-
PDF
- Split View
-
Views
-
Cite
Cite
Jing Yang, Lv-xia Dai, Xing Pan, Hongren Wang, Bei Li, Jie Zhu, Ming-yuan Li, Xin-li Shi, Bao-ning Wang, Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB, Pathogens and Disease, Volume 73, Issue 5, July 2015, ftv026, https://doi.org/10.1093/femspd/ftv026
Close - Share Icon Share
Chronic gastric infection by the Gram-negative bacterium Helicobacter pylori (H. pylori) is strongly associated with gastritis, gastric ulcer and the development of distal gastric carcinoma and gastric mucosal lymphoma in humans. Antibiotic treatment of H. pylori is becoming less effective because of increasing antibiotic resistance; other treatment approaches such as specifically targeted methods, etc. to destroy this organism would be beneficial. An epitope vaccine is a promising option for protection against H. pylori infection. In this study, a multi-epitope vaccine was constructed by linking cholera toxin B subunit (CTB), two antigenic fragments of H. pylori urease I subunit (UreI20–29, UreI98–107) and four antigenic fragments of H. pylori urease B subunit (UreB12–23, UreB229–251, UreB327–400, UreB515–561), resulting in the recombinant CTB-UreI-UreB (BIB). Its protective effect against H. pylori infection was evaluated in BALB/c mice. Significant protection against H. pylori challenge was achieved in BALB/c mice immunized with BIB (15/18, 83.3%), rIB plus rCTB (6/18, 33.3%) and rIB (2/18, 11.1%) separately, while no protective effect was found in the mice immunized with either adjuvant rCTB alone or PBS. The induction of significant protection against H. pylori is possibly mediated by specific serum IgA and mucosal sIgA antibodies, and a mixed Th1/Th2/Th17 cells response. This multi-epitope vaccine might be a promising vaccine candidate that helps to control H. pylori infection.
INTRODUCTION
Helicobacter pylori is associated with gastrointestinal illnesses such as chronic gastritis, peptic ulcer, gastric lymphoma and gastric cancer (Strugatsky et al.2012). Currently, about half of the Chinese population are infected with H. pylori, most of them don't experience symptoms, but it is causing an increased incidence of gastrointestinal problems in China (Liu, Xie and Cheng 2012). Based on the available epidemiological evidence, the World Health Organization's International Agency for Research on Cancer classified H. pylori as a group I carcinogen in 1994 (Wang Yuan and Hunt 2007). The most used standard treatment consists of two antibiotics and a proton pump inhibitor, sequential therapy or conventional levofloxacin containing quadruple therapy now becomes less successful because of antibiotic resistance (Malfertheiner et al.2012). Therefore, this problem combined with high percentage rates of reinfection and low hygiene standards in developing countries makes the production of effective vaccines a high priority (Graham and Fischbach 2010; Czinn and Blanchard 2011; Malfertheiner et al.2012; Zawahir et al.2013). There is not an effective vaccine against H. pylori which was recognized so far, although several vaccine candidates have been tested.
Different kinds of vaccines have been formulated aiming at protecting against H. pylori infection, including whole-cell vaccine (Adrian and Chen 1994), recombinant subunit antigen vaccine (Arêas et al.2004; Liu et al.2011) and DNA vaccine (Chen et al.2012); however, no major breakthrough has been achieved. Comparing to traditional vaccines, epitope vaccines display many advantages. The most outstanding advantage for an epitope vaccine is that it can avoid the disadvantages of virulence recovery. In addition, an epitope vaccine is non-toxic and stable. Therefore, the epitope vaccine meets the developing direction of future vaccines. Epitope-vaccine design requires epitope screening, the optimization of epitope combination and construction, and assembly of adjuvant factors and epitope stability factors, etc. (Sette and Fikes 2003). Combining epitopes from special antigens, epitope-based vaccines are designed to induce more specific and potent immune responses than the whole antigens (Sette and Fikes 2003; Yang et al.2013).
