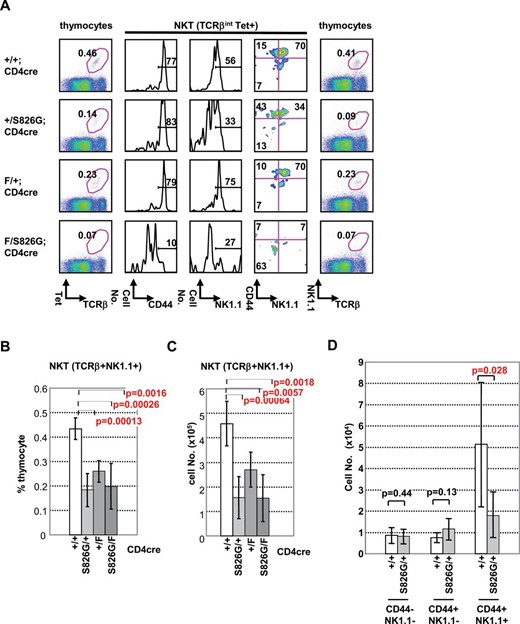
PLZF-expressing NKT and γδT cells secrete IL-4 and thereby promote the differentiation of innate CD8SP thymocytes by the bystander effect (7, 9, 10). Therefore, we considered that the increase of innate CD8SP thymocytes in Bcl11bF/S826GCD4cre mice may be a result of an alteration of PLZF-expressing cells, probably NKT cells because NKT, but not γδ T, cells develop from DP thymocytes after positive selection. In fact, however, development of γδT cells did not differ between Bcl11bF/S826GCD4cre and Bcl11b+/+ mice (Supplementary Figure 8 is available at International Immunology Online). Thus, we investigated NKT development by examining the expression of α-galactosylceramide (αGalCer)-CD1d tetramer, TCRβ, CD44 and NK1.1 in thymocytes. TCRβint CD1d tetramer+ cells are first produced from DP cells, and the cells express CD44 and then NK1.1 to generate mature NKT cells (39). Bcl11bF/S826GCD4cre mice did not have an expanded NKT population, but instead completely failed to generate TCRβint CD1d tetramer+ cells (Fig. 4A; Supplementary Figure 9 is available at International Immunology Online), consistent with the result of Bcl11bF/FCD4cre mouse analysis (28), indicating that the increase of innate CD8SP thymocytes is not due to increased NKT cell generation. Indeed, Bcl11b+/S826G and Bcl11bF/+CD4cre mice also generated reduced amounts of those innate cells (Fig. 4B and C), implying that their production is very sensitive to Bcl11b dosage.
Impaired NKT development at two steps in Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre and Bcl11b+/S826G mice. (A) FCM analysis of thymocytes from Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre, Bcl11b+/S826GCD4cre and Bcl11b+/+CD4cre mice using αGalCer-CD1d tetramer (Tet) and anti-TCRβ, anti-CD44 and anti-NK1.1. The markers used for staining are indicated below each panel. The second, third and fourth panels are the staining for TCRβint Tet+ cells, and the other panels are that for total thymocytes. (B, C) The average percentages and numbers, respectively, of TCRβint NK1.1+ cells in Bcl11bF/S826GCD4cre (n = 8), Bcl11bF/+CD4cre (n = 10), Bcl11b+/S826GCD4cre (n = 10), and Bcl11b+/+CD4cre (n = 6) mice. (D) The average numbers of CD44− NK1.1−, CD44+ NK1.1− and CD44+ NK1.1+ cells are shown in Bcl11b+/S826GCD4cre (n = 10) and Bcl11b+/+CD4cre (n = 6) mice.
The reduction of NKT cells in Bcl11bF/+CD4cre mice may be due to impaired positive selection. Of interest, however, Bcl11b+/S826G mice showed less expression of NK1.1 in TCRβint CD1d tetramer+ CD44high cells than Bcl11b+/+ and Bcl11bF/+CD4cre mice did (Fig. 4B–D). This result cannot be due to a requirement for Bcl11b in the expression of the NK1.1 gene, since at earlier stages of T-cell development Bcl11b deletion actually increases the yield of NK1.1+ NK cells. Thus, the effect on NKT cells suggests that the presence of the Bcl11bS826G allele specifically prevents the maturation of this lineage from the CD44high NK1.1− to the CD44high NK1.1+ stage. These results suggest that Bcl11b is required for multiple steps in the maturation of NKT cells.
