-
PDF
- Split View
-
Views
-
Cite
Cite
V. Remorgida, N. Ragni, S. Ferrero, P. Anserini, P. Torelli, E. Fulcheri, How complete is full thickness disc resection of bowel endometriotic lesions? A prospective surgical and histological study, Human Reproduction, Volume 20, Issue 8, August 2005, Pages 2317–2320, https://doi.org/10.1093/humrep/dei047
Close - Share Icon Share
Abstract
BACKGROUND: This study aims to evaluate the completeness of full thickness disc resection in the treatment of deep endometriotic bowel lesions. METHODS: This study comprised 16 women with bowel endometriotic lesions requiring segmental resection. For the purpose of the study, before intestinal resection, nodulectomy was performed. The presence of endometriotic infiltration in direct continuity with the removed nodule and the presence of fibrosis in the area surrounding the nodule were histologically evaluated. RESULTS: In seven out of 16 cases (43.8%; 95% CI, 19.8–70.1), endometriosis was found in the bowel wall adjacent to the site of nodulectomy; the infiltration was visible in the muscular layer in all cases. In cases of incomplete nodulectomy, the muscular layer of the bowel segment surrounding the endometriotic nodule contained limited or no fibrosis. CONCLUSIONS: Full thickness disc resection is not complete in ≥40% of women with bowel endometriosis. Our finding that fibrosis in the muscular layer, the main landmark during surgical resection, does not always surround bowel endometriotic lesions might explain why incomplete resection may occur.
Introduction
Intestinal involvement occurs in five to 27% of women with pelvic endometriosis, usually affecting the rectosigmoid colon (Weed and Ray, 1987; Jerby et al., 1999; Redwine, 1999; Chapron et al., 2003). A distinction must be drawn between the presence of superficial endometriotic foci on the large bowel serosa which do not cause specific symptoms, and deep infiltrating lesions which can be associated with severe gastrointestinal symptoms (i.e. obstructive symptoms, severe pain and heavy transit disturbances) (Azzena et al., 1998; Mussa et al., 2001; Yantiss et al., 2001; Varras et al., 2002; Weizman and Sullivan, 2003) or less severe disturbances mimicking the irritable bowel syndrome (Kumar, 2004; Remorgida et al., 2005).
The management of colorectal endometriosis is controversial. When surgery is judged to be required, deep infiltrating endometriotic lesions of the bowel can be removed either by full thickness disc resection or by segmental resection (Verspyck et al., 1997; Duepree et al., 2002; Redwine, 2004). For lesions producing partial obstruction, most authors advocate bowel resection (Weed and Ray, 1987; Prystowsky et al., 1988) while full thickness disc resection is the recommended approach to less extensive lesions (Redwine, 2004).
Up to now, the choice between the two different approaches has been more focused on the considerations concerning the reconstruction of bowel wall, rather than on the completeness of surgery. The main aim of the current study is to evaluate the completeness of disc resection in the treatment of deeply infiltrating endometriotic bowel lesions. We also investigated whether laparotomy and laparoscopy yield a different effectiveness in the removal of bowel endometriotic lesions.
Materials and methods
Between October 2003 and December 2004 patients with bowel endometriotic lesions requiring segmental resection were asked to participate in this study. In order to classify patients' bowel habits and complaints, the Rome II Criteria (Thompson et al., 1999) were used under the conditions previously described (Remorgida et al., 2005).
The study included patients requiring bowel resection on the basis of the following criteria: single lesion ≥3 cm in diameter, single lesion infiltrating ≥50% of the bowel wall, and ≥3 lesions infiltrating the muscular layer.
