-
PDF
- Split View
-
Views
-
Cite
Cite
A. Eley, S. Hosseinzadeh, H. Hakimi, I. Geary, A.A. Pacey, Apoptosis of ejaculated human sperm is induced by co-incubation with Chlamydia trachomatis lipopolysaccharide, Human Reproduction, Volume 20, Issue 9, 1 September 2005, Pages 2601–2607, https://doi.org/10.1093/humrep/dei082
Close - Share Icon Share
Abstract
BACKGROUND: Previous work has shown that co-incubation of human sperm with Chlamydiatrachomatis serovars E and LGV leads to premature sperm death and that this is due primarily to chlamydial lipopolysaccharide (LPS). Here, we investigated the possible involvement of apoptosis in this premature sperm death. METHODS: Highly motile preparations of sperm from normozoospermic patients were co-incubated for 6 h with extracted LPS from C. trachomatis serovars E and LGV. Three different methods were used to determine if LPS-treated sperm underwent apoptosis, including: (i) flow cytometry; (ii) measurement of ADP:ATP ratios; and (iii) measurement of mono- and oligonucleosomal DNA fragments. Caspase activity was also investigated by fluorimetry and by use of a pan-caspase inhibitor and caspase-3 inhibitor. RESULTS: All three methods used for detection indicated that C. trachomatis LPS induced some apoptosis in sperm after 6 h when compared with a staurosporine (apoptosis-positive) control. Moreover, a greater degree of apoptosis was seen with C. trachomatis serovar E than with serovar LGV. It was also shown that C. trachomatis LPS-induced apoptosis of sperm could be blocked with a pan-caspase inhibitor and a caspase-3 inhibitor. Moreover, by using a fluorogenic substrate, apoptosis was shown to be caspase-mediated. CONCLUSIONS: In general it is believed that apoptosis does not occur in C. trachomatis-infected host cells. However, using three different methods, our findings clearly indicate that co-incubation of sperm with C. trachomatis LPS results in cellular death which is in part due to apoptosis and is caspase-mediated. These findings provide an explanation as to how C. trachomatis can mediate premature death in human sperm.
Introduction
Conventionally, all death in ejaculated sperm is considered to be as a result of necrosis since vital stains penetrate sperm through alterations in their plasma membrane (Blanc-Layrac et al., 2000). However, it is generally accepted that apoptosis is a major mechanism in regulating spermatogenesis and that in males with abnormal semen parameters, the presence of Fas-labelled sperm in the ejaculate is indicative of an ‘abortive apoptosis’ having taken place (Sakkas et al., 1999). More controversially, the occurrence of apoptosis in ejaculated human sperm has been reported (Gorczyca et al., 1993; Baccetti et al., 1996; Blanc-Layrac et al., 2000; Oosterhuis et al., 2000; Shen et al., 2002; Paasch et al., 2004a,b) although criticisms have been made about the criteria and techniques used to demonstrate apoptosis. Caspase activity has been shown to be present in human sperm in support of the above (Weng et al., 2002; Paasch et al., 2004a,b). Moreover, in infertile men a higher percentage of sperm with activated caspases was seen, confirming the existence of a caspase-dependent apoptotic pathway in ejaculated human sperm (Paasch et al., 2003). In contrast, membrane changes (that are not Fas-mediated) assessed by annexin V staining in ejaculated sperm do not correlate with semen abnormalities (Ricci et al., 2002), and may instead be associated with sperm capacitation (De Vries et al., 2003).
Chlamydial infection is a major cause of subfertility in both males and females (Westrom, 1996). The mechanism by which this occurs is thought to be via the acute inflammatory reaction associated with infection, leading to permanent scarring and functional impairment of the infected mucous membranes (Schachter, 1990). In women this is typically associated with salpingitis, which can lead to the blockage of Fallopian tubes (Kossein and Brunham, 1986). In men, chlamydial infection is associated with epididymitis and/or prostatitis that can lead to stenosis of the duct system, orchitis, or an impairment of accessory gland function (Purvis and Christiansen, 1995). Few studies, however, have considered whether exposure to C. trachomatis itself may compromise gamete function directly and thereby lead to subfertility by a route which is independent of any damage to the reproductive epithelium.
