-
PDF
- Split View
-
Views
-
Cite
Cite
Carole Marchetti, Nathalie Jouy, Brigitte Leroy-Martin, André Defossez, Pierre Formstecher, Philippe Marchetti, Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility, Human Reproduction, Volume 19, Issue 10, October 2004, Pages 2267–2276, https://doi.org/10.1093/humrep/deh416
Close - Share Icon Share
Abstract
BACKGROUND: Sperm motility evaluation is associated with fertility in IVF programmes. The visual estimation of sperm motility is extremely subjective. Hence, alternative methods are required. Among them, determination of mitochondrial membrane potential (Δψm) changes of spermatozoa using potentiometric dyes may be a reliable test to determine sperm quality. However, the use of the potentiometric dyes in sperm samples has not been compared. METHODS: We have studied sperm samples from 28 infertile patients enrolled in an IVF programme in flow cytometry after staining of spermatozoa with four commonly used potentiometric dyes. Sperm motility was evaluated visually. RESULTS: As expected, JC-1 seems to detect specifically Δψm changes, CMX-Ros, DiOC6(3) and TMRE fluorescence is easily analysed and the latter three fluorochromes are particularly suitable for multiparametric staining. Irrespective of the Δψm-dependent fluorochromes used to stain spermatozoa, a positive correlation was found between the percentage of Δψmhigh cells and forward motility and also with high fertilization rates after IVF. CONCLUSION: The four fluorochromes may be useful for evaluation of sperm samples from infertile patients. The choice of the potentiometric dyes will depend on their fluorescence characteristics in order to use them in combination with other fluorescent markers.
Introduction
Failed fertilization happens in 5–10% of IVF cycles and may result from defective spermatozoa and/or oocytes. In the IVF setting, most cases result from male factor deficiencies (Liu and Baker, 2000). Consequently, a variety of tests assessing sperm quality may be useful in determining the likelihood of successful IVF (for a review see Mahutte and Arici, 2003). Among them, standard semen analysis including sperm count, motility and morphology is the most commonly used as a fundamental indicator of male fertility. However, semen analysis has limited clinical value for predicting IVF since 50% of couples with failed fertilization have a normal pre-IVF semen analysis (Liu and Baker, 2000). Due to extreme variability in estimating sperm motility, it is important to develop objective measurements. Thus, other sperm function tests are needed to improve the clinical management of patients and to allocate them to the best assisted reproductive technology programme (IVF or ICSI).
Analysing mitochondrial function may offer a means of assessing the motility of sperm. This can be achieved by determining the inner mitochondrial membrane potential (Δψm) in sperm cells. The Δψm is a sensitive indicator for the energetic state of the mitochondria and the cell, and can be used to assess the activity of the mitochondrial respiratory chain, electrogenic transport systems and the activation of the mitochondrial permeability transition (for a review see Ly et al., 2003). Thus, determination of Δψm is widely used for characterization of cellular metabolism, viability and apoptosis in various cellular models. In human, a correlation exists between poor sperm mitochondrial function detected by reduction of Δψm, and diminished motility and reduced fertility (Troiano et al., 1998; Donnelly et al., 2000; Marchetti et al., 2002; Piasecka and Kawiak, 2003; Wang et al., 2003).
Several fluorimetric methods using cationic lipophilic dyes have been utilized to measure the Δψm. The cationic lipophilic dyes accumulate in mitochondria depending on Δψm, and the fluorescence of the accumulated fluorochromes corresponds to this potential. During the past decades, rhodamine 123 (Rh123) has been widely used as a fluorescent probe to assess Δψm. However, contradictory data indicated that this probe was not fully satisfactory to measure Δψm because of the existence of several energy-independent Rh123-binding sites (Lopez-Mediavilla et al., 1989).
To overcome these drawbacks, several potential sensitive dyes were developed including rosamines, rhodamine and carbocyanine derivatives: (i) Chloromethyl-X-rosamine (CMX-Ros) dye, that contains a mildly thiol-reactive chloromethyl moiety, is much more photostable than Rh123 and constitutes a valuable dye to analyse mitochondrial morphology and function (Poot et al., 1996); (ii) The rhodamine derivative tetramethylrhodamine ethyl ester (TMRE) which has reduced hydrophobic character, also exhibits less potential-independent binding to cells than other rhodamines and has been described as one of the best fluorescent dyes for Δψm measurements in living cells (Loew et al., 1993). However, like other rhodamines, TMRE used at high concentration induces fluorescence quenching so that an increase in mitochondrial fluorescence corresponds to depolarization (O'Reilly et al., 2003); (iii) The carbocyanine derivative 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] offers the important advantage of not causing quenching effects (Metivier et al., 1998) but DiOC6(3) uptake depends on both mitochondrial membrane and plasma membrane potentials (Salvioli et al., 1997); (iv) The carbocyanine fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) has been proposed to evaluate changes in Δψm accurately (Salvioli et al., 1997).
Thus, interpreting and evaluating changes in Δψm may be somewhat confusing because there are substantial variations between these dyes depending on various susceptibilities to the surrounding environment. Therefore, comparing results obtained with different potentiometric dyes is useful to select the most accurate probe for a particular application.
In recent years, many investigators have used lipophilic cationic fluorochromes including Rh123 (Troiano et al., 1998), JC-1 (Donnelly et al,. 2000; Piasecka and Kawiak, 2003) and DiOC6(3) (Marchetti et al., 2002; Wang et al., 2003) for Δψm determinations in sperm samples. A comparison of the ability of these potentiometric dyes to evaluate Δψm in sperm samples has not been made. To our knowledge, fluorescence from CMX-Ros or TMRE has never been analysed in human spermatozoa. Flow cytometry, when used in conjunction with Δψm-dependent fluorochromes, could be an ideal method to study the mitochondrial potential in sperm samples. Indeed, flow cytometry provides a rapid, accurate and reliable estimation of the Δψm in a large number of cells and is of considerable relevance for laboratory practice.
Therefore, we have studied sperm samples from infertile patients enrolled in an IVF programme in flow cytometry after concomitantly staining with CMX-Ros, DiOC6(3), TMRE and JC-1.
