-
PDF
- Split View
-
Views
-
Cite
Cite
Deeptankar DeMazumder, Worawan B. Limpitikul, Miguel Dorante, Swati Dey, Bhasha Mukhopadhyay, Yiyi Zhang, J. Randall Moorman, Alan Cheng, Ronald D. Berger, Eliseo Guallar, Steven R. Jones, Gordon F. Tomaselli, Entropy of cardiac repolarization predicts ventricular arrhythmias and mortality in patients receiving an implantable cardioverter-defibrillator for primary prevention of sudden death, EP Europace, Volume 18, Issue 12, December 2016, Pages 1818–1828, https://doi.org/10.1093/europace/euv399
Close - Share Icon Share
The need for a readily available, inexpensive, non-invasive method for improved risk stratification of heart failure (HF) patients is paramount. Prior studies have proposed that distinct fluctuation patterns underlying the variability of physiological signals have unique prognostic value. We tested this hypothesis in an extensively phenotyped cohort of HF patients using EntropyXQT, a novel non-linear measure of cardiac repolarization dynamics.
In a prospective, multicentre, observational study of 852 patients in sinus rhythm undergoing clinically indicated primary prevention implantable cardioverter-defibrillator (ICD) implantation (2003–10), exposures included demographics, history, physical examination, medications, laboratory results, serum biomarkers, ejection fraction, conventional electrocardiographic (ECG) analyses of heart rate and QT variability, and EntropyXQT. The primary outcome was first ‘appropriate’ ICD shock for ventricular arrhythmias. The secondary outcome was composite events (appropriate ICD shock and all-cause mortality). After exclusions, the cohort (n = 816) had a mean age of 60 ± 13 years, 28% women, 36% African Americans, 56% ischaemic cardiomyopathy, and 29 ± 16% Seattle HF risk score (SHFS) 5-year predicted mortality. Over 45 ± 24 months, there were 134 appropriate shocks and 166 deaths. After adjusting for 30 exposures, the hazard ratios (comparing the 5th to 1st quintile of EntropyXQT) for primary and secondary outcomes were 3.29 (95% CI 1.74–6.21) and 2.28 (1.53–3.41), respectively. Addition of EntropyXQT to a model comprised of the exposures or SHFS significantly increased net reclassification and the ROC curve area.
EntropyXQT measured during ICD implantation strongly and independently predicts appropriate shock and all-cause mortality over follow-up. EntropyXQT complements conventional risk predictors and has the potential for broad clinical application.
EntropyXQT, a novel, non-invasive, non-linear parameter, easily and inexpensively measured in ambulatory settings, complements conventional risk predictors and provides unique insight into cardiac repolarization dynamics.
Higher EntropyXQT, which reflects reduced repetition of fluctuation patterns of ventricular repolarization in sinus rhythm, strongly and independently predicts the incidence of sustained ventricular tachyarrhythmias and all-cause mortality in patients with heart failure.
Dynamic non-linear measures based on newer concepts of entropy estimation, e.g. EntropyXQT, may help identify patients with advanced subclinical disease before clinical deterioration and has the potential for broad clinical application.
Introduction
Cardiac arrest claims a quarter million lives per year in the USA. Implantable cardioverter-defibrillators (ICDs), the first line of therapy in high-risk patients, deliver shocks to prevent cardiac arrest from lethal ventricular arrhythmias.1 However, the selection of patients for primary prevention ICD implantation based primarily on ejection fraction (EF) criteria lacks sensitivity and specificity.2 Those at risk for pulseless electrical activity (PEA) or asystole are even more challenging to identify, have less favourable outcomes, and benefit from early implementation of advanced cardiac life support. The identification of improved methods for risk stratification remains a clinical and public health priority.
A large body of evidence indicates that repetition of patterns within the variability of physiological signals reflects interdependences between distinct physiological processes and provides unique prognostic insight.3–7 This conceptually simple approach has been used to describe signatures of health and disease in particular clinical circumstances, such as the surface electrocardiographic (ECG) patterns associated with respiratory sinus arrhythmia compared with Kussmaul breathing. Small clinical studies have proposed that more subtle non-linear changes in physiological variability have prognostic value that is incremental to conventional risk predictors.8–13 This strategy has potential utility for improved risk stratification of patients.
In the present study of heart failure (HF) patients referred for primary prevention ICD implantation, our goal is to identify non-linear signatures of advanced disease by building upon new concepts of entropy estimation. We introduce EntropyXQT, a novel, non-invasive, easily determined non-linear parameter for quantifying the repetition of fluctuation patterns underlying the variability of cardiac repolarization. We test the hypothesis that EntropyXQT measured before ICD implantation predicts lethal ventricular arrhythmias and all-cause mortality, independent of established risk factors and conventional measures of variability.