Helicobacter pylori can produce a large amount of urease that catalyzes the hydrolysis of urea. The active H. pylori urease has a polymeric structure that comprises two subunits, UreA and UreB (Strugatsky et al.2012). UreB is the subunit responsible for enzyme activity, which is critical for the virulence of H. pylori (Zhang et al.2011). The antibody against UreB capable of destroying urease activity can protect the stomach from colonization by H. pylori (Fujii et al.2004; Li et al.2010; Qiu et al.2010; Yang et al.2013). Therefore, an antigenic fragment containing the active center of urease might serve as a good vaccine candidate (Guo et al.2012b; John et al.2013). Another clinical target, the inner membrane protein UreI of H. pylori, which is the proton-gated inner-membrane urea channel, is a key factor for colonization of H. pylori in acidic mammalian stomach (Strugatsky et al. 2012).
Cholera toxin B subunit (CTB) is the non-toxic portion of cholera toxin (CT) and can bind to the GM1 ganglioside receptor, a glycolipid that is expressed in most cells in human body, including immune cells (Arêas et al.2004). Coupling the antigen to CTB increased the immunogenicity as it helped receptor-mediated uptake and subsequent presentation by the antigen presenting cells (APCs) (Guo et al.2014; Price, McFann and Holmes 2013).
In this study, a multi-epitope vaccine was constructed by linking CTB, two antigenic fragments of H. pylori urease I subunit (UreI20–29, UreI98–107) and four antigenic fragments of H. pylori urease B subunit (UreB12–23, UreB229–251, UreB327–400, UreB515–561), resulting in the recombinant CTB-UreI-UreB (BIB). Its immunogenicity, specificity, ability to induce neutralizing antibodies against H. pylori urease, and prophylactic efficacy were evaluated in BALB/c mice.
MATERIALS AND METHODS
Animals
Specific pathogen-free (SPF) BALB/c mice, 6–8 weeks of age, 16 ± 2 g, half male and half female, were purchased from the Experimental Animal Center of Sichuan University and were bred in an axenic environment. All experiments were approved by the Animal Ethical and Experimental Committee of Sichuan University.
Bacteria and culture conditions for infection
Mouse-adapted H. pylori strain SS1 was obtained from the National Center for Disease Control and Prevention (NCDC) (collection number: CPU-BS-09). The bacteria were cultured on Columbia blood agar plates enriched with 10% defibrinated horse blood (OXOID, UK) under microaerobic conditions (5% O2, 10% CO2, 85% N2) at 37°C for 3–4 days. Helicobacter pylori were harvested and resuspended in Brucella Broth (OXOID) and the final concentration was adjusted to 1 × 109 colony forming units (CFU) mL–1 before inoculation.
Epitope vaccine design, construction and purification
The theoretically optimal combination of the intramolecule adjuvant CTB (GenBan:NP_231099.1), the linkers, the antigenic fragments of urease B subunit (GenBank:NP_222789.1) and the antigenic fragments of urease I subunit (GenBank:NP_222788.1) was established on the basis of modeling and prediction using RANKPEP, DNASTAR software and molecular operating environment (MOE).
In order to construct the expression vector pET28a(+)/ctB-ureI-ureB expressing the fusion protein BIB, a DNA fragment named ctB-ureI-ureB containing tandem copies of the selected B-cell epitopes, T-cell epitopes and ctB was synthesized. The fusion gene ctB-ureI-ureB was cloned into pET28a(+) through BamHI and Xho I sites, generating the plasmid pET28a(+)/ctB-ureI-ureB. The recombinant vector pET28a(+)/ctB-ureI-ureB was transformed into Escherichia coli coli BL21(DE3) for the expression of the BIB. After inducing IPTG (Takara, Japan), bacteria were harvested by centrifugation, and inclusion body pellets were isolated by centrifugation and dissolved. BIB was purified by cation exchange chromatography using SP Sepharose XL (GE Healthcare, USA) in 20 mM sodium phosphate (pH 7.0) and was eluted with phosphate buffer including 0.5 M NaCl (pH 7.0). After refolding, the samples were dialyzed against PBS (20 mM sodium phosphate, pH 7.0) and finally concentrated and stored at −70°C. The purity of the BIB was assessed by 12% SDS-PAGE and high-pressure liquid chromatography. The N-terminus sequence of BIB was determined by Edman degradation on an Applied Biosystem Procise N-terminal 494 protein Sequencer (APT, Shanghai, China).