We have compared the results obtained with these dyes and have established the correlation with the quality of sperm evaluated by conventional light microscopic analysis in spermatozoa prepared for IVF. This approach allowed us to discuss the advantages and limitations of Δψm-dependent cytofluorometric assays for assessment of sperm quality in the reproductive biology laboratory.
Materials and methods
Materials
Ferticult medium was purchased from Fertipro NV (Beernem, Belgium) and PureSperm gradient from NidaCon International AB (Gothenburg, Sweden). DiOC6(3), JC-1, TMRE, CMX-Ros, propidium iodide (PI) and YOPRO-1 were obtained from Molecular Probes Inc. (Eugene, OR). All other reagents were purchased from Sigma Chemical Co. (St Louis, MO).
Collection of semen samples
We studied 28 male subjects who underwent seminal fluid evaluation at the Laboratory of Reproductive Biology (CHRU, Lille). All subjects were the partners of women who had failed to conceive after 2 years of unprotected intercourse. Patient information remained confidential and within the institution. This study was conducted according to guidelines established for research on human subjects (Ethical committee, CHRU Lille). The samples were collected by masturbation into sterile plastic jars, after 3–5 days of sexual abstinence.
Preparation of semen samples
To isolate spermatozoa, an aliquot of semen was purified using a three-step discontinuous Pure Sperm gradient (90–70–50%) diluted in Ferticult medium. After centrifugation at 300 g for 20 min, purified populations of highly motile spermatozoa (from the 90% layer) were recovered, washed in Ferticult medium, and resuspended in 1 ml of the same medium. Prepared spermatozoa were counted and the percentage of forward motile spermatozoa was calculated. Prepared sperm was used for IVF and aliquots taken for cytofluorometric experiments.
Conservation of spermatozoa before Δψm labelling
Typically, purified spermatozoa were subjected to flow cytometry within 1 h. In some experiments, we set up conditions of spermatozoa conservation that allow for the retention of the Δψm. For this experiment, samples were stored at 4°C, room temperature or 37°C in medium until the times (0, 30, 60 and 120 min) that the staining with the potentiometric dyes were performed (see below).
Cytofluorometric assessment of mitochondrial membrane potential
Stock solutions of JC-1 (2.5 mmol/l), TMRE (1 mmol/l) and CMX-Ros (1 mmol/l) were made in dimethylsulphoxide (DMSO). DiOC6(3) (4 mmol/l) was dissolved in ethanol. Stock solutions were stored in small aliquots at −20°C, and subsequent working solutions [dilutions 1:250 for JC-1, 1:40 for TMRE; 1:200 for CMX-Ros; 1:2000 for DiOC6(3)] were made in experimental medium immediately before use. A total of 5 × 105 spermatozoa were incubated in the Ferticult medium with the fluorochromes at 37°C, followed by analysis on a cytofluorometer. Except when indicated for Figures 1 and 2, we used optimal conditions of staining defined as 50 nmol/l CMX-Ros for 20 min, 20 nmol/l DiOC6(3) for 20 min, 250 nmol/l TMRE for 20 min, and 1 μmol/l JC-1 for 30 min. All flow cytometry experiments were performed on a Coulter XL cytofluorometer (Coulter Corp., Hialeah, FL). Data were acquired using Expo 32 software (Coulter). The analyser threshold was adjusted on the forward scatter channel to exclude subcellular debris. Forward and side scatters were gated on the major population of normal size cells and a minimum of 10 000 cells was analysed. The fluorescent probes DiOC6(3), JC-1, CMX-Ros and TMRE were excited with the 488 nm argon laser. Signals from the DiOC6(3) fluorescence were collected through the FL1 detector (525±5 nm band pass filter), CMX-Ros through the FL3 detector (620±5n m band pass filter) and TMRE through the FL2 channel (575±5 nm band pass filter). The fluorescence signals of JC-1 monomers and aggregates were detected through the FL1 (525±5 nm band pass filter) and FL2 channels (575±5 nm band pass filter), respectively. Control experiments were performed in the presence of carbamoylcyanide m-chlorophenylhydrazone (ClCCP) or the K+ ionophore valinomycin. ClCCP is a protonophore that uncouples oxidation from phosphorylation by dissipating the chemiosmotic gradient and induces dissipation of Δψm. Spermatozoa were incubated with 0.5 μmol/l ClCCP or 100 nmol/l valinomycin for 30 min at 37°C then stained with the potentiometric dyes as described above.
Double staining of Δψm and cell viability
YOPRO-1 and PI were used as supravital fluorescent stains. Both YOPRO-1 and PI are membrane-impermeant nuclear fluorescent dyes. When plasma membrane integrity is altered, these dyes enter cells (non-viable cells), bind to nucleic acids and exhibit fluorescence. YOPRO-1 and PI were excited at 488 nm. YOPRO-1 fluorescence was monitored in FL1 (525±5 nm band pass filter) and PI in FL3 (620±5 nm band pass filter). These emission wavelengths allow us to define double staining with potentiometric dyes. We used the following probe combinations: CMX-Ros and YOPRO-1; DiOC6(3) and PI; and TMRE and YOPRO-1. Preliminary results shown that Δψm fluorescence did not change with YOPRO-1 or PI addition.
Spermatozoa were exposed for 20 min at 37°C to CMX-Ros (50 nmol/l) and YOPRO-1 [200 nmol/l in phosphate-buffered saline (PBS)], to DiOC6(3) (20 nmol/l) and PI (5 μg/ml) or to TMRE (250 nmol/l) and YOPRO-1 (200 nmol/l in PBS). Immediately after staining, spermatozoa were analysed by flow cytometry.
Fluorescence microscopy
Immediately after the Δψm staining procedure, counterstaining of nuclei was performed with Hoescht 33342 1 μg/ml for 10 min in the dark. Then, cells were washed once in PBS and resuspended in Vectashield H-100 mounting medium (Vector Laboratories, Burlingame, CA), coverslipped and analysed with a Leica DMLR epifluorescence microscope, using a Leica 63×/1.32 HCX PL APO objective (Leica S.A., Rueil Malmaison, France). Images were captured using Leica software.