Bowel resection was performed through a laparotomic suprapubic incision after laparoscopic mobilization of the bowel segment(s) involved. For the purpose of the study, before intestinal resection, nodulectomy was performed. During laparoscopy or laparotomy, nodulectomy was performed with electrosurgery (either uni- or bipolar) cutting the serosa around the ‘tip’ of the nodule leaving ≥1 cm of macroscopically normal tissue. After the first incision, the nodule was removed following the ‘cleavage plane’. When the surgeons were satisfied with the completeness of the nodulectomy, segmental resection was performed with automatic stapler. Both the nodule and the resected bowel segment were sent to the pathologist in order to evaluate the presence of endometriosis in the nodule and in the bowel wall adjacent to the removed nodule (Figure 1). The margins of the resected bowel segment were also evaluated for endometriotic infiltration. The anatomical distributions of the removed nodules and of the resected segments were recorded; the largest diameter of the removed nodules and the length of the resected segments were measured.
This study was approved by the local Institutional Review Board. All patients included in the study were informed before surgery of the presence of bowel endometriotic lesions requiring segmental resection. They were informed on the experimental design of this study and provided a written informed consent.
Histological and immunohistochemical evaluation
All surgical specimens were histologically evaluated in a standardized fashion as previously described (Remorgida et al., 2005). The specimens were immediately fixed in 4% formaldehyde for 12 h. The nodules were macroscopically oriented along the intestinal wall (from the serosa towards the mucosa) and cut in macro-sections of 2 mm thickness. From each macrosection, tissue blocks of ± 1.5 cm length were obtained in variable number according to the size of the lesion. Each tissue block was embedded in paraffin and a 5 μm section was obtained for microscopical evaluation. Bowel segments were opened longitudinally through their entire length. Two millimetre longitudinal bands of bowel wall, reaching the two resection margins, were cut (Figure 1). These bands were sampled in tissue blocks and 5 μm sections were obtained for microscopical evaluation. These sections were stained with haematoxylin and eosin and examined histologically.
The purified murine monoclonal antibody against muscle-specific actin (clone HHF 35, dilution 1/50; Biogenex, USA) was used to recognize smooth muscle cells; this antibody does not recognize other muscle filament proteins and it is non-reactive for other mesenchymal or epithelial cells. Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections. After overnight incubation with a 1:50 dilution of the murine monoclonal antibody against muscle-specific actin, slides were rinsed with phosphate-buffered saline (PBS) and incubated with a biotinylated anti-mouse immunoglobulin G (Dako, USA). After rinsing again with TBS, preformed avidin and biotinylated horseradish peroxidase macromolecular complex (Vectastain Elite ABC kit; Vector Laboratories, Inc., USA) was applied for 30 min at room temperature. The antigen–antibody reaction was visualized using diaminobenzidine. Negative control sections were processed by omitting the primary antibody. Positive controls consisted in uterine leiomyomas and normal myometrium.
The histopathological criteria for determining the presence and extension of bowel endometriotic lesions were the presence of both ectopic endometrial glands and stroma. The presence of endometriotic infiltration in the bowel segment representing an extension of the removed nodule was evaluated. The presence of fibrosis in the area surrounding the resected endometriotic nodule was examined. The degree of fibrosis was evaluated in all bowel wall layers. In particular, muscular layer fibrosis was divided into five grades according to the ratio of the fibrotic area to the entire tissue area: grade (G) 0, no fibrosis; G1, <25%; G2, 25–49%; G3, 50–75%; G4, >75%; as previously performed by Itoga et al. (2003) in rectovaginal endometriosis. Two experienced pathologists independently evaluated the degree of fibrosis in all the specimens; in the case of discrepancy between the two, the specimen was examined in a joint session and assigned a final grade agreed by both observers.
Statistical analysis
Statistical analysis was performed by using Fisher's exact test. The analysis was performed using the Statistical Packages for the Social Sciences (SPSS, USA). P<0.05 was considered statistically significant.
Results
Sixteen patients with bowel endometriotic lesions fitting the criteria for segmental resection volunteered to participate in this study.
The diagnosis of bowel endometriosis was suspected on the basis of clinical symptoms and confirmed by using helicoidal computerized tomography. The baseline characteristics of the patients included in the study are listed in Table I.