Western blot
Purified fusion proteins BIB, previously prepared rUreB, rIB, rUreI and rCTB (SCWK, Sichuan, China), were applied to 12% SDS-PAGE under denaturing conditions and electrotransfered onto a nitrocellulose membrane at 80 mA for 2 h. Non-specific binding sites were blocked overnight at 4°C in blocking buffer (5% non-fat milk in PBST, pH 7.4, with 0.05% Tween 20). Membranes were washed three times for 15 min with PBST and further incubated with a 1:2000 dilution of mouse polyclonal anti-BIB serum, a 1:10 000 dilution of mouse monoclonal anti-rCTB serum (SCWK, Sichuan, China) or normal mouse serum at 37°C for 1 h. After washing three times with PBST, the membrane was incubated in horseradish peroxidase HRP-conjugated goat antimouse IgG (ZCGB-BIO, Beijing, China) at 37°C for 1 h. The protein bands were detected using ECL chemiluminescence reagents (Pierce, USA).
Immunization and infection
SPF BALB/c mice were randomized into five groups (n = 18 mice, half male and half female) and were respectively vaccinated intragastrically with 200 μg of antigen (BIB, rIB plus rCTB, rIB, rCTB or PBS) in 0.2 M sodium hydrogen carbonate buffer for three times at 2 week intervals. Two weeks after the final booster vaccination, all mice were inoculated with H. pylori SS1 dilution (0.2 mL, 108 CFU mL–1) by oral gavage for three times during a 5 day period with one day between each gavage. The mice were bred for 4 weeks and evaluated the protection against H. pylori infection.
Microbial analysis of infection
This protocol was adapted from a published method (Zhou et al.2009; Houghton 2012). Briefly, mice were sacrificed 4 weeks after H. pylori challenged. The stomach was opened along the greater curvature, rinsed in PBS and the gastric mucosa was used for microbial culture, biopsy, urease test and bacterial DNA detection.
Helicobacter pylori cells were cultured on selective plates containing Columbia blood agar plates containing 10% horse blood, 5 μg mL−1 trimethoprim, 0.33 μg mL−1 polymyxin B sulfate, 10 μg mL−1 vancomycin hydrochloride, 5 μg mL−1 Cefsulodin sodium salt, and 8 μg mL−1 amphotericin B (Sigma, USA) under microaerobic conditions. After 4–6 days culture, H. pylori was confirmed by characteristic colony morphology and catalase, oxidase and urease activities. Colonies were counted and the number of CFU per stomach was calculated. The urease test was used for rapid detection of H. pylori in the gastric mucosa. Helicobacter pylori DNA were prepared from gastric tissue by QIAGEN DNA Tissue Mini Kit. Polymerase chain reaction (PCR) was used for the detection of H. pylori as described by Houghton JM (Houghton 2012).
To investigate the immunoprotectivity of the recombinant protein BIB after the challenge, three methods, i.e., biopsy tissue direct rapid smear method and culture method, PCR, rapid urease test, were used to detect H. pylori colonization in gastric tissue of the mice. If two of the three tests were positive, positive H. pylori infection was judged. Data were expressed as percentage protection. The percentage protection was calculated according to the following formula. Protection rate (%) = (uninfected in the group/survived in the group) × 100%.
Assay for H. pylori-specific antibody
The antibody levels were assessed by ELISA (Zhou et al.2009). Helicobacter pylori SS1 were harvested from the Columbia plate, washed twice with PBS and were lysed by sonication. The supernatant was collected after centrifugation at 13 000 × g for 15 min. ELISA plates were coated with H. pylori lysates (1 μg mL−1, 100 μL well−1) at 4°C overnight and were blocked with 5% bovine serum albumin (Sigma, USA). On day 28 after the last challenge, the mice were sacrified, and stomach tissue in the mice was sampled under sterile condition. To measure the serum IgG, blood was collected immediately before sacrifice. The serum was isolated and diluted 1:1000 before assay. To assess specific mucosal IgA production, half the stomach was homogenized in 1 mL PBS containing 2 mM phenylmethylsulfonyl fluoride, 0.05 M ethylenediaminetetraacetic acid and 0.1 mg mL−1 of soybean trypsin inhibitor (Invitrogen, USA). The supernatant was collected and diluted 1:5 for analysis of specific IgA antibodies. Each sample was added to the H. pylori lysates-coated plate. Then HRP-conjugated goat antimouse IgG or IgA antibody (Sigma, USA) was added. The plate was incubated at 37°C for 1 h. After three times washed with PBS containing 0.05% Tween 20, the conjugated HRP was visualized using the O-phenylenediamine as substrate and the absorbance at 490 nm was measured. Each sample was tested three times.