The possibility that gametes may be directly affected by C. trachomatis arises as a result of the unique development cycle of the chlamydia. Briefly, the bacterium exists in two forms: alternating between an extracellular but metabolically inactive infectious form called the elementary body (EB) and an intracellular metabolically active reproducing form called the reticulate body (RB) which uses the intracellular machinery of a host cell to reproduce. Consequently, the epithelium of an infected individual of either sex will periodically release large numbers of EBs which themselves will further release lipopolysaccharide either the EBs or the lipopoly saccharide may then be encountered by any gametes within the reproductive tract.
We have shown that co-incubation of human sperm with C. trachomatis serovar E, and to a lesser extent serovar LGV, causes a significant decline in the percentage of motile sperm and results in premature sperm death (Hosseinzadeh et al., 2001). It is known that serovar E is the one most commonly seen in the UK as a cause of genital infection (Eley et al., 1993). In addition, we showed (Hosseinzadeh et al., 2003) that C. trachomatis-induced death of human sperm is primarily caused by lipopolysaccharide (LPS).
Bacterial components such as LPS are known to induce apoptosis in a variety of cell types (Zhang et al., 1993; Lakics and Vogel, 1998). Indeed, Gorga et al. (2001) have reported that both Salmonella and Pasteurella LPS can induce apoptosis in sperm. However, only one method for determining apoptosis [terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL)] was used and semen samples were tested rather than purified sperm cells.
In this study we wanted to investigate further the mode of sperm death upon exposure to C. trachomatis LPS. We did this by comparing three different methods for demonstrating apoptosis in sperm and by looking at the role of caspases.
Materials and methods
Sample preparation
Semen samples that were identified as normozoospermic by World Health Organization (1999) criteria were obtained from patients attending the Andrology Laboratory (Jessop Wing, Royal Hallamshire Hospital, Sheffield, UK) for diagnostic analysis. Ethical approval for the use of semen samples in this study was granted by the South Sheffield Research Ethics Committee (project number 02/337). From each sample, a highly motile suspension of sperm was obtained by density centrifugation of a 1 ml aliquot of liquefied semen through a Percoll gradient as described previously (Hosseinzadeh et al., 2000). The final working concentration of sperm obtained was adjusted to ∼20×106 sperm per ml (unless otherwise stated) in Earle's balanced salt solution (EBSS) containing 0.3% (w/v) bovine serum albumin fraction V (Sigma, Poole, UK) prior to being prepared for use in the experiments described below.
LPS extraction and quantification
LPS was extracted from C. trachomatis serovars E and LGV as previously described (Hosseinzadeh et al., 2003). A Limulus Amebocyte Lysate (Cambrex Biosciences, Wokingham, UK) assay was used to quantify chlamydial LPS. The method was performed as recommended by the manufacturer.
Apoptosis and necrosis controls
To find the optimal induction of apoptosis in sperm, actinomycin (2.5–10 μg/ml), cycloheximide (0.50–2.0 μg/ml) and staurosporine (1 μmol/l–1 mmol/l) were added to 5×106/ml sperm, incubated for 6 h at 37°C/5% CO2 and samples were investigated by flow cytometry. Necrotic sperm were produced by heat treatment at 56°C for 1 h. In all experiments, untreated Percoll-prepared sperm (Moohan and Lindsay, 1995) were used as a control.
Exposure of sperm to LPS
A total of 5×106 sperm were incubated at 37°C/5% CO2 for 6 h with 0.1 μg/ml LPS from both serovars of C. trachomatis. In caspase inhibition experiments, prior to adding LPS, 200 μmol/l of pan-caspase inhibitor (Z-VAD-FMK) (Calbiochem, Darmstadt, Germany) or caspase 3 inhibitor (C3I) (Calbiochem) were added to the cells and incubated for 1 h at room temperature.
To prepare the samples (including controls) for flow cytometry, sperm were washed twice with annexin binding buffer (BD Biosciences Pharmingen, San Diego, USA) and diluted to give a final concentration of 1×106/ml sperm.
Detection of apoptosis using flow cytometry
The technique used for the annexin V assay and its characteristics are described elsewhere (Oosterhuis et al., 2000). To remove in vivo bound annexin V, 200 μl Percoll-prepared sperm was washed twice with annexin V binding buffer.