Cell intrinsic effect of Bcl11b on innate CD8+ T cell expansion
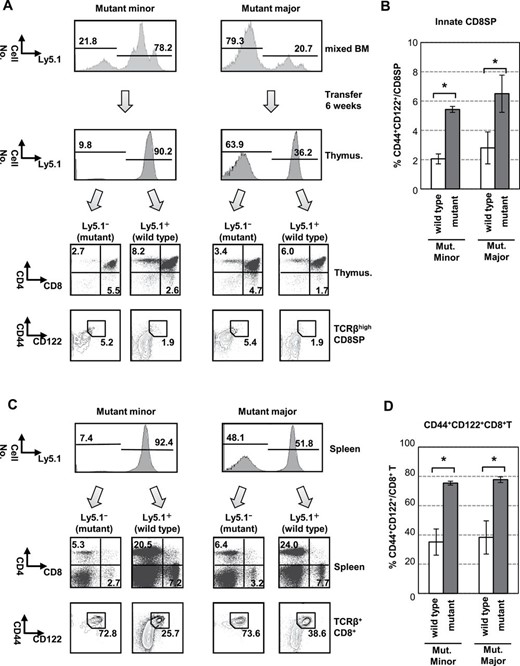
It was suggested that the increase of innate CD8+ T cells is independent of the bystander effect of IL-4. To confirm this, we carried out unequal mixed BM chimera experiments (7). Bcl11bF/S826GCD4cre Ly5.2+ BM cells were mixed with wild-type Ly5.1+ BM cells at the ratio of 1:4 (mutant minor) or 4:1 (mutant major) and transferred into lethally irradiated Ly5.1+ congenic mice. Analysis of mutant-minor chimeric mice at 6 weeks after transplantation showed that the increase of the innate CD8+ T cell population was more in the Ly5.1− cell fraction enriched with mutant cells than in the Ly5.1+ cell fraction enriched with wild-type cells (Fig. 5A and B). Essentially the same result, that is more in the Ly5.1− cell fraction, was obtained in the experiment of mutant-major chimeric mice, indicating no driving effect of wild-type thymocytes. Analysis of the spleen showed that most CD8+ T cells in the Ly5.1− cell fraction consisted of CD44highCD122high cells in not only mutant majority chimeras but also mutant minority chimeras (Fig. 5C and D). This suggests that the innate cell expansion in the periphery is not caused by the homeostatic expansion that is induced in lymphopenic conditions. Collectively, these results indicate that the expansion of innate CD8SP T cells within the thymus is cell intrinsic, independent of the bystander effect.
Expansion of innate CD8+ T cells is cell intrinsic. (A, C) BM cells from Bcl11b+/+ (Ly5.1) and Bcl11bF/S826GCD4cre mice (Ly5.2) were mixed at a ratio of 1:4 (mutant minority) and 4:1 (mutant majority), and transferred into irradiated host mice. Cell surface markers were analyzed by FCM for Ly5.1− and Ly5.1+ fractions: mixed BM cells (upper of A) and 6 weeks (below the panels) after transfer and splenocytes 6 weeks after (C). The ratio of Ly5.1− and Ly5.1+ cells is shown in indicated fraction by lines. Dot-plots in each quadrant shown in lower panels in A and C are CD4 versus CD8 in thymocytes and CD44 versus CD122 in TCRβhighCD8SP thymocytes. The percentage of CD44highCD122+ cell populations is shown near the square. (B, D) Histograms show the percent of CD44highCD122+ cells in CD8SP cells: (B) thymus, (D) spleen. Obtained from two independent experiments (n = 4). *P < 0.01.