Helicobacter pylori urease neutralization assay
A urease neutralization test was performed as previously described (Guo et al.2012a). Briefly, mice IgG in the antiserum was purified using a Protein A Sepharose column (GE Healthcare, USA). The H. pylori lysates containing urease (50 μL) were incubated with 50 μL serial dilutions of purified antiserum IgG (0.5–24 μg mL−1) from different groups in 96-well microtiter plates overnight at 4°C, then 50 μL of 50 mM phosphate buffer (pH 6.8) containing 0.5 M urea, 0.02% phenol red and 0.1 mM DTT was added to each well. The plates were incubated at 37°C. Color development was measured at 578 nm at 30 min intervals over a period of 3 h. Percentage inhibition was determined by the following equation: [(activity without antiserum−activity with antiserum)/(activity without antiserum)] × 100%.
Cellular immune responses
For the proliferation assay (Zhou et al.2009; Houghton 2012), lymphocyte suspensions were prepared from spleen, and cultured (2 × 105 cells well−1) with the antigen (BIB, rIB plus rCTB, rIB, rCTB) in RPMI-1640 in 96-well round-bottom plates at 37°C, 5% CO2 for 72 h. To determine proliferation, the cells were pulsed with 1 μCi of [3H] thymidine (3H-TdR) (Amersham Bioscience, UK) for the last 6–8 h of culture. The cellular DNA was collected with a cell harvester (Skatron, Norway) on glass fiber filters and assayed for 3H incorporation using a liquid scintillation counter 10 (Beckman, Sweden). The results were expressed as stimulation indices (SI).
To determine cytokine production, lymphocytes were isolated from spleens, and cultured (2 × 105 cells well−1) with H. pylori lysates (0.5 μg mL−1) in RPMI-1640 in 96-well flat-bottom plates at 37°C in 10% CO2 for 96 h. After that, the supernatants were collected, and interleukin-4 (IL-4), interferon gamma (IFN-γ) and interleukin-17 (IL-17) were assayed by ELISA Kits (R&D, USA).
Histological analysis
One set of biopsies was fixed in 4% buffered formaldehyde and then embedded in paraffin. Sections 4 μm thick were cut, dewaxed and stained with hematoxylin and eosin (HE). For evaluation of gastritis, HE-stained sections were scored based on the degree of infiltrating lymphocytes, plasma cells and neutrophils (Houghton 2012).
Statistical analyses
One-way ANOVA and the non-parametric Mann-Whitney U-test were used to evaluate the difference among groups. Data were expressed as mean ± standard deviation (SD). P < 0.05 was considered as statistically significant. (*P < 0.05, **P < 0.01, ***P < 0.001; ns: not significant).
RESULTS
Design of multi-epitope vaccine BIB
Urease B subunit (UreB) was the most effective and common immunogen of all strains of H. pylori, which was widely used as a potential vaccine antigen (Li et al.2010; Qiu et al.2010; Wang et al.2010; Liu et al.2011; Zhang et al.2011; John et al.2013; Yang et al.2013). UreI (urea membrane channel protein) is another highly conserved H. pylori protein and can be used as antigen and a good molecular marker for identification of H. pylori (Strugatsky et al.2012). In order to retain the immunogenicity of the three proteins UreB, UreI and CTB, we constructed the multi-epitope vaccine BIB on the basis of prediction and modeling using RANKPEP, DNASTAR, MOE software and MHC class-II binding peptide prediction server. Comprehensive analysis of the results forecast led us to select three fragments, CTB25–124, UreI (UreI20–29, UreI98–107) and UreB (UreB12–23, UreB229–251, UreB327–400, UreB515–561), theoretically strong antigenic fragments that include MHC Class-II binding regions. A three-amino-acid spacer, EFM was used at linkage sites between CTB and UreI to decrease the interaction between them. The linker between UreI fragments and UreB fragments was selected as KK. By adding the appropriate spacer sequence between epitopes, the epitopes in the vaccine could function independently, and the formation of new epitopes could be avoided. KK is the target sequence for lysosomal protease, which is an important enzyme for MHC-II antigen presentation. Two epitopes connected by KK could be cut properly and play effect independently during antigen presentation. EFM helps recognize T cell and induce T-cell proliferation. The peptide linker is non-immunogenic to humans. (Yano et al.2005; Mackiewicz et al.2010).