Statistical analysis
Results were analysed using GraphPad Prism version 3.00 (GraphPad Software, San Diego CA). The Pearson rank correlation test was used to calculate the correlation coefficient between flow cytometric analyses. The Spearman rank correlation test was employed to evaluate the relationship between semen analysis parameters and cytofluorometric results. Statistical significance was set at P<0.05. To test the reproducibility of the assays, 10 replicates from a single sample were processed and acquired by the same operator for each staining, then typical intra-assay precision tests were performed.
Results
Conventional semen analysis
Table I shows patients' age and contains results of classical semen analysis performed by light microscopy with samples from a total of 28 men consulting for sterility.
Typical representation of cytofluorometric profiles after spermatozoa staining
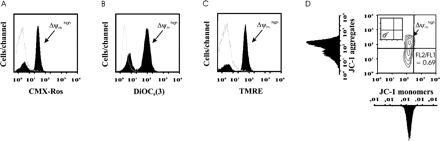
Figure 1 represents an example of cytofluorometric profiles of spermatozoa stained with the four putative Δψm-sensitive fluorescence probes used in this study. All these potentiometric probes are lipophilic cationic fluorochrome able to detect Δψm in viable cells. They distribute passively between the cytosol and mitochondria according to the Nernst equation, where transmembrane distribution depends on the mitochondrial membrane potential. Flow cytometric analysis of spermatozoa stained with either CMX-Ros (Figure 1A), DiOC6(3) (Figure 1B) or TMRE (Figure 1C) revealed in each case two cellular subsets displaying different levels of fluorescence. These two subpopulations had distinct incorporation of fluorochromes into spermatozoa. One subpopulation that incorporated more (1–1.5 log more) fluorochrome which corresponded to spermatozoa with high fluorescence signals [CMX-Roshigh, DiOC6(3) or TMREhigh] were called Δψmhigh cells (Figure 1A, B and C, respectively). For each fluorochrome, the percentage of cells with Δψmhigh was determined in sperm samples and used for the study (see below).
To monitor the Δψm, we have also chosen the carbocyanine dye JC-1 which has been shown to be more reliable than other fluorescent dyes for detecting changes in Δψm due to its dual emission characteristics (Cossarizza et al., 1996). Mitochondria with Δψmhigh concentrate JC-1 into aggregates (red-orange fluorescence in the FL2 channel), while in depolarized mitochondria JC-1 forms mainly monomers (green fluorescence in the FL1 channel). A two-parameter fluorescence display of JC-1-stained spermatozoa reveals that most of the cells emitted relatively high levels of both green and orange-red fluorescence, whereas a subpopulation exhibited a reduced JC-1 aggregation and a decrease in the orange-red fluorescence emission, a finding that indicates a drop in Δψm (Figure 1D). By flow cytometry, a high correlation has been found previously between mitochondrial membrane potential values in isolated mitochondria and fluorescence ratio (mean red-orange fluorescence intensity/mean green fluorescence intensity corresponding to the FL2/FL1 ratio) (Cossarizza et al,. 1996). Therefore, in order to determine the population of cells with Δψmhigh in sperm samples after JC-1 staining, we evaluated both the percentage of cells which concentrate JC-1 into aggregates (high fluorescence of JC-1 red-orange in the upper left quadrant) called Δψmhigh cells and the values of the fluorescence ratio (JC-1 red-orange/JC-1 green or FL2/FL1 ratio) (Figure 1D).
Optimization of conditions for spermatozoa labelling
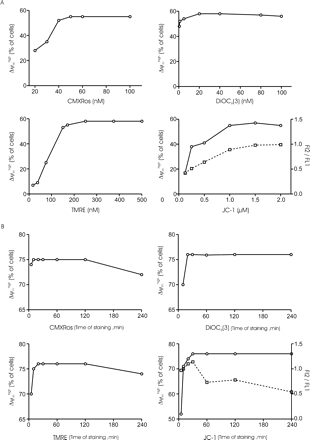
Incubation of spermatozoa with different concentrations of fluorochromes revealed that the percentage of cells emitting high fluorescence was influenced by the concentration of dye used (Figure 2A). This study indicated that the minimal dose of dye required to achieve an effective spermatozoa loading was 50 nmol/l of CMX-Ros, 20 nmol/l of DiOC6(3), 250 nmol/l of TMRE and 1 μmol/l of JC-1.
Figure 2B represents the time course for the uptake of dyes used at optimal concentrations. Under these conditions, a 20 min period of incubation was enough for DiOC6(3), CMX-Ros and TMRE to equilibrate into the cells. For JC-1 staining, a 30 min period of incubation was needed and allowed a better separation of the high fluorescence peak of JC-1 red-orange (data not shown). These patterns of cell fluorescence were stable and remained unchanged for at least 120 min of incubation in the medium at 37°C, except for the FL2/FL1 ratio.
In the optimal conditions of staining based on the above results, fluorescence microscopy (Figure 3) was used to verify that each dye accurately measured the correct sperm compartment. The spermatozoa stained with the Δψm-sensitive fluorescence probes in conditions defined above were counterstained with the DNA marker Hoechst 33342. In each case, a high level of fluorescence was associated with the sperm midpiece where mitochondria are located. No other portion of spermatozoa displayed fluorescence that could be detected microscopically, indicating a characteristic mitochondrial uptake of all fluorochromes.
Sensitivity of potentiometric dyes to Δψm changes
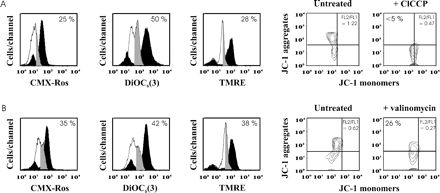
Under the optimal conditions of staining defined above, we used mitochondria-targeted drugs to ascertain that the fluorochromes were able to measure Δψm variations in spermatozoa. Exposure of spermatozoa to ClCCP significantly reduced fluorochrome incorporation into cells by 0.5–1 logs for all fluorochromes used (Figure 4A).