Concerning the topographic distribution of the endometriotic lesions, 11 were located on the rectosigmoid and five on the sigmoid. In three cases the removal of the nodules required vaginal resection.
Nodulectomy was performed at laparoscopy in 10 cases, and at laparotomy in the remaining six women. The endometriotic nature of the removed nodules was confirmed at histology in all cases. The mean ( ± SD) larger diameter of the resected nodule was 2.9 ± 0.7 cm; the mean ( ± SD) length of the resected segments was 17.0 ± 9.2 cm.
In seven out of 16 cases (43.8%; 95% CI, 19.8–70.1), endometriosis was still present in the bowel wall adjacent to the site of nodulectomy (incomplete nodulectomy); the infiltration reached the muscular layer in all the cases. No significant difference was observed in the presence of persistent disease after laparoscopic (n=4, 40.0%) and laparotomic (n=3, 50.0%) nodulectomy (P=0.549) (Table II). The endometriotic lesions spread laterally in the bowel wall up to 2.6 cm beyond the limit of nodulectomy (mean ± SD, 1.6 ± 0.6 cm).
In the case of incomplete nodulectomy, the muscular layer of the bowel segment surrounding the endometriotic nodule contained G2 fibrosis in one case, G1 fibrosis in three cases, and no fibrosis (G0) in three cases (Figure 2, Table III). Fibrosis in the submucosal layer was found in five cases (71.4%); of those, two were in the G0 group.
In one case the margin of the resected bowel was infiltrated by endometriosis.
Discussion
To the best of our knowledge, this is the first study evaluating the completeness of full thickness disc resection in the treatment of bowel endometriosis. Our findings suggest that full thickness disc resection is not complete in ≥40% of women with bowel endometriosis.
A similar rate of persistent disease was observed after laparoscopic (40%) and laparotomic (50%) full thickness disc resection; suggesting that neither the augmented vision associated with laparoscopy nor the tactile feedback allowed by laparotomy improve the completeness of nodulectomy. This observation is limited by the small number of patients included in the study, which does not allow drawing definitive conclusions on the efficacy of the two surgical techniques.
In the current study, the histological criteria for the diagnosis of bowel endometriosis consisted of the presence of both ectopic endometrial glands and stroma. A rate of incomplete nodulectomy higher than the observed 43.8% could have been found if endometriosis was diagnosed on the basis of less strict criteria. In particular, the presence of endometrial stromal cells in absence of endometrial glands could be relevant considering that these cells are an important infiltrating component of endometriosis. CD10, which is a reliable and sensitive immunohistochemical marker of endometrial stroma both at eutopic and ectopic sites (McCluggage et al., 2001; Sumathi and McCluggage, 2002; Toki et al., 2002), could be used to identify the infiltration of endometrial stromal cells not associated with endometrial glands.
One possible limitation of the current study may be that nodulectomy could have been suboptimally performed. However, all the procedures were performed by a gynaecologist (V.R.) and a gastrointestinal surgeon (P.T.) who have extensive experience in the treatment of bowel endometriosis. Another apparent limitation of the current study may reside in the size of the resected nodules which may be seen as an indication for bowel resection, as the choice between resection and nodulectomy is generally based on considerations concerning the reconstruction of bowel wall, rather than on the completeness of surgery. However, considering that bowel resection was already planned to be performed in all cases, it seems unlikely that surgeons might have been limited in their ‘radicality’ for fear of not being able to adequately suture the bowel wall.
Ideal conditions for performing a complete nodulectomy are the presence of a lesion with the shape of a plaque and completely surrounded by fibrosis which allows the surgeon to detect its limit. Unfortunately, we have demonstrated the absolute absence of fibrosis in 18.8% of the cases and the presence of only mild fibrosis (G1) in 18.8% of the cases. In addition, endometriotic lesions may not have the shape of a plaque; this hypothesis is consistent with the involvement of the margins after bowel resection reported by others (Kavallaris et al., 2003) and found also in our experience. Even more importantly, Anaf et al. (2004) demonstrated that endometriotic lesions infiltrate the large bowel wall preferentially along the nerves, even at distance from the palpated lesion; this observation is consistent with our hypothesis that bowel nodules, rather than having the shape of a plaque, may follow the enteric nervous system and may spread laterally to the point of serosal invasion.