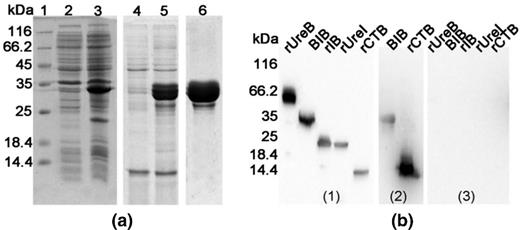
BIB expression, purification and antigenicity
The fusion protein BIB was expressed and purified as previously described research (Yang et al.2015; Yang et al.2013; Wang et al.2011). After purification, the purity of the fusion protein BIB, analyzed by 12% SDS-PAGE and computer scan, was about 92.3% of total protein (Fig. 1a). N-terminal amino-acid sequencing confirmed that the BIB was expressed as expected. Western blot was performed to assess the antigenicity of the BIB. As shown in Fig. 1b, the antiserum induced by the BIB reacted with rCTB, rUreI and rUreB while a negative serum did not. This indicated that BIB had good immunogenicity and immunoreactivity, and also suggested that CTB, UreI and UreB were present in the fusion protein. In addition, BIB also reacted with mouse monoclonal anti-CTB serum (Fig. 1b).
Expression, purification and antigenicity of BIB (a) Expression and purification of BIB analyzed by 12% SDS-PAGE. Lane 1 Protein marker, lanes 2 the proteins of E. coli BL21(DE3), lanes 3 the proteins of E. coli BL21(DE3) expressing BIB (33 kDa), lanes 4 the soluble body proteins of E. coli BL21(DE3) expressing BIB, lane 5 BIB in inclusion bodies washed with phosphate buffer, lane 6 BIB purified by SP Sepharose XL chromatography.( b) Western blotting of multi-epitope vaccine. rUreB, BIB, rIB, rUreI, rCTB were separated by 12% SDS-PAGE and transferred to a nitrocellulose membrane. Mouse polyclonal anti-BIB serum (1), mouse monoclonal anti-rCTB serum (2), and normal mouse serum (3) were collected for detecting immunoreactivity to rUreB, BIB, rIB, rUreI and rCTB.
Protective effect of BIB on H. pylori infection
To follow the effects of vaccination of BIB against H. pylori infection, BALB/c mice were immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS for three times at 2 week intervals, and challenged with H. pylori on day 14 after the last immunization. Compared with oral immunization with rIB, rCTB or PBS, oral immunization with BIB (log10 CFU g−1,1.13 ± 0.26) or rIB plus rCTB (2.53 ± 0.36) dramatically reduced the bacterial load in the stomachs of H. pylori-infected mice (3.53 ± 0.55, P < 0.01). In addition, the BIB-vaccinated group was significantly better protected than the rIB plus rCTB-vaccinated group (P < 0.01), and almost no protective effect was found in the mice immunized with either adjuvant rCTB alone or PBS.
Humoral immune response elicited by BIB immunization
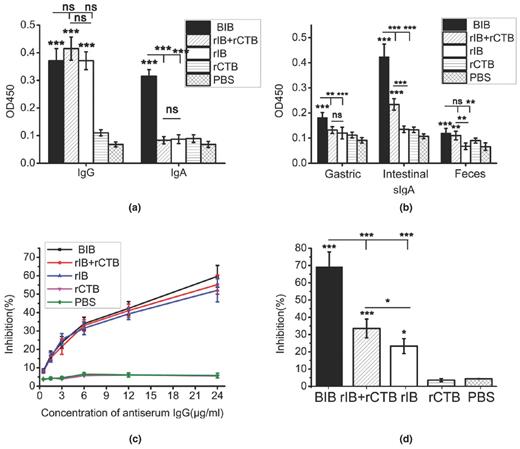
The H. pylori-specific antibody levels were measured by ELISA. As shown in Fig. 2a, oral immunization with BIB, rCTB plus rIB or rIB showed significantly increased level of specific IgG, compared with rCTB or PBS (P < 0.001). The levels of IgA against H. pylori lysates in serum, gastric mucosal, intestinal mucosal or feces samples were tested by ELISA as well. Oral immunization with BIB markedly elevated the level of specific serum IgA and mucosal sIgA antibodies compared with other groups (P < 0.05, Fig. 2b).