A total of 106 sperm/ml were incubated with 0.1 mg ml–1 fluorescein isothiocyanate (FITC)–labelled annexin V, incubated at 37°C/5% CO2 for 30 min in 5% CO2 in the dark followed by adding 50 mg ml–1 propidium iodide (PI) (Sigma, UK). The sperm were then analysed in a FACScalibur flow cytometer (Becton Dickinson, Oxford, UK). A minimum of 10 000 sperm were examined for each experimental group. The sperm population was gated by using forward-angle light scatter. However, side-angle light scatter was used to exclude electronic noise and debris. The FITC-labelled annexin V-positive sperm cells were measured in the FL1 channel and the PI-labelled cells were measured in the FL2 channel of the flow cytometer.
Detection of apoptosis by measurement of ADP:ATP ratios
ATP was measured using a commercial kit (Bradbury et al., 2000), following the manufacturer's instructions. The ApoGlow™ kit (Cambrex Biosciences, UK) is based upon the bioluminescent measurement of ATP. The method uses luciferase enzyme which catalyses the formation of light from ATP and luciferin according to the following reaction: ATP+luciferin+O2→(luciferase/Mg2+)→oxyluciferin+AMP+Ppi+CO2+light. Measurement of ADP:ATP ratios is used to differentiate cells undergoing apoptosis or necrosis.
The emitted light intensity is linearly related to the ATP concentration and is measured using a luminometer. The non-adherent cell assay was used in our experiments due to the nature of Percoll-prepared sperm. A volume of 100 μl of the cell suspension was transferred into a fresh 96-well opaque white microtitre plate using a multichannel pipette. The microtitre plate was then loaded into the luminometer and the protocol initiated. Measurement was then recorded.
Detection of apoptosis by measurement of mono- and oligonucleosomal DNA
Apoptosis was determined using the ‘Cell Death Detection ELISA’ (enzyme-linked immunosorbent assay) kit from Roche (Diagnostics Ltd, Lewes, UK) following the manufacturer's instructions on a final concentration of sperm adjusted to 5×106 per ml.
The assay is based on a quantitative sandwich enzyme immunoassay principle using mouse monoclonal antibodies directed against DNA and histones respectively. This allows the specific determination of mono- and oligonucleosomes in the cytoplasmatic fraction of cell lysates.
Quantification of caspase activity
Sperm lysates were prepared as described previously (Parvathenani et al., 1998). An aliquot of sperm lysate was diluted with a solution containing interleukin 1B converting enzyme (ICE) buffer and the fluorogenic substrate N-acetyl-aspartate-glutamate-valine-aspartate, 7-amino-4-trifluorome-thyl coumarin (Ac-DEVD-afc). Fluorescent emission (excitation 400 nm and emission 505 nm) was measured after incubation for 45 min at 37°C. Blanks without sperm were evaluated to determine background fluorescence. Standards containing 0–500 pmol/γ AFC were diluted to determine the amount of fluorochrome released. Fluorescence was measured at χmax=505 nm using a fluorimeter (Perkin–Elmer, Beaconsfield, UK). Caspase activity was expressed as pmol/min/mg protein. Apoptotic human neutrophils treated with 1 mmol cycloheximide were used as positive controls.
Statistical analysis
The results of all experiments in this study were analysed using GraphPad InStat software version 3.0 (GraphPad Software Inc, USA). The statistical significance of differences between each experimental pair was evaluated by one-way analysis of variance (ANOVA) (Figures 2, 3 and 4), or by Student's t-test (Figures 5 and 6). P<0.05 was considered significant.
Results
Staurosporine but not actinomycin or cycloheximide induces apoptosis in sperm
At concentrations of up to 10 μg/ml actinomycin and 2 μg/ml cycloheximide, no noticeable apoptosis could be detected by flow cytometry (data not shown). However, increasing concentrations of staurosporine from 1 μmol/l to 1 mmol/l demonstrated a rise in apoptosis which reached a maximum level of 35–50% with 1 mmol/l (data not shown). In all further experiments, staurosporine at 1 mmol/l was used as a positive control for induction of apoptosis.