Impaired Bcl11b alters selection processes
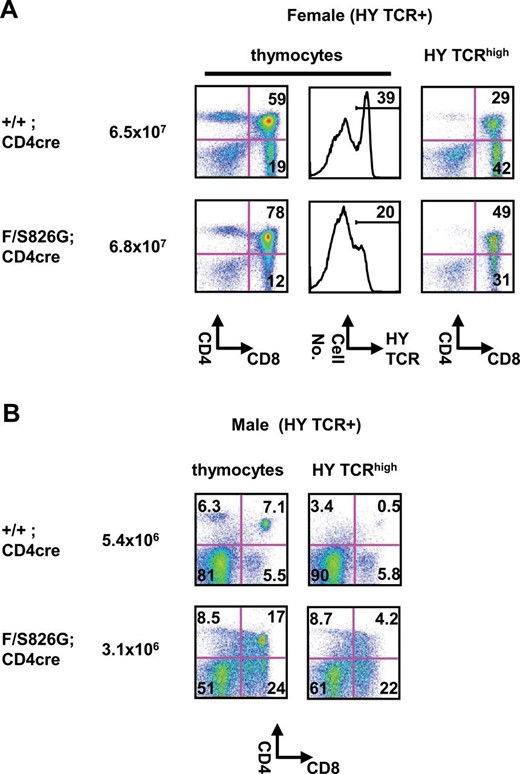
Since CD8SP thymocytes in Bcl11bF/S826GCD4cre mice acquired innate phenotypes during the transition from DP to CD8SP cells, which is a phase that coincides with T-cell positive selection, we examined whether Bcl11b abnormality alters negative selection in addition to positive selection. An MHC class I-restricted HY TCR transgene (40) was introduced into the Bcl11bF/S826G background to generate Bcl11bF/S826GCD4cre mice with the HY TCR transgene (Bcl11bF/S826GCD4cre HY). Those female mice showed decreases in the percentage of CD8SP cells and HY TCRhigh cells in total thymocytes (Fig. 6A). Also, a decrease was observed in the percentage of CD8SP cells in the HY TCRhigh thymocytes. These confirmed impaired positive selection in Bcl11bF/S826GCD4cre mice.
Reduced negative selection in Bcl11bF/S826GCD4cre mice. FCM analysis of thymocytes in Bcl11b+/+CD4cre and Bcl11bF/S826GCD4cre mice expressing the MHC class I-restricted H-Y TCR transgene. (A) Expression of H-Y TCR, CD4 and CD8 is shown in total thymocytes (left) and H-Y TCRhigh thymocytes (middle) of female mice. Total cell number is shown next to genotypes. The percentage of DP and CD8SP thymocytes is shown in quadrants and the percentage of H-Y TCRhigh cells is shown above lines (middle). (B) Expression of H-Y TCR, CD4 and CD8 in male mice: total thymocytes (left) and H-Y TCRhigh thymocytes (right). Total cell number is shown next to genotypes. The percentage of DP and CD8SP thymocytes is shown in quadrants.
To evaluate negative selection, we analyzed thymocytes of male Bcl11bF/S826GCD4cre HY mice. The HY TCR transgenic mice undergo early deletion at the DN and DP stages due to the presence of a male-specific self-antigen (41). As expected, therefore, the number of DP thymocytes was much lower in male Bcl11bF/S826GCD4cre HY mice than the corresponding male mice (Fig. 6B). However, the percentage of DP thymocytes was higher in male Bcl11bF/S826GCD4cre HY mice than male Bcl11b+/+CD4cre HY mice (Fig. 6B). In addition, the percentage of CD8SP cells was increased in these mice. The results indicated less efficiency of negative selection in Bcl11bF/S826GCD4cre mice. Supplementary Figure 10, available at International Immunology Online, shows decreased CD69 expression, indicative of impaired TCR signaling, in TCRβhigh thymocytes of Bcl11bF/S826GCD4cre mice. These confirmed that the combination of Bcl11b null and S826G alleles impairs not only positive but also negative selection.