Serum and mucosal antibody responses against H. pylori lysates after orally immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS. Data are mean ± SD. P < 0.05 was considered as statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. (a) The levels of specific IgG and IgA against H. pylori lysates in serum samples were tested by ELISA. (b) The levels of sIgA against H. pylori lysates in gastric mucosal, intestinal mucosal or feces samples were tested by ELISA. (c) Inhibition of H. pylori urease activity by serum IgG. The H. pylori lysates was preincubated with a serial dilution of IgG from mice immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS immunized mice. The optical density of the mixture was determined at 578 nm by the indicator of phenolsulfonphthalein. The data are expressed as percentage inhibition. (d) The supernatants of homogenized stomachs were collected for detecting the levels of specific IgA against H. pylori lysates.
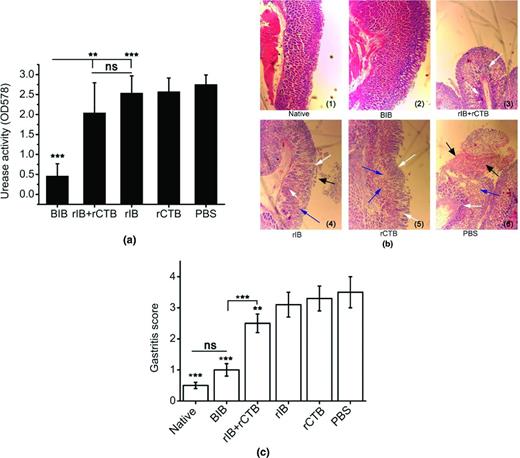
To test the effects of those antibodies, urease neutralization assay was performed. The H. pylori lysates was preincubated with a serial dilution of IgG or the supernatants of homogenized stomachs from mice immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS immunized mice for 3 h. The optical density of the mixture was determined at 578 nm by the indicator of phenolsulfonphthalein. The data are expressed as percentage inhibition. IgG from mice immunized with BIB, rCTB plus rIB or rIB inhibited the urease activity dose-dependently, whereas the IgG from mice immunized with rCTB or PBS gave no obvious inhibition (Fig. 2c). In addition, the supernatants of homogenized stomachs were collected for detecting the levels of specific IgA against H. pylori lysates, and oral immunization with BIB, rIB plus IB or rIB markedly elevated the inhibition of the urease activity (P < 0.05, Fig. 4a).
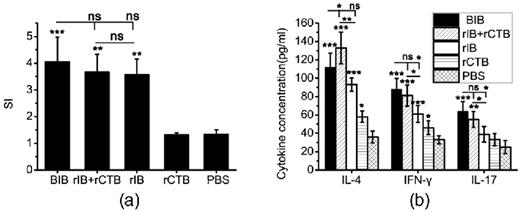
H. pylori specific lymphocyte responses after immunization. Data were expressed as mean ± SD. P < 0.05 was considered as statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. (a) Assessment on proliferation of specific lymphocytes. Splenic lymphocytes from mice immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS were stimulated with H. pylori lysates (0.5 μg mL−1) for 5 days, and proliferation was detected by 3H-TdR incorporation. The results were expressed as stimulation indices (SI). SI represents the ratio between the proliferation rates of cells stimulated with antigen and with medium alone. (b) The concentrations of cytokines in the supernatants of lymphocytes cultures were determined by ELISA. Splenic lymphocytes from mice immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS were stimulated with H. pylori lysates for 96 h, and the supernatants were collected and cytokine production was tested by ELSA.
Evaluation of H. pylori infection after vaccination (HE stain, × 100). The mice were orally immunized with BIB, rIB plus rCTB, rIB, rCTB or PBS and infected with H. pylori SS1. Data are expressed as mean ± SD. P < 0.05 was considered as statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. (a) Urease activity in the stomach after oral immunization. (b) Gastric histology in BALB/c mice after challenge with H. pylori SS1. (c) Assessment on prophylactic efficacy of the epitope vaccine BIB based on gastritis scores. Pathological examination showed that gastric mucosal glands of the rats from PBS, rCTB and rIB group were significantly reduced (blue arrow), and the submucosa showed necrosis and exfoliation (black arrow). In addition, mice vaccinated with rIB plus rCTB, rIB, rCTB or PBS also showing severe inflammatory infiltrates in the mucosa and submucosa (white arrow). In contrast, mice immunized with BIB showing only a few leukocytes scattered in the deep mucosa.