We have previously shown that, in subjects with true bowel endometriosis (penetrating beyond the serosal layer), the surgical removal of the lesions by nodulectomy or by bowel resection is associated with a significant improvement of gastrointestinal symptoms at 1 year (Remorgida et al., 2005); however, this study was characterized by a small number of patients evaluated and by limited length of follow-up. The proper indications for bowel surgery in women with endometriosis and the effect of extreme radicality in effectiveness of cure still remain debatable and were beyond the aims of this study.
Although our findings suggest that full thickness disc resection of bowel endometriotic lesion is often incomplete, it is well known that bowel resection carries the risk of major post-operative complications such as bowel perforations, rectovaginal fistula, perineal abscess and, in some patients, a protective colostomy may be required (Darai et al., 2005). In the light of the fact that women with endometriosis, besides pain, might suffer from infertility and that they do not usually have a life-threatening condition, the surgeons must always weigh the risk of potential complications of surgery against the benefit of the complete removal of bowel endometriotic lesions. To date, no clear guideline exists for the pre-operative assessment of patients with suspected endometriosis; therefore bowel resections should only be performed after a careful pre-operative evaluation of patients' symptoms and a radiological examination of the bowel.
In conclusion, for the first time, this study shows that at least one-third of patients with bowel endometriosis treated by full thickness disc resection have persistent disease. The clinical implications of our finding are still unclear; further investigations should be aimed to evaluate the long-term follow-up of patients treated by the intestinal resection and nodulectomy.
Formaldehyde-fixed bowel nodule and segment. Two millimetre longitudinal bands of bowel wall, reaching the two resection margins, were cut. These bands were sampled in tissue blocks and 5 μm sections were obtained for microscopical evaluation.
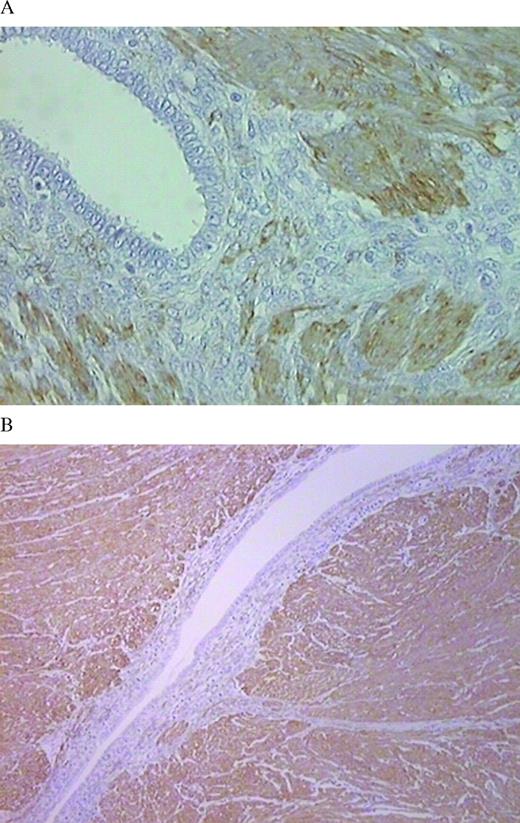
Muscle-specific actin immunohistochemistry of the resected bowel in a case of incomplete nodulectomy. The endometriotic lesions are completely surrounded by smooth muscle cells; there is no fibrosis (G0) in the muscular layer. Original magnification: (A) ×25; (B) ×10.