Co-incubation of sperm with C. trachomatis LPS leads to apoptosis as demonstrated by three methods
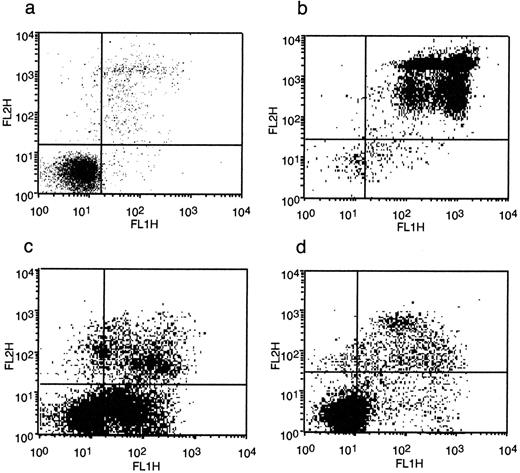
In the first experiment, apoptosis was quantified by measuring phosphatidylserine (PS) externalization using annexin V binding and flow cytometry. Untreated sperm typically showed a small number of necrotic and apoptotic cells (Figure 1a). When sperm were heat-shocked, the majority of cells became necrotic with a slight increase in apoptotic cells (Figure 1b). Staurosporine at 1 mmol/l showed a marked apoptotic effect although there was also a substantial increase in necrotic cells (Figure 1c). When sperm were exposed to C. trachomatis LPS from serovar E they showed an increased number of both apoptotic and necrotic cells (Figure 1d).
Quantification of apoptotic sperm by flow cytometry clearly showed a marked difference in the necrosis and apoptosis controls as would be expected (Figure 2). The data also clearly showed a predominantly apoptotic response of sperm when exposed to C. trachomatis LPS from either serovar E or LGV 1.
Similar to what has been previously reported (Bradbury et al., 2000) we found that heat-shocked (i.e. necrotic) rather than staurosporine (i.e. apoptotic)-treated sperm had higher ADP:ATP ratios (Figure 3). As the ADP:ATP ratios can serve to differentiate cells which are either necrotic or apoptotic, these ratios allowed us to determine that sperm exposed to C. trachomatis LPS from serovar E were predominantly apoptotic but that a larger population of necrotic cells was seen in sperm exposed to C. trachomatis LPS from serovar LGV1.
Our final method of determining apoptosis was to look for DNA fragmentation which is an end-stage marker of apoptosis. Using an ELISA format, there was a marked difference between staurosporine or heat-treated sperm, with high levels of DNA fragmentation seen with staurosporine treatment (Figure 4). Sperm when exposed to C. trachomatis LPS from serovar E showed levels of DNA fragmentation indicative of apoptosis. However, when sperm were exposed to C. trachomatis LPS from serovar LGV1, there was only a slight apoptotic effect and overall findings were similar to those of necrotic (heat-treated) sperm.
C. trachomatis LPS-induced apoptosis of sperm can be blocked with a pan-caspase inhibitor
To give us an indication as to whether caspases were implicated in the apoptosis of ejaculated sperm exposed to C. trachomatis LPS, we preincubated cells to the pan-caspase inhibitor (Z-VAD-FMK) and looked for apoptosis by flow cytometry and annexin V binding.
Not surprisingly, the inhibitor had a negligible (statistically insignificant) effect on both untreated and heat-treated sperm (Figure 5). In contrast, there was statistically significant inhibition of apoptosis in sperm which were treated with staurosporine or C. trachomatis LPS from either serovars E or LGV1. These findings suggest that caspases play an important role in those sperm in which apoptosis is induced.
Confirmation that C. trachomatis LPS-induced apoptosis of sperm is caspase-mediated using a fluorogenic substrate
When the fluorogenic substrate Ac-DEVD-afc was added to sperm exposed to staurosporine, increased levels of fluorescent emission were detected indicating increased caspase activity (Figure 6) in comparison to heat-shocked sperm. In addition, increased caspase activity was also seen following the exposure of sperm to C. trachomatis LPS from both serovar E and LGV1. Moreover, this was significantly reduced in the presence of the caspase-3 inhibitor C3I, confirming an important role for caspase-3 in the apoptotic pathway of ejaculated sperm.
Discussion
Over the last few years there has been a series of publications showing both anti- and proapoptotic activities occurring during chlamydial infection (Fan et al., 1998; Gibellini et al., 1998; Ojcius et al., 1998; Coutinho-Silva et al., 2001; Dean and Powers, 2001; Rajalingham et al., 2001; Perfettini et al., 2003; Byrne and Ojcius, 2004). In an attempt to try and resolve the conflicting observations, Greene et al. (2004) performed a series of experiments and concluded that biologically significant apoptosis did not occur in infected host cells. However, Greene et al. (2004) also suggested that toxic materials released by chlamydia (such as LPS) could play some role in promoting apoptosis.