Discussion
In this article, we examined thymocytes at and after the DP stage in Bcl11bF/S826GCD4cre, Bcl11bF/+CD4cre and Bcl11b+/S826G mice that exhibited impaired Bcl11b activity. Results showed that Bcl11b impairment leads to three developmental abnormalities of thymocytes: (i) an increased population of TCRαβhighCD44highCD122high innate CD8SP thymocytes, (ii) impaired positive and negative selection accompanying a reduction in the number of CD8SP cells and (iii) developmental arrests of NKT cells. The innate CD8SP thymocytes express Eomes and secrete IFN-γ after stimulation with PMA and ionomycin, possessing the same phenotypes to those of the innate CD8SP cells that were found in gene-deficient mice such as Itk, CBP, Id3, KLF2 genes and SLP76Y145F mutant mice [reviewed in ref. (10)]. Of note, generation of the innate CD8SP cells in Bcl11bF/S826GCD4cre mice is not due to the bystander effect of IL-4, but is cell intrinsic. This is because in the mixed BM chimera experiment we observed expansion of the innate cell population more in the Bcl11bF/S826GCD4cre-enriched cell fraction than the wild-type-enriched cell fraction that might have the bystander effect. That is further supported by the observation that no increase was detected in PLZF+ NKT cells or γδ T cells that produce and secrete IL-4.
Innate CD8SP cells and CD8+ T cells are present in BALB/c mice at a certain level, though their physiological role is not well known. Innate CD8+ T cells expanded in Klf2-deficient mice (7) can produce IFN-γ upon stimulation with IL-12 or IL-18 in an antigen-independent manner and are supposed to play a role in the acute phase of various bacterial, fungal, parasite and viral infections via production of IFN-γ (10). The transcriptional profile of the innate CD8SP thymocytes in Bcl11b-attenuated mice showed upregulation of Il12rb1, Il18r1 and Il18rap, which encode components of IL-12 receptor and IL-18 receptor, implicating a capability of IL-12 and IL-18 dependent IFN-γ production. Therefore, these innate CD8+ T cells may also play a role in the acute phase of infection.
Acquisition of the innate phenotype in Bcl11bF/S826GCD4cre CD8SP cells accompanied impairments in positive and negative selection and in CD8SP development. The percentage of TCRβhighCD24low mature thymocytes was reduced in Bcl11bF/S826GCD4cre mice together with the lower ratio of CD8SP/CD4SP cells. Also we observed increases in the percentage of DP and CD8SP thymocytes in male Bcl11bF/S826GCD4cre mice bearing a transgenic TCR specific for an HY peptide presented by MHC class I.
Developmental arrest in NKT cells was observed in Bcl11bF/S826GCD4cre and Bcl11b+/S826G mice. Bcl11bF/S826GCD4cre mice failed to generate TCRβint CD1d tetramer+ NK1.1− cells, the initial precursor of mature NKT cells. The result may be at least in part due to impaired positive selection, as reported previously (28). Of note, however, Bcl11b+/S826G mice produced CD44+ NK1.1+ cells less than Bcl11bF/+CD4cre mice did, indicating that the modulated Bcl11b activity leads to the developmental arrest at the TCRβint CD1d tetramer+ cell maturation. The Bcl11bS826G allele probably possesses a property distinct from the Bcl11b null allele, which might be explained by the property of the SWI/SNF complex (see below). This different property is also noted in a stronger effect on CD8SP development than CD4 development. Collectively, those results suggest that Bcl11b is required for NKT development at initial and maturation stages after positive selection. The arrest between TCRβint CD1d tetramer+ and CD44+ NK1.1+ stages was found in mice lacking RelA, a member of the NF-κB complexes that are considered to act under the TCR signaling in NKT cell development (42). It was reported that the loss of Bcl11b at the DN1 stage results in conversion of DN2 immature thymocytes to NK cells (24, 25) and conditional deletion of Bcl11b in mature CD8SP cells in vitro leads to the generation of innate-like cells called ITNK cells (25), which are similar to NK cells, one of the innate lymphoid cells (43). These results suggest that Bcl11b acts for preventing or controlling the conversion from T cell progenitors to other cells with innate phenotypes.