Baseline characteristics of the study population (n=16)
| Age (years) (mean ± SD) | 33.8 ± 2.1 | |
| Body mass index (kg/m2) (mean ± SD) | 20.8 ± 1.5 | |
| Previous surgery for endometriosis, n (%) | 5 (31.1) | |
| Pre-operative gastrointestinal symptomsa , n (%) | ||
| Irritable bowel syndrome predominantly diarrhoea | 3 (18.8) | |
| Irritable bowel syndrome predominantly constipation | 5 (31.1) | |
| Functional abdominal pain syndrome | 6 (37.5) | |
| Unspecified functional abdominal pain | 2 (12.5) | |
| Age (years) (mean ± SD) | 33.8 ± 2.1 | |
| Body mass index (kg/m2) (mean ± SD) | 20.8 ± 1.5 | |
| Previous surgery for endometriosis, n (%) | 5 (31.1) | |
| Pre-operative gastrointestinal symptomsa , n (%) | ||
| Irritable bowel syndrome predominantly diarrhoea | 3 (18.8) | |
| Irritable bowel syndrome predominantly constipation | 5 (31.1) | |
| Functional abdominal pain syndrome | 6 (37.5) | |
| Unspecified functional abdominal pain | 2 (12.5) | |
Classified according to the Rome II criteria (Thompson et al., 1999).
Baseline characteristics of the study population (n=16)
| Age (years) (mean ± SD) | 33.8 ± 2.1 | |
| Body mass index (kg/m2) (mean ± SD) | 20.8 ± 1.5 | |
| Previous surgery for endometriosis, n (%) | 5 (31.1) | |
| Pre-operative gastrointestinal symptomsa , n (%) | ||
| Irritable bowel syndrome predominantly diarrhoea | 3 (18.8) | |
| Irritable bowel syndrome predominantly constipation | 5 (31.1) | |
| Functional abdominal pain syndrome | 6 (37.5) | |
| Unspecified functional abdominal pain | 2 (12.5) | |
| Age (years) (mean ± SD) | 33.8 ± 2.1 | |
| Body mass index (kg/m2) (mean ± SD) | 20.8 ± 1.5 | |
| Previous surgery for endometriosis, n (%) | 5 (31.1) | |
| Pre-operative gastrointestinal symptomsa , n (%) | ||
| Irritable bowel syndrome predominantly diarrhoea | 3 (18.8) | |
| Irritable bowel syndrome predominantly constipation | 5 (31.1) | |
| Functional abdominal pain syndrome | 6 (37.5) | |
| Unspecified functional abdominal pain | 2 (12.5) | |
Classified according to the Rome II criteria (Thompson et al., 1999).
Presence of persistent disease after laparoscopic and laparotomic nodulectomy
| Full thickness disc resection . | Location of the lesion . | Persistent disease in the resected segment . | ||
|---|---|---|---|---|
| Laparoscopy (n=10) | ||||
| Rectosigmoid | 7 | 2 | ||
| Sigmoid | 3 | 2 | ||
| Laparotomy (n=6) | ||||
| Rectosigmoid | 4 | 2 | ||
| Sigmoid | 2 | 1 | ||
| Full thickness disc resection . | Location of the lesion . | Persistent disease in the resected segment . | ||
|---|---|---|---|---|
| Laparoscopy (n=10) | ||||
| Rectosigmoid | 7 | 2 | ||
| Sigmoid | 3 | 2 | ||
| Laparotomy (n=6) | ||||
| Rectosigmoid | 4 | 2 | ||
| Sigmoid | 2 | 1 | ||
Presence of persistent disease after laparoscopic and laparotomic nodulectomy
| Full thickness disc resection . | Location of the lesion . | Persistent disease in the resected segment . | ||
|---|---|---|---|---|