In an attempt to understand how sperm became non-viable in the presence of C. trachomatis LPS (Hosseinzadeh et al., 2003), we performed three different types of experiments to explore the possible induction of apoptosis in sperm caused by exposure to C. trachomatis LPS. Although there were some differences in the results by the different methodologies, in general terms LPS from serovar E led to a greater amount of cell death through apoptotic mechanisms, whereas co-incubation with LPS from serovar LGV generally led to sperm death by the process of necrosis.
Further experiments demonstrated caspase activation, and inhibition by a pan-caspase inhibitor and a caspase-3 inhibitor confirm that caspases play a central role in C. trachomatis LPS-induced apoptosis of sperm. Of all downstream caspases, caspase-3 is the most important among them and works by generating DNA strand breaks (Paasch et al., 2004a).
Although the concept of apoptosis in ejaculated human sperm has been controversial, there is an increasing body of evidence to show that it is possible (Said et al., 2004). Indeed, recent work by Weng et al. (2002) clearly demonstrates that caspases are present in ejaculated sperm undergoing apoptosis. Moreover, it has already been reported that lipopolysaccharide from both Salmonella and Pasteurella can induce apoptosis in human sperm as demonstrated by TUNEL (Gorga et al., 2001). Similarly, Helicobacter pylori LPS has also been shown to induce caspase-mediated apoptosis in other cell types (Kawahara et al., 2001).
Finally, an interesting question to arise from this work is why human sperm should respond differently to the LPS molecule purified from two closely related serovars of C. trachomatis. Theoretically, the structure of the LPS molecule of serovars E and LGV are very similar (Heine et al., 2003) although detailed studies of the structure of LGV1 LPS have not yet been carried out. Following the results of this study we would propose that, given the importance of LPS in compromising assisted reproduction (Snyman and Van der Merwe, 1986; Fishel et al., 1988), future research efforts should focus on the molecular structure of LPS in relation to its spermicidal properties.
In conclusion, our work strongly suggests that in vitro human sperm can undergo caspase-mediated apoptosis and that LPS from C. trachomatis is able to initiate this effect. These findings provide an explanation as to why C. trachomatis, when co-incubated with sperm, causes their death (Hosseinzadeh et al., 2001, 2003). They also support the suggestion of Greene et al. (2004) that LPS is a toxic material released by chlamydia and, as our results show, this appears to play a role in promoting apoptosis. Why sperm should undergo apoptosis in the presence of C. trachomatis is an interesting question with possible relevance to human fertility. It is plausible that the exposure of sperm to chlamydia either in the male or female genital tract, or both, could lead to sperm dysfunction. Furthermore, it is interesting to speculate that apoptosis in sperm may have evolved to prevent the transmission of genetic defects, as sperm infected by C. trachomatis may be sufficiently damaged to prevent the successful development of embryos should fertilization be successful. It is clear that further work is warranted to see if these in vitro findings can also be observed in infected individuals with a subsequent effect on semen quality.
Detection of apoptotic and necrotic sperm when exposed to the following over a 6 h incubation period: (a) no addition (control), (b) heat-shock, (c) staurosporine (1 mmol/l), (d) C. trachomatis serovar E lipopolysaccharide (LPS) (0.1 μg/ml). The fluorescein isothiocyanate (FITC)-labelled annexin V-positive sperm cells were measured in the FL1 channel and the propidium iodide-labelled cells were measured in the FL2 channel of the flow cytometer. Data shown are an example from one patient.
Percentage apoptotic sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. ***P<0.001.
ADP:ATP ratios of sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV 1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. NS=non-significant; **P<0.01; ***P<0.001.
Measurement of mono- and oligonucleosomal DNA of sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV 1 LPS, 0.1 μg/ml). Data shown are the mean+SEM of incubations with sperm preparations from six patients. NS=non-significant; *P<0.05.
Percentage apoptotic sperm in the presence and absence of PCI (pan-caspase inhibitor) when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Untransformed data shown are the mean±SEM of incubations with sperm preparations from six patients. ***P<0.001.
Caspase activity of sperm in the presence and absence of C3I (caspase-3 inhibitor) when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. NS=non-significant; **P<0.01; ***P<0.001.