Bcl11b is a subunit of the SWI/SNF chromatin remodeling complex that regulates gene expression by utilizing the energy of ATP hydrolysis to modulate chromatin structure (44, 45). Mammalian SWI/SNF complexes (also known as BAF in mammals) comprise at least 14–20 subunits consisting of three different kinds (46, 47). The first is one of the two mutually exclusive catalytic ATPase subunits, BRG1/SMARCA4 and BRM/SMARCA2, and the second is a set of widely expressed core subunits. The third is a large number of lineage-restricted subunits such as Bcl11b. Loss of one Bcl11b allele probably reduces the percentage of the Bcl11b-containing SWI/SNF complexes within a cell whereas the presence of the Bcl11bS862G allele gives rise to the SWI/SNF complexes possessing a mutant Bcl11b. Total SWI/SNF complexes comprising these two distinct subsets affect gene expression probably in different manners, and the effects may include the transcription of signaling components downstream of TCR signaling and/or a pathway that impacts TCR signaling (22, 26).
The effect of Bcl11b on TCR-downstream pathways may play a key role in the promotion of innate CD8SP thymocytes and NKT cell development accompanying positive and negative selection. TCR signaling leads to the stimulation of the Ras-MEK-extracellular signal-regulated kinase (ERK) pathway and calcium mobilization, resulting in the activation of transcription factors, AP-1 and NFAT4, respectively (48, 49). NF-κB was another transcription factor downstream of TCR signaling (50, 51), and inactivation of NF-κB was shown to lead to a dramatic loss of CD8SP cells (52). The degree of activation of these three transcription factors may determine the activation state of a T cell (51). It was reported that loss of Bcl11b decreases the activity of ERK signaling and this may abrogate positive selection (26). Decreased ERK signaling explains the impaired positive selection in Bcl11bF/S826GCD4cre mice. However, it is inconsistent with the decrease of CD8SP cells in Bcl11bF/S826GCD4cre mice, because the decrease of ERK signaling leads to the reduction of CD4SP cells more than CD8SP cells (53). It is more plausible that Bcl11b impairment negatively affects NF-κB signaling or works parallel to it. As described above, there are other similarities of observed consequences between Bcl11bF/S826GCD4cre mice and NF-κB1-deficient mice; the increase of innate CD8SP cell generation in a cell intrinsic manner and the impaired maturation of NKT cells at a stage between TCRβint CD1d tetramer+ and CD44+ NK1.1+ cells. However, our microarray analysis of Bcl11bF/S826GCD4cre CD8SP thymocytes failed to detect any marked changes in mRNA expression of components of NF-κB signaling. This suggests that Bcl11b may work parallel to NF-κB signaling or affect a pathway that impacts its signaling, rather than directly control signaling its signaling components. It may be also possible that NF-κB signaling affects TCR signaling by controlling Bcl11b activity. However, this possibility is less likely because of the much lower effect on positive selection of NF-κB than Bcl11b (27, 54). The relationship between Bcl11b and NF-κB activity will be a rich area for future investigation.
Funding
JSPS KAKENHI Grant (23310036 to R.K., 21790462 and 24790465 to S.H. and 26460570 to M.T.), Health Labour Sciences Research Grant (201313001A to R.K.), Grant for Promotion of Niigata University Research Projects and Tsukada Medical Foundation (S.H.).
Acknowledgement
The authors thank Dr. Ellen V Rothenberg for critical reading of the manuscript, Dr. Takashi Saito (RIKEN RCAI) for HY TCR mice, Dr. Ichiro Taniuchi (RIKEN RCAI) for CD4cre transgenic mice, the staff of the animal facility of Niigata University, especially Yoshitaka Yamamoto for mouse care, Kazuyasu Takizawa, Ryota Ishizawa, Akiko Kudo and Fumi Yuzawa for technical assistance, Isako Sakai, Keiko O-shima and Yasuko Kokabu for secretarial work.
Conflict of interest statement: The authors have no financial conflicts of interest.
References