Cellular immune responses elicited by BIB immunization
To determine the capacity of BIB to induce an antigen specific lymphocyte response, splenic lymphocytes were stimulated in vitro with H. pylori lysates. Compared with lymphocytes from mice orally immunized with rCTB, lymphocytes from mice orally immunized with BIB, rIB plus rCTB or rIB displayed significantly high proliferation after stimulation with H. pylori lysates. In contrast, lymphocytes from mice orally immunized with rCTB had no significant proliferation compared with cells from PBS controls (Fig. 3a).
The production of IFN-γ, IL-4 and IL17 in the supernatants of splenic lymphocytes cultures was measured by ELISA. Stimulation of splenic lymphocytes from mice after vaccination with BIB, rIB plus rCTB or rIB resulted in significantly higher levels of the IFN-γ, IL-4 and IL17 than stimulation of cells from rCTB or PBS immunized mice (P < 0.05, Fig. 3b).
DISCUSSION
Although 32 years have elapsed since the discovery of H. pylori, non-effective vaccine against H. pylori has been fully developed (Sutton and Chionh 2013). The urease of H. pylori is widely conserved among the various H. pylori strains. It has been used as an antigenic component of potential vaccines in human tests. UreB was the most effective and common immunogen of all strains of H. pylori, which was widely investigated as a potential antigen for the development of prophylactic and therapeutic vaccines against H. pylori infection (Li et al.2010, 2011; Qiu et al.2010; Wang et al.2010; Zhang et al.2011; John et al.2013; Yang et al.2013). UreI is a proton-gated inner-membrane urea channel or urea transporter, It is essential for survival of H. pylori in the acidic environment of the stomach. The channel is closed at neutral pH and opens at acidic pH to allow the rapid access of urea to cytoplasmic urease. The ureI gene is highly conserved among H. pylori strains and can be used as a good molecular marker for identification of H. pylori. Each UreI protomer encloses a channel formed by a twisted bundle of six transmembrane helices (Strugatsky et al.2012). The first human study on H. pylori vaccine development showed that oral administration of enzymatically inactive recombinant H. pylori urease without adjuvant was safe but minimally immunogenic in infected humans. It is worth noting that Michetti recorded a significant reduction in bacterial load in a group of patients who had received vaccination with H. pylori urease plus E. coli enterotoxin (Michetti 1998; Michetti et al.1999; Banerjee et al.2002). Consisting of immunodominant epitopes from different protective antigens, epitope-based vaccines are likely to induce strong, comprehensive protective immunity as well as preventing undesired side effects (Li et al.2012). Epitope-based vaccines have been developed to protect against certain infections, such as HIV (Jin et al.2009; Paul et al.2010), Neisseria meningitides (Gupta et al.2010; Malito et al.2013) and Mycobacterium tuberculosis (Christy et al.2012). Epitope-based vaccines may also be a potential preventive strategy in H. pylori infection (Chen et al.2013; Koch, Meyer and Moss 2013). In this study, we constructed a multi-epitope vaccine named BIB composing of the mucosal adjuvant CTB, two antigenic fragments from UreI and four antigenic fragments from UreB. It contains 11 confirmed or predicted epitopes.
Our results demonstrated that BALB/c mice can be successfully protected from challenge infection with H. pylori SS1 by prophylactic oral vaccination with BIB. The induction of significant protection against H. pylori was associated with a concomitant reduction in gastric pathology (Fig. 4).
It has been reported that although antibodies may have a role in vaccine-mediated immunity, they are not required to elicit immune protection, but some studies support that humoral immune response is important to clear H. pylori (Gorrell et al.2013). In our study, 4 weeks post-challenge, almost no protective effects were observed in rIB (2/18, 11.1%), rCTB (0/18,0%) or PBS (0/18,0%) group, although high levels of serum IgG antibody were elicited. In contrast, oral immunization with BIB induced significantly higher H. pylori-specific serum IgA and mucosal sIgA secretion than immunization with other groups, and observed mice were well protected. Nevertheless, anti-BIB antibodies can inhibit urease activity, which might breakdown the microenvironment colonized by H. pylori and contribute to clearance of H. pylori. These results suggest that the serum IgG antibody level was not directly associated with the protective effect, and serum IgA and gastric sIgA antibody would play a role in blocking infection which has already been noted in many vaccination studies (Lee et al.1995).