References
Baccetti B, Collodel G and Piombini P (
Blanc-Layrac G, Bringuier A-F, Guillot R and Feldmann G (
Bradbury DA, Simmons TD, Slater KJ and Crouch SPM (
Byrne GI and Ojcius DM (
Couthinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A and Ojcius DM (
Dean D and Powers VC (
De Vries KJ, Wiedmer T, Sims PJ and Gadella BM (
Eley A, Khalili M, Abbot M, Patel R and Kinghorn GR (
Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH and Zhong G (
Fishel S, Jackson P, Webster J and Faratian B (
Gibellini D, Panaya R and Rumpianesi F (
Gorczyca W, Traganos F, Jesionowska H and Darzynkiewicz Z (
Gorga F, Galdiero M, Buommino E and Galdiero E (
Greene W, Xiao Y, Huang Y, McClarty G and Zhong G (
Heine H, Muller-Loennies S, Brade L, Lindner B and Brade H (
Hosseinzadeh S, Brewis IA, Pacey AA, Moore HDM and Eley A (
Hosseinzadeh S, Brewis IA, Eley A and Pacey AA (
Hosseinzadeh S, Pacey AA and Eley A (
Kawahara T, Teshima S, Kuwano Y, Oka A, Kishi K and Rokutan K (
Kossein M and Brunham RC (
Lakics V and Vogel SN (
Moohan JM and Lindsay KS (
Ojcius DM, Souque P, Perfettini JL and Dautry-Varsat A (
Oosterhuis GJE, Mulder AB, Kalsbeek-Batenburg E, Lambalk CB, Schoemaker J and Vermes I (
Paasch U, Grunewald S, Fitzl G and Glander H-J (
Paasch U, Grunewald S, Agarwal A and Glander H-J (
Paasch U, Sharma RK, Gupta AK, Grunewald S, Mascha EJ, Thomas AJ, Glander H-J and Agarwal A (
Parvathenani LK, Buescher ES, Chacon-Cruz E and Beebe SJ (
Perfettini JL, Ojcius DM, Andrews CW Jr, Korsmeyer SJ, Rank RJ and Darville T (
Purvis K and Christiansen E (
Rajalingham K, Al-Younes H, Muller A, Meyer TF, Szczepek AJ and Rudel T (
Ricci G, Perticarari S, Fragonas E, Giolo E, Canova S, Pozzobon C, Guaschino S and Presani G (
Said TM, Paasch U, Glander H-J and Agarwal A (
Sakkas D, Mariethoz E and St John JC (
Schachter J (
Shen HM, Dai J, Chia SE, Lim A and Ong CN (
Snyman E and Van der Merwe JV (
Weng S-H, Taylor SL, Morshedi M, Schuffner A, Duran EH, Beebe S and Oehninger S (
World Health Organization (
Author notes
1Division of Genomic Medicine, Floor F, University of Sheffield Medical School, Beech Hill Road, Sheffield S10 2RX and 2Academic Unit of Reproductive and Developmental Medicine, The Jessop Wing, Central Sheffield University Hospitals Trust, Sheffield S10 2SF, UK


![Percentage apoptotic sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. ***P<0.001.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/20/9/10.1093_humrep_dei082/2/m_148029f2.jpeg?Expires=1716477985&Signature=MrtwWO64vV09Qm57R-QatFX1JPcabGtP4XvDgtaqKrPhzv3yxP4FZtzZBhuJJ79g4K7dOeIEBVUH7Z-MKuZaW9vc8tESE-LyAN9URb8Gp~2PPfU1nfgHwjlwYpE~YngURjVeMlj5HlcOK76JCOd~axScBZgBBN8ZGeCc~fpVEK3XxfEUKbntI5c2HGM5OPN0JUEB-4JGZufwTNRvESXsHg1k8ImBudfXxr4aEahxPvonzj9vuHNZ0AN36cJwFT91uOwcYXGlzcqZNt8mNTrLBWzQacXjDHPSqYnCGlXk24hisgRO76vq88TWLW6qU9CK0j-w9T-8KIkE-rTwqlgi~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ADP:ATP ratios of sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV 1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. NS=non-significant; **P<0.01; ***P<0.001.