To confirm the ability of fluorochromes to detect changes in Δψm, we also used valinomycin, which is able to collapse Δψm (Figue 4B). After 30 min of incubation with valinomycin, spermatozoa stained with CMX-Ros, DiOC6(3), TMRE or JC-1 changed their fluorescence pattern, as observed after ClCCP incubation.
These results indicate that under our experimental conditions, the four potentiometric fluorochromes reveal a Δψm variation in spermatozoa. However, when we displayed the percentage of ionophore-treated spermatozoa which retain a high Δψm (grey profiles in Figure 4A and B), the remaining fluorescence was much lower in JC-1-stained spermatozoa than in cells labelled with the other fluorochromes.
We also found a highly significant relationship between all four cytofluorometric methods in samples (Table II), confirming that any potential-sensitive fluorochromes detect Δψm changes of spermatozoa.
To test the reproducibility of the assays, 10 replicates of a sperm sample were analysed by each staining. The mean percentage of Δψmhigh was 95±0.4 by CMX-Ros staining, 91.8±1.4 by DiOC6(3) staining, 92.6±1 by TMRE staining and 88.5±1 by JC-1 staining. We found that the coefficients of variation (CVs) of these flow cytometric assays were 0.5% for the CMX-Ros staining, 1.6% for the DiOC6(3) staining, 1.1% for the TMRE staining and 1.2% for the JC-1 staining.
Conservation of spermatozoa before Δψm labelling
Results showed that spermatozoa stored at 4°C undergo a decrease in the percentage of Δψmhigh cells irrespective of the Δψm-dependent dye used to stain spermatozoa (Figure 5). In any case, prepared spermatozoa stored at room temperature or at 37°C maintained a constant proportion of Δψmhigh cells at least for the first 60 min.
Simultaneous determination of Δψm and cell viability
A double staining procedure was developed to assess Δψm and cell viability simultaneously in spermatozoa (Figure 6). We used the following probe combinations: CMX-Ros and YOPRO-1 (Figure 6A), DiOC6(3) and PI (Figure 6B) and TMRE and YOPRO-1 (Figure 6C), in order to have compatible emission wavelengths. All non-viable spermatozoa (YOPRO-1- or PI-marked spermatozoa) had low Δψm [CMX-Roslow, DiOC6(3)low or TMRElow], whereas viable spermatozoa (YOPRO-1- or PI-negative) contained two distinct subpopulations, one that exhibited a reduction in Δψm comparable with non-viable spermatozoa and the other that displayed high Δψm (Figure 6). This suggest that the Δψm collapse occurs at an early stage before the loss of viability.
Correlation between Δψmhigh cells and sperm motility
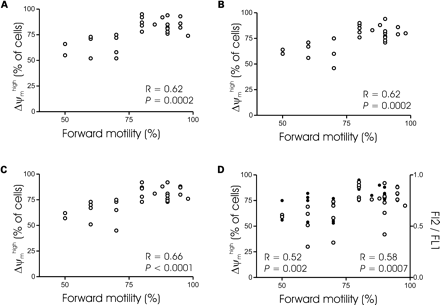
Regarding the relationship between the percentage of spermatozoa that were highly motile and the percentage of Δψmhigh spermatozoa measured by the four fluorochromes, a positive correlation was found to be significant for all Δψm-dependent dyes (Figure 7).
Correlation between Δψmhigh cells and the fertilization rate
We also determined the correlation between the percentage of Δψmhigh spermatozoa determined by the four potentiometric dyes and fertilization rates (Table III). Fertilization rate correlated positively with Δψmhigh cells for all Δψm-dependent dyes.
Discussion
The assessment of Δψm in intact spermatozoa is attracting growing interest since functional mitochondria have been related mainly to sperm motility. Thus, the assessment of Δψm could represent an important test to determine the quality of sperm of infertile men enrolled in an assisted reproductive programme. Cationic lipophilic fluorochromes have been widely used to assess the functionality of mitochondria in numerous cells including spermatozoa (Garner et al., 1997; Troiano et al., 1998; Gravance et al., 2000, 2001; Marchetti et al., 2002; Piasecka and Kawiak, 2003; Wang et al., 2003). These fluorochromes are permeable to the plasma membrane and therefore can be easily used on living cells to assess Δψm changes. However, the behaviour of the dyes may depend on environmental events independent of the Δψm. It has been observed that some of these fluorochromes (i) are highly toxic and interfere with the bioenergetic function of mitochondria (Modica-Napolitano et al., 1996); (ii) may undergo self-quenching upon accumulation in the mitochondrial matrix which is responsible for a paradoxical relationship between fluorochrome concentration and fluorescence (Metivier et al., 1998); (iii) may show non-specific interaction with lipids or thiols (Ferlini et al., 1998); (iv) may be influenced by the multidrug resistance-associated proteins (Kuhnel et al., 1997); and (v) may depend on the magnitude of plasma membrane potential (Δψp) (Rottenberg and Wu, 1998).
Thus, determining advantages and limitations of different Δψm-sensitive fluorescence probes in spermatozoa is an essential step to develop efficient test(s) to evaluate sperm quality.
We first optimized the conditions of dye labelling. To increase the actual contribution of Δψm to cell fluorescence, it has been suggested to reduce dye concentrations, i.e. the dye/cell ratio. Indeed, lowering the dye/cell ratio reduces the toxicity, the quenching effect and the importance of the Δψp in cell fluorescence (Rottenberg and Wu, 1998). Thus, we have chosen the lowest concentration of fluorochromes (and the shortest incubation time) that do not reduce the percentage of Δψmhigh spermatozoa and that allow us to discriminate unambiguously between spermatozoa with high and low Δψm. However, even in these optimal conditions, we cannot exclude that Δψm-independent processes could contribute at least in part to the fluorescence observed.