Helicobacter pylori infection involves a strong T helper 1 (Th1), T helper 2 (Th2) and/or T helper 17 (Th17) cell response that promotes a mixed lymphocyte and granulocyte in the gastric mucosa (Salama, Hartung and Müller 2013). Inducing these types of H. pylori-specific T cells is the crucial step in generating immunity to this infection. CD4+ T cells recognize antigenic epitopes in the context of MHC II molecules on APCs. Previous investigations of H. pylori infection have demonstrated that the protective immune response is mediated by CD4+ T cells but not by CD8+ T cells (Sayi et al.2009). CD4+ T cells are classified as Th1, Th2 and Th17 cell subsets on the basis of their cytokine secretion and immune regulatory function. Th1 cells predominantly secrete IFN-γ and IL-2, and Th2 cells secrete IL-4, IL-5 and IL-10. Th17 cells produce the proinflammatory cytokines IL-17, IL-17F and IL-22. Studies concerning CD4+ T cell responses against H. pylori have predominantly focused on the Th1/Th2 paradigm (Chao Wu et al.2008; Wang et al.2010). Extensive studies have demonstrated that H. pylori infection results in a Th1-dominant response, recent studies have demonstrated that H. pylori infection can induce a Th17 cell response and this response could play a role in the pathogenesis of H. pylori infection (Amedei et al.2014; Bhuiyan et al.2014; Munari et al.2014). In addition, Th17 cell response is reported to be involved in vaccine-induced protection against H. pylori infection (Zhang et al.2011; Flach et al.2010). We therefore selected three Th2 epitopes and one Th1 epitope from UreB to construct the epitope vaccine BIB. The lymphocyte cell proliferation assay showed that BIB could stimulate high proliferative responses, and analysis of the cytokines showed that IFN-γ, IL-4 and IL-17 were all significantly induced by BIB. These results indicate that BIB induced a mixed Th1/Th2/Th17 immune response as expected, which might contribute to the successful protection. The precise mechanism of Th17 cell function in the H. pylori infection is not clear, and the protective and pathogenic functions of IL-17-producing Th cells were both reported in infections. Activated Th17 cells could produce IL-17. rBIB protein contains T-cell epitope and B-cell epitope, some epitopes are predicted and not confirmed by careful research, it maybe induce some variability of IL-17. Our result shown rBIB induced IL-17 level change, which suggests some epitope of rBIB could be Th17 epitope. The detail and its mechanism need further investigation.
Several studies have shown that various arrangements of amino-acid sequences may affect protein properties (Abdel-Aal et al.2010; Kulkarni et al.2013). The optimal sequences of the seven antigenic fragments in tandem were selected by RANKPEP software. The linkers (EFM, KK) were designed to retain the immunologic competence of each antigenic fragment. The software did not predict new conjugation epitope, and epitope functions should be retained. CTB was selected as the intramucosal adjuvant to increase the immunogenic activity of the vaccine. Immunoblotting and GM1 ganglioside enzyme-linked immunosorbent assay results (data not shown) indicated that prediction of the combination order in the epitope vaccine by RANKPEP and DNASTAR software was successful.
In summary, we have designed and constructed a multi-epitope vaccine (BIB) against H. pylori urease B and I subunits, and oral prophylactic immunization with it gave satisfactory protection against H. pylori infection in BABL/c mice. In addition, the induction of significant protection against H. pylori is possibly mediated by specific serum IgA and mucosal sIgA antibodies, and a mixed Th1/Th2/Th17 cells response. This study suggests that the multi-epitope vaccine BIB is a promising candidate for protection against H. pylori infection. In future studies, the selected epitopes from UreI and UreB should be synthesized to further detect whether they are retained their functions. We will also evaluate the efficacies of BIB with other adjuvants including vaccine carriers such as attenuated Salmonella and lactic acid bacteria as well as different immunization routes.
We thank Prof Wan-yi Li (Sichuan University) for providing language help and revising the manuscript.
AUTHORS' CONTRIBUTIONS
Xin-li SHI and Bao-ning WANG designed the research; Jing YANG and Lv-xia DAI performed the experiments; Jing YANG wrote the manuscript with contribution from other authors.
FUNDING
This research was financially supported by Supporting Technology Project (No. 15ZC2000, 2014SZ0036), Application of Infrastructure Projects (No. 2014JY0201), Science & Technology Department of Sichuan Province.
Conflict of interest. None declared.
REFERENCES