| Laparoscopy (n=10) | ||||
| Rectosigmoid | 7 | 2 | ||
| Sigmoid | 3 | 2 | ||
| Laparotomy (n=6) | ||||
| Rectosigmoid | 4 | 2 | ||
| Sigmoid | 2 | 1 | ||
| Full thickness disc resection . | Location of the lesion . | Persistent disease in the resected segment . | ||
|---|---|---|---|---|
| Laparoscopy (n=10) | ||||
| Rectosigmoid | 7 | 2 | ||
| Sigmoid | 3 | 2 | ||
| Laparotomy (n=6) | ||||
| Rectosigmoid | 4 | 2 | ||
| Sigmoid | 2 | 1 | ||
Muscular layer fibrosis in the resected bowel segment surrounding the lesions removed by nodulectomy
| . | Complete nodulectomy (n=9) . | Incomplete nodulectomy (n=7) . |
|---|---|---|
| G0, no fibrosis | 0 | 3 |
| G1, <25% | 0 | 3 |
| G2, 25–49% | 2 | 1 |
| G3, 50–75% | 4 | 0 |
| G4, >75% | 3 | 0 |
| . | Complete nodulectomy (n=9) . | Incomplete nodulectomy (n=7) . |
|---|---|---|
| G0, no fibrosis | 0 | 3 |
| G1, <25% | 0 | 3 |
| G2, 25–49% | 2 | 1 |
| G3, 50–75% | 4 | 0 |
| G4, >75% | 3 | 0 |
Muscular layer fibrosis in the resected bowel segment surrounding the lesions removed by nodulectomy
| . | Complete nodulectomy (n=9) . | Incomplete nodulectomy (n=7) . |
|---|---|---|
| G0, no fibrosis | 0 | 3 |
| G1, <25% | 0 | 3 |
| G2, 25–49% | 2 | 1 |
| G3, 50–75% | 4 | 0 |
| G4, >75% | 3 | 0 |
| . | Complete nodulectomy (n=9) . | Incomplete nodulectomy (n=7) . |
|---|---|---|
| G0, no fibrosis | 0 | 3 |
| G1, <25% | 0 | 3 |
| G2, 25–49% | 2 | 1 |
| G3, 50–75% | 4 | 0 |
| G4, >75% | 3 | 0 |
References
Anaf V, El Nakadi I, Simon P, van de Stadt J, Fayt I, Simonart T and Noel JC (
Azzena A, Litta P, Ferrara A, Perin D, Brotto M, Chiarelli S and Sandei F (
Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, Vacher-Lavenu MC and Dubuisson JB (
Darai E, Thomassin I, Barranger E, Detchev R, Cortez A, Houry S and Bazot M (
Duepree HJ, Senagore AJ, Delaney CP, Marcello PW, Brady KM and Falcone T (
Itoga T, Matsumoto T, Takeuchi H, Yamasaki S, Sasahara N, Hoshi T and Kinoshita K (
Jerby BL, Kessler H, Falcone T and Milsom JW (
Kavallaris A, Kohler C, Kuhne-Heid R and Schneider A (
Kumar D (
McCluggage WG, Sumathi VP and Maxwell P (
Mussa FF, Younes Z, Tihan T and Lacy BE (
Prystowsky JB, Stryker SJ, Ujiki GT and Poticha SM (
Redwine DB (
Redwine DB (
Remorgida V, Ragni N, Ferrero S, Anserini P, Torelli P and Fulcheri E (
Sumathi VP and McCluggage WG (
Toki T, Shimizu M, Takagi Y, Ashida T and Konishi I (
Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ and Muller-Lissner SA (
Varras M, Kostopanagiotou E, Katis K, Farantos C, Angelidou-Manika Z and Antoniou S (
Verspyck E, Lefranc JP, Guyard B and Blondon J (
Weizman DA and Sullivan P (
Author notes
1Department of Obstetrics and Gynaecology, 2Department of General Surgery and Transplant, San Martino Hospital and University of Genoa, Largo R. Benzi 1, 16132 Genoa and 3Di.C.M.I., Unit of Anatomy and Histopathology, San Martino Hospital and University of Genoa, Via De Toni 14, 16132 Genoa, Italy