For an ideal Δψm-sensitive fluorescence probe, specific alterations of the Δψm should result in decreased uptake of the fluorescence probe in the mitochondrial matrix. To test the capacity of the dyes to detect Δψm variations, we pre-incubated spermatozoa with uncouplers (Figure 4). We used the protonophore ClCCP and the K+ ionophore valinomycin because it has been demonstrated that ClCCP may change the Δψp in human spermatozoa (Guzman-Grenfell et al., 2000). Valinomycin was used at concentrations that modulate the Δψm without collapsing the Δψp (Rottenberg and Wu, 1998). In these experimental conditions, we confirmed that the four classical dyes, DiOC6(3), CMX-Ros, TMRE and JC-1, are sensitive enough to detect changes in Δψm induced by mitochondrial uncouplers. ClCCP and valinomycin caused a complete modification of JC-1 fluorescence, suggesting that JC-1 had an exclusive distribution to mitochondria. This observation is consistent with the fact that JC-1 is considered as a fluorochrome which can measure Δψm with great accuracy in intact cells (Salvioli et al., 1997) including cardiomyocytes (Mathur et al., 2000) and spermatozoa (Troiano et al., 1998). In contrast, the uncoupler-induced decrease in CMX-Ros, DiOC6(3) and TMRE fluorescence was much smaller as the peak was in an intermediate position between those of spermatozoa with high and low fluorescence (Figure 4), indicating that CMX-Ros, DiOC6(3) and TMRE staining respond not only to Δψm changes but also to other non-specific (Δψm-independent) processes. Thus, JC-1 appears to be the best probe to detect specifically Δψm changes in spermatozoa.
For an ideal probe suitable for explicit determination of Δψm in clinical samples, the resolution between the Δψmhigh and Δψmlow fluorescence peaks should be maximal. As shown here, cytofluorometric profiles of spermatozoa stained with either CMX-Ros, DiOC6(3) or TMRE unambiguously revealed two distinct populations with Δψmhigh and Δψmlow. In any case, the determination of the percentage of Δψmhigh spermatozoa was easily done by setting markers on histograms and using statistical functions of the software. JC-1 differs from rhodamines and other carbocyanines because it produces two fluorescence emission peaks that reflect the existence of two forms of the dye. The JC-1 monomers which emit green fluorescence are predominant at low Δψm, while the JC-1 aggregates (red-orange fluorescence) are predominant at high Δψm. Typically, it is described that upon lowering the Δψm, the JC-1 aggregates dissipate into monomers and lead to a shift from red to green fluorescence. In fact, the intensity of the green fluorescence from the JC-1 monomer form seems to be insensitive to Δψm changes (Cossarizza et al., 1996) and was instead used to monitor changes in mitochondrial mass (Mancini et al., 1997). In spermatozoa, we found that disruption of Δψm does not lead to a significant increase in green fluorescence even after incubation with valinomycin (Figure 4B). Consequently, we have relied on the red-orange fluorescence emission of JC-1 aggregates to monitor changes in Δψm. Nevertheless, as shown in Figure 1, the discrimination of spermatozoa with reduced and high Δψm was rather difficult since the resolution between the two fluorescence peaks of JC-1 aggregates was weak in many samples (see Figure 1D). Thus, it is more subjective to set markers on the JC-1 aggregate histograms. In our study, the use of JC-1 would lead to variations in the determination of the percentage of Δψm and could explain why the correlation factor among Δψm-dependent fluorochromes is lower with JC-1 (Table II). One other possibility is to employ JC-1 as a ratiometric probe since a strict correlation has been found between the FL2/FL1 ratio and the Δψm in isolated mitochondria (Cossarizza et al., 1996). Consistent with previous results (Gravance et al., 2000; Mathur et al., 2000), assessment of JC-1 staining by a ratiometric analysis did not provide better results. This is not surprising because variations in the mitochondrial mass, which can influence the FL2/FL1 ratio independently of changes in Δψm, have been demonstrated in some cases of asthenospermia (Piasecka and Kawiak, 2003). Thus, the analysis of the percentage of Δψmhigh stained with JC-1 seems to be more subjective than after staining with other potential-sensitive dyes.
To develop objective measurements of Δψm in semen samples, it is fundamental to achieve standardized protocols. First, we demonstrated acceptable reproducibility. Secondly, it is important to establish what, if any, is the influence on the Δψm of storage conditions of spermatozoa before flow cytometric analysis. Indeed, Δψm-dependent fluorochromes are used on living cells and inadequate storage could seriously alter the Δψm of spermatozoa. The results of our study indicate that a conservation period at 4°C of spermatozoa in culture medium decreases the percentage of Δψmhigh spermatozoa irrespective of the fluorochromes used. The recommendations from our study are that spermatozoa should be stored in culture medium at room temperature or 37°C for a maximum period of 60–120 min before flow cytometric analysis if they cannot be used immediately.
We compared the evaluation of Δψmhigh by four fluorochromes with respect to their ability to correlate with forward motility. Prepared sperm with high Δψm correlated with forward motility, thus confirming the strong link between the functional status of mitochondria and sperm cell quality (Marchetti et al., 2002; Wang et al., 2003). Whereas JC-1 and DiOC6(3) have been used previously for sperm sample evaluation (Donnelly et al., 2000; Marchetti et al., 2002; Piasecka and Kawiak, 2003; Wang et al., 2003), it is, to our knowledge, the first report describing CMX-Ros and TMRE as valuable probes to measure Δψm in sperm samples. Importantly, we found that all Δψm-dependent fluorochromes were able to predict successful IVF, providing additional evidence supporting the importance of a flow cytometric Δψm-based test in evaluation of spermatozoa for clinical studies.