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/20/9/10.1093_humrep_dei082/2/m_148029f3.jpeg?Expires=1716477985&Signature=gFXdLyeORN-VxDD0UeCQxSwkNQyjRF7eQh1CiPabCDxbbwHLbCldzb5Tk0hB2AW5L7gp2pOYmVrYfwtU~iS-Py9GGebAWsXneBPNkIHrOg57s9K22Juvj4uW3A8l2W~DPAHvZip0GwDNWWyvyPmjmijuCRPUs53YvkHFbsU0S0DpSVmrSbvxhJ9ResS4THeaJh7XYyl8~3zwvK1hIWx-HqUnurJ~nlhq6J4adfv5VSCfBgyIeiCfD0MEiWv77m5VXr1~rcLVO6TMaquT9xNlRRF0pPP5kLpO3UwXFlBA3vkX~C4vx0H1ViakJKq8DLnkcCQW8bD5wIQm6rM~9~LznQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Measurement of mono- and oligonucleosomal DNA of sperm when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV 1 LPS, 0.1 μg/ml). Data shown are the mean+SEM of incubations with sperm preparations from six patients. NS=non-significant; *P<0.05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/20/9/10.1093_humrep_dei082/2/m_148029f4.jpeg?Expires=1716477985&Signature=Z6FKJMkIJ3YflYy0P8u2bP-33ZIhjKmPPCrzREU3MM3ZaRBX5onuAtutMqEy8sjk2q2sIkcQd7sdfAqJin-4y-~UJ4oV1at7m-YhLy2N~p1YoaTKbvCksfyFLAN900zRMGCZ0S1fETqXJYQ9VuXylmEeqT056qg04rZBNrxUHYkQnc2LFBSIrZBELf2XAdBYMCG65-l~Ip4GkZRfvWK2-Xwgqcvka4RO0LdMH0eUXwXJNWcjo0WqfNzU7KousVe9kA2GqM2bP14WJtUxotD-7zf3WCo~G-l-8CDqs7ZfyiIHiNoJ3wRSZpPh20z3aenJCfH5nBYdtkaeRcREKAAybA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Percentage apoptotic sperm in the presence and absence of PCI (pan-caspase inhibitor) when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Untransformed data shown are the mean±SEM of incubations with sperm preparations from six patients. ***P<0.001.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/20/9/10.1093_humrep_dei082/2/m_148029f5.jpeg?Expires=1716477985&Signature=1gkLlmJMxjD4sD6UG5KOHaqSI8WCpj4v5PJMmGJ0gg5JD4Ixw~Hp41DxKAO3Vpvu~bB4Xeic8Dwl1NiznB2YVshxOVKJgXuY~tGdwoaUEHXC0nOqocINyBheciuvvhuLuwYmSg2DnUETfpnIW~ru2sjD8spFE1WUwuXMGJsazSXsACdQzmwS-gyy3AvevmZf2cGv20xTbmdQwnQ3TZmGGpYRTCI73KDWjrQWBRwuwOvLuWUgXqUF69MFJ4n5I-gAgMmrs9cS6Nm0LThJ8juipH~u17i6b-533xYbmpaNz-YeFQrxgiYElXvK1mIcIdbUoLcCtDBFsaOHM7ub8roDuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Caspase activity of sperm in the presence and absence of C3I (caspase-3 inhibitor) when exposed to the following over a 6 h incubation period: Control (no addition), H/S (heat-shock), S (staurosporine, 1 mmol/l), E [C. trachomatis serovar E lipopolysaccharide (LPS), 0.1 μg/ml], L (C. trachomatis serovar LGV1 LPS, 0.1 μg/ml). Data shown are the mean±SEM of incubations with sperm preparations from six patients. NS=non-significant; **P<0.01; ***P<0.001.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/20/9/10.1093_humrep_dei082/2/m_148029f6.jpeg?Expires=1716477985&Signature=rIuZZNDOPz14olSKw2Z6K8-RF6JVLAAcOQKuebwc~LXCi49t9DlvRKCWu3aXG701P9YNUH6EjcUi4ilKilmNBecQTmUknxyZMJP44bM-jFM1OHDOAZzwZ0k4PvmOpsVNfKihIqwxonJhkjOFsU7Fm9R-rg4cHizgU3Hw9LtMVpiHnn1u81qre4FJkYWQzOAPqiwi2mo8pTyZwV~1cP9A19adO23YOWaR4eh611v2TVagPWCbdytxkdfJJIjWDq2VPRBUq6dQqWzM0u97J~56tS71VYOlcXEVjOq6ijAWDs~-VeenykMcwwteIAxygneWr39on3nAboPgJpx~q0-xhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