Spermatozoa need to possess many attributes including a high motility in order to fertilize an oocyte, and sperm may be infertile for numerous reasons. Therefore, measuring multiple sperm parameters simultaneously on individual spermatozoa should provide a better indication of fertilizating capacity than one single parameter. One advantage of the flow cytometry is the possibility of evaluating, in combination, multiple fluorescent markers associated with individual spermatozoa in a population (Graham, 2001). Thus, flow cytometry should be used to analyse multiple sperm parameters (including the determination of Δψm and cell viability) to enhance the capacity to estimate the fertilizing potential of semen samples. For this reason, it is important to have several reliable Δψm-dependent probes emitting in different fluorescence channels, which can be used in combination with other probes evaluating different sperm attributes. In order to evaluate Δψm and cell viability simultaneously, we developed double staining protocols. We used CMX-Ros, DiOC6(3) and TMRE as potential-sensitive dyes because they produce a single fluorescent emission peak allowing the combination with supravital fluorochromes. In contrast, JC-1 emits two fluorescence peaks (green and orange-red) which preclude simultaneous assessment of cell viability by commonly used supravital probes, because of fluorescence overlap. However, it should be noted that JC-1 has been used recently in combination with the impermeant dye TOTO-3 to investigate cell death (Zuliani et al., 2003). Nevertheless, the major limitation of this method is the need to use a cytofluorometer equipped with multiple lasers to excite both TOTO-3 and JC-1. Thus, in contrast to JC-1, the fluorochromes CMX-Ros, DiOC6(3) or TMRE permit the development of a simple method in combination with other probes for multiparametric evaluation of sperm quality.
In conclusion, our results indicate that four classical Δψm-dependent fluorochromes provide valuable tests assessing changes in mitochondrial membrane potential of human spermatozoa and may be usable for evaluation of sperm sample quality from infertile patients. These Δψm changes can be easily detected using cytoflurometric analysis of spermatozoa. Whereas JC-1 detects Δψm changes in spermatozoa more specifically than other dyes tested, CMX-Ros, DiOC6(3) and TMRE fluorescence is easily analysed and these fluorochromes are particularly suitable for multiparametric staining. The choice of the Δψm-dependent fluorochromes will depend on their fluorescence characteristics in order to use them in combination with other sperm attribute-dependent fluorescent markers.
Representative examples of stained spermatozoa. Cytofluorometric analysis of histograms of spermatozoa from one sample stained with the following potentiometric dyes: (A) CMX-Ros 50 nmol/l for 20 min, (B) DiOC6(3) 20 nmol/l for 20 min and (C) TMRE 300 nmol/l for 20 min (black profiles). Unlabelled sample is represented by white profiles. (D) Example of a contour plot (and corresponding histograms) of JC-1-stained spermatozoa (JC-1 1.5 μmol/l for 20 min). The insert shows a contour plot of unlabelled spermatozoa.
Variations of the percentage of Δψmhigh spermatozoa depending on experimental conditions. (A) Representative dose response of the loading concentration of fluorochromes. Spermatozoa were incubated in medium for 30 min with different doses of CMX-Ros (upper left panel), DiOC6(3) (upper right panel), TMRE (lower left panel) or JC-1 (lower right panel) and analysed by flow cytometry. The results are representative of three samples, each from a different patient. (B) Kinetics of fluorochrome uptake. Spermatozoa were incubated with 50 nmol/l CMX-Ros (upper left panel), 20 nmol/l DiOC6(3) (upper right panel), 250 nmol/l TMRE (lower left panel) or 1 μmol/l JC-1 (lower right panel). At the indicated times, cells were analysed by flow cytometry. The results are representative of five samples, each from a different patient. The percentage of Δψmhigh cells was calculated as previously described. When cells are stained with JC-1 (lower right panel in A and B), the open circles indicate the values of the percentage of Δψmhigh spermatozoa and the open squares represent the values of the FL2/FL1 ratio. (A) and (B) refer to different samples.
Photomicrographs of spermatozoa labelled with fluorochromes. The sample was stained with either 50 nmol/l CMX-Ros for 20 min (A), 20 nmol/l DiOC6(3) for 20 min (B), 250 nmol/l TMRE for 20 min (C) or 1 μmol/l JC-1 for 30 min (D), then counterstained with the nuclear dye, Hoechst 33342. Spermatozoa that were stained with CMX-Ros or TMRE displayed midpieces (where mitochondria are located) that fluoresced bright red, and DiOC6(3)-stained midpieces appeared green. After JC-1 staining (D), the left and right panels show the monomer (green) and aggregate (red) fluorescence, respectively. In this case, a perfect co-localization of green and red fluorescence signals was observed. Original magnification ×630.
Cytofluorometric analysis of Δψm in spermatozoa treated with depolarizing agents. Fluorescence pattern of spermatozoa treated with ClCCP (A) or valinomycin (B) then stained with either CMX-Ros, DiOC6(3), TMRE or JC-1 according to staining procedures defined in Figure 3. Fluorescence histograms (left) of spermatozoa kept untreated (black profiles) or treated with depolarizing agents (white profiles) are represented when spermatozoa are stained with either CMX-Ros, DiOC6(3) or TMRE. When cells are stained with JC-1, contour plots are indicated (right). Quadrant boundaries were set with reference to untreated spermatozoa. Grey profiles correspond to the ionophore-treated spermatozoa which maintain a high Δψm. Numbers indicate the percentage of ionophore-treated spermatozoa with Δψmhigh (grey profiles). The results are representative of five samples, each from a different patient. (A) and (B) refer to different samples.
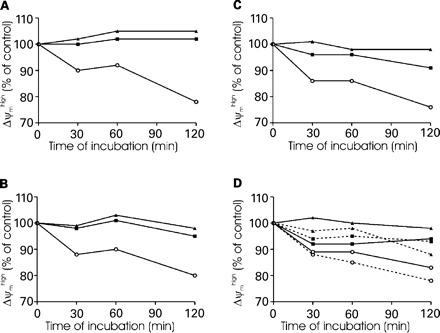
Effect of sample storage on Δψm. Spermatozoa were kept at 4°C (○), room temperature (▪) or 37°C (▴) over time (120 min). At the indicated times, cells were stained with either CMX-Ros (A), DiOC6(3) (B), TMRE (C) or JC-1 (D) according to staining procedures defined in Figure 3. Results are expressed as a percentage of Δψmhigh cells at time zero (control). When cells are stained with JC-1 (D), the results are presented both as a percentage of Δψmhigh cells at time zero (solid lines) and as a percentage of the FL2/FL1 ratio at time zero (dotted lines). Data are representative of three samples, each from a different patient.
Representative examples for simultaneous Δψm measurement and cell viability in spermatozoa. Spermatozoa were stained with either CMX-Ros and YOPRO-1 (A), DiOC6(3) and PI (B), or TMRE and YOPRO-1 (C) as described in Materials and methods. The abscissa indicates the fluorescence intensity of spermatozoa stained with potentiometric dyes [CMX-Ros, DiOC6(3) or TMRE] and the ordinate indicates the fluorescence intensity of spermatozoa stained with impermeant nuclear dyes (YOPRO-1 or PI). Numbers indicate the percentage of spermatozoa in each quadrant. Results are representative of two samples, each from a different patient.
Relationship between forward motility and the percentage of Δψmhigh spermatozoa. Spermatozoa from 28 subjects were stained with either CMX-Ros (A), DiOC6(3) (B), TMRE (C) or JC-1 (D) according to staining procedures defined in Figure 3. Results are expressed as a percentage of Δψmhigh cells. R indicates the Spearman correlation factor. When cells are stained with JC-1 (D), open circles indicate the values of the percentage of Δψmhigh spermatozoa and closed circles represent the values of the FL2/FL1 ratio. R and P indicate the statistical values when results are expressed as a percentage of Δψmhigh cells (left) or as a FL2/FL1 ratio (right).
Patient age, and sperm characteristics of semen and prepared spermatozoa from infertile patients
| . | No. of samples analysed . | Mean±SEM . | Minimum . | Median . | Maximum . |
|---|---|---|---|---|---|
| Patient age | 28 | 35±1 | 20 | 34.5 | 55 |
| Sperm concentration (×106/ml) | 28 | 101±17 | 25 | 74 | 232 |
| Progressive motility (a+b) in neat semen (%) | 28 | 30±3 | 5 | 30 | 50 |
| Forward motility (a) in prepared spermatozoa (%) | 28 | 79±3 | 50 | 80 | 98 |
| Normal morphology by David's criteria (%) | 28 | 43±3 | 10 | 44 | 72 |
| . | No. of samples analysed . | Mean±SEM . | Minimum . | Median . | Maximum . |
|---|---|---|---|---|---|
| Patient age | 28 | 35±1 | 20 | 34.5 | 55 |
| Sperm concentration (×106/ml) | 28 | 101±17 | 25 | 74 | 232 |
| Progressive motility (a+b) in neat semen (%) | 28 | 30±3 | 5 | 30 | 50 |
| Forward motility (a) in prepared spermatozoa (%) | 28 | 79±3 | 50 | 80 | 98 |
| Normal morphology by David's criteria (%) | 28 | 43±3 | 10 | 44 | 72 |
Patient age, and sperm characteristics of semen and prepared spermatozoa from infertile patients
| . | No. of samples analysed . | Mean±SEM . | Minimum . | Median . | Maximum . |
|---|---|---|---|---|---|
| Patient age | 28 | 35±1 | 20 | 34.5 | 55 |
| Sperm concentration (×106/ml) | 28 | 101±17 | 25 | 74 | 232 |
| Progressive motility (a+b) in neat semen (%) | 28 | 30±3 | 5 | 30 | 50 |
| Forward motility (a) in prepared spermatozoa (%) | 28 | 79±3 | 50 | 80 | 98 |
| Normal morphology by David's criteria (%) | 28 | 43±3 | 10 | 44 | 72 |
| . | No. of samples analysed . | Mean±SEM . | Minimum . | Median . | Maximum . |
|---|---|---|---|---|---|
| Patient age | 28 | 35±1 | 20 | 34.5 | 55 |
| Sperm concentration (×106/ml) | 28 | 101±17 | 25 | 74 | 232 |
| Progressive motility (a+b) in neat semen (%) | 28 | 30±3 | 5 | 30 | 50 |
| Forward motility (a) in prepared spermatozoa (%) | 28 | 79±3 | 50 | 80 | 98 |
| Normal morphology by David's criteria (%) | 28 | 43±3 | 10 | 44 | 72 |
Correlations (Pearson correlation test) among flow cytometric methods for the detection of Δψm changes in spermatozoa
| r . | DiOC6(3)a . | CMX-Rosb . | TMREc . | JC-1d . | JC-1 ratioe . |
|---|---|---|---|---|---|
| DiOC6(3)a | 0.94* | 0.97* | 0.78* | 0.66* | |
| CMX-Rosb | 0.96* | 0.70* | 0.65* | ||
| TMREc | 0.74* | 0.64* | |||
| JC-1d | 0.60* |
| r . | DiOC6(3)a . | CMX-Rosb . | TMREc . | JC-1d . | JC-1 ratioe . |
|---|---|---|---|---|---|
| DiOC6(3)a | 0.94* | 0.97* | 0.78* | 0.66* | |
| CMX-Rosb | 0.96* | 0.70* | 0.65* | ||
| TMREc | 0.74* | 0.64* | |||
| JC-1d | 0.60* |
Percentage of DiOC6(3)high.
Percentage of CMX-Roshigh.
Percentageof TMREhigh.
Percentage of JC-1 aggregateshigh.
Ratio of JC-1 aggregates/JC-1monomers.
*P<0.05.
Correlations (Pearson correlation test) among flow cytometric methods for the detection of Δψm changes in spermatozoa
| r . | DiOC6(3)a . | CMX-Rosb . | TMREc . | JC-1d . | JC-1 ratioe . |
|---|---|---|---|---|---|
| DiOC6(3)a | 0.94* | 0.97* | 0.78* | 0.66* | |
| CMX-Rosb | 0.96* | 0.70* | 0.65* | ||
| TMREc | 0.74* | 0.64* | |||
| JC-1d | 0.60* |
| r . | DiOC6(3)a . | CMX-Rosb . | TMREc . | JC-1d . | JC-1 ratioe . |
|---|---|---|---|---|---|
| DiOC6(3)a | 0.94* | 0.97* | 0.78* | 0.66* | |
| CMX-Rosb | 0.96* | 0.70* | 0.65* | ||
| TMREc | 0.74* | 0.64* | |||
| JC-1d | 0.60* |
Percentage of DiOC6(3)high.
Percentage of CMX-Roshigh.
Percentageof TMREhigh.
Percentage of JC-1 aggregateshigh.
Ratio of JC-1 aggregates/JC-1monomers.
*P<0.05.
Correlations of cytofluorometric Δψm markers in spermatozoa with fertilization rate
| Percentage spermataozoa . | n . | Spearman coefficient r . | P-value . |
|---|---|---|---|
| CMXRoshigh | 28 | 0.40 | 0.02 |
| DiOC6(3)high | 28 | 0.44 | 0.01 |
| TMREhigh | 28 | 0.36 | 0.03 |
| JC-1high | 28 | 0.36 | 0.03 |
| JC-1 ratio | 28 | 0.34 | 0.03 |
| Percentage spermataozoa . | n . | Spearman coefficient r . | P-value . |
|---|---|---|---|
| CMXRoshigh | 28 | 0.40 | 0.02 |
| DiOC6(3)high | 28 | 0.44 | 0.01 |
| TMREhigh | 28 | 0.36 | 0.03 |
| JC-1high | 28 | 0.36 | 0.03 |
| JC-1 ratio | 28 | 0.34 | 0.03 |
Correlations of cytofluorometric Δψm markers in spermatozoa with fertilization rate
| Percentage spermataozoa . | n . | Spearman coefficient r . | P-value . |
|---|---|---|---|
| CMXRoshigh | 28 | 0.40 | 0.02 |
| DiOC6(3)high | 28 | 0.44 | 0.01 |
| TMREhigh | 28 | 0.36 | 0.03 |
| JC-1high | 28 | 0.36 | 0.03 |
| JC-1 ratio | 28 | 0.34 | 0.03 |
| Percentage spermataozoa . | n . | Spearman coefficient r . | P-value . |
|---|---|---|---|
| CMXRoshigh | 28 | 0.40 | 0.02 |
| DiOC6(3)high | 28 | 0.44 | 0.01 |
| TMREhigh | 28 | 0.36 | 0.03 |
| JC-1high | 28 | 0.36 | 0.03 |
| JC-1 ratio | 28 | 0.34 | 0.03 |
We thank Professor Remi Neviere and Dr Hubert Andre for critical reading of the manuscript. This work was supported by grants from IFR114-IMPRT, INSERM, Faculté de médecine-Université de Lille II and CHRU de Lille.
References
Cossarizza A, Ceccarelli D and Masini A (
Donnelly ET, O'Connell M, McClure N and Lewis SE (
Ferlini C, Scambia G and Fattorossi A (
Garner DL, Thomas CA, Joerg HW, DeJarnette JM and Marshall CE (
Graham JK (
Gravance CG, Garner DL, Baumber J and Ball BA (
Gravance CG, Garner DL, Miller MG and Berger T (
Guzman-Grenfell AM, Bonilla-Hernandez MA and Gonzalez-Martinez MT (
Kuhnel JM, Perrot JY, Faussat AM, Marie JP and Schwaller MA (
Liu DY and Baker HW (
Loew LM, Tuft RA, Carrington W and Fay FS (
Lopez-Mediavilla C, Orfao A, Gonzalez M and Medina JM (
Ly JD, Grubb DR and Lawen A (
Mahutte NG and Arici A (
Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB and Hockenbery DM (
Marchetti C, Obert G, Defossez A, Formstecher P and Marchetti P (
Mathur A, Hong Y, Kemp BK, Barrientos AA and Erusalimsky JD (
Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I and Kroemer G (
Modica-Napolitano JS, Koya K, Weisberg E, Brunelli BT, Li Y and Chen LB (
O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA and Walsh JV Jr (
Piasecka M and Kawiak J (
Poot M, Zhang YZ, Kramer JA, Wells KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL and Haugland RP (
Rottenberg H and Wu SL (
Salvioli S, Ardizzoni A, Franceschi C and Cossarizza A (
Troiano L, Granata AR, Cossarizza A, Kalashnikova G, Bianchi R, Pini G, Tropea F, Carani C and Franceschi C (
Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T and Agarwal A (
Author notes
1INSERM U459, 3Laboratoire d'Histologie, 4IFR 114-IMPRT, Faculté de Médecine 1, Place Verdun, 59045 Lille Cedex and 2Laboratoire de Biologie de la Reproduction, Hôpital Jeanne de Flandre, 59037 Lille Cedex, France




![Representative examples for simultaneous Δψm measurement and cell viability in spermatozoa. Spermatozoa were stained with either CMX-Ros and YOPRO-1 (A), DiOC6(3) and PI (B), or TMRE and YOPRO-1 (C) as described in Materials and methods. The abscissa indicates the fluorescence intensity of spermatozoa stained with potentiometric dyes [CMX-Ros, DiOC6(3) or TMRE] and the ordinate indicates the fluorescence intensity of spermatozoa stained with impermeant nuclear dyes (YOPRO-1 or PI). Numbers indicate the percentage of spermatozoa in each quadrant. Results are representative of two samples, each from a different patient.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/19/10/10.1093/humrep/deh416/2/m_111362f6.gif?Expires=1716381182&Signature=M6ETBBD20VPijZumgDqQCfRPxTiWqSIJ0I1AcXKH9SQz38MBV3tS5k-gUO-iBM-sWw30cBlpQncvV3ZwNfHoHBfvMHlDnHuiTDNx6tmxSN~wsDS~dGfp4jToY34NYlYHGr~Dco3JZcFJFxPJ9uYE9SLnswEiYN5lOmsOtCG29piiDWMGgFYiUIDfsjPAmZED2b0IdAc4GEFpwhuR7YViSuru9NtDGJwXitDTVKcU4j2bmH18vMtQqdLuDBMZs3ImO4dBMvJT4Itf3eCB0bc~7~EzryLuzidmNB6QJy8pOKGYkepzGMBkkKOKmNKs3YY6q7U8lgO0-sPhjEDRzUjkmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)