Methods
Study sample
The Prospective Observational Study of Implantable Cardioverter-Defibrillators (PROSE-ICD) is a multicentre prospective cohort study (clinicaltrials.gov #NCT00733590) of patients undergoing clinically indicated ICD implantation for primary prevention of sudden death in four clinical centres: Johns Hopkins Hospital, University of Maryland Hospital, Washington Hospital Center, and Virginia Commonwealth University Hospital.14 We included patients with HF of ischaemic or non-ischaemic aetiology, EF ≤35%, New York Heart Association (NYHA) Class I–III symptoms, normal sinus rhythm at enrollment, and no history of ventricular tachycardia (VT) or fibrillation (VF) (except in the acute post-myocardial infarction phase). We did not include patients with a permanent pacemaker or a pre-existing Class 1 indication for a permanent pacemaker. ICDs were programmed at the discretion of implanting physicians, although high VT/VF cut-off zones were encouraged as previously described.14
Between December 2003 and December 2010, PROSE-ICD recruited 852 patients. We excluded one patient who died from VF secondary to ICD malfunction at 1.5 months and 35 patients (4.1%) for missing results. The final number of participants in this analysis was 816. The study was approved by the Institutional Review Boards of all participating centres. All patients provided written informed consent.
Data collection
Demographics, medical history, physical examination, EF assessed by echocardiography or ventriculography, clinical laboratory analyses [serum sodium, potassium, blood urea nitrogen (BUN), creatinine], serum biomarkers [N-terminal prohormone of brain natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hsCRP), troponin T (cTNT)], prescribed medications, standard 12-lead ECG, and high-resolution 5-min ECG rhythm strips were collected at the time of ICD implantation. The Seattle HF risk score (SHFS) was calculated as previously described.15 The ECGs and the accuracy of automated detection of RR and QT intervals were manually reviewed with the aid of a graphical display using applications written in MATLAB (MathWorks, Inc., Natick, MA, USA), as previously described.16 Briefly, for each record, the lead with the most prominent and morphologically consistent set of T waves was used for analysis. In the selected lead, several QT intervals were designated as a template and the start of the QRS and end of the T wave were manually identified. The custom-designed software identified the apex of the T wave in the QT windows by searching for a zero slope point between the increasing and decreasing limbs, and identified the end of the T wave by the decreasing slope relative to the maximal tangential slope of the descending T wave limb. This, in combination with the onset of the QRS and offset of the T wave, was used by the software to calculate variations in the QT interval. A major advantage of this method is that it is less dependent on the actual measured QT interval and provides a precise measure of changes in QT interval. Throughout this process, manual checks were routinely performed over all QT intervals to identify physiologically unlikely changes in any of the measurements and noise that impeded QT measurement. The analyses for extracting RR and QT intervals were performed by three of the co-authors blinded to the study results and one-third of the dataset analysed by each of these operators was overlapped with those assigned to the other two. Comparison of results between operators yielded consistent measurements.
Conventional electrocardiographic analyses
The 12-lead ECGs were analysed for rate, rhythm, morphology, and length of the RR, QRS, JT, and QT intervals, and evidence for myocardial infarction. Detailed quantitative analyses of the high-resolution 5-min ECGs included RR variance (average of the squared differences from the mean), JT variance, QT variance, QT/RR slope, and QT variability index (QTVI).16 The RR variance was log-transformed to normalize the distribution. The analysis of the power spectra of RR and QT intervals was performed to assess coherence of the spectra over the physiological frequency range, and the cross spectrum was generated using the Blackman–Tukey method and coherence was calculated as previously reported.16 Heart rate variability (HRV) in the time domain was calculated as the standard deviation of NN intervals (SDNN) over 5 min. Heart rate variability in the frequency domain was calculated using fast Fourier transform and Lomb–Scargle periodogram with bands of high frequency (0.15–0.4 Hz) and low frequency (0.04–0.15 Hz).17 A range of normal values for HRV analysed in the time and frequency domains have been established.17 We16,18 and others19,20 previously generated a normal range for the QTVI and studied changes in patients with HF16 and ventricular arrhythmia.18 Standard signal-averaged ECG (SAECG) measures were recorded including high-frequency QRS (HFQRSD), voltage of the terminal 40 ms of HFQRS (RMS40), and duration of terminal signals <40 µV in amplitude (LAS40).
EntropyXQT
The information entropy of a time series can be used to quantify patterns within the variability of a signal and often is characterized as complexity.5,9 EntropyXQT examines changes in patterns of cardiac repolarization (as indexed by the QT interval) such that the presence of more frequent and more similar patterns leads to lower EntropyXQT values. EntropyXQT exploits information in the order of the times between ventricular repolarization and quantifies the degree to which patterns of fluctuation of ventricular repolarization repeat themselves. More self-similar fluctuations imply increased coupling of periodic processes among organ systems that typically are a signature of health,5,21 such as respiratory sinus arrhythmia.
The EntropyX algorithm is conceptually simple and computationally straightforward (refer to Supplementary materials online, Methods) and builds upon new concepts of entropy estimation.7,22 Unlike conventional measures of variability,16,17 EntropyX analysis of a time series does not require equally sampled time intervals or extensive preprocessing of data. Unlike sample entropy or approximate entropy, the EntropyX computational algorithm is insensitive to the degree of tolerance allowed for matching templates and less sensitive to the presence of outlying points7,22 such as ectopic beats or noise. Unlike approximate entropy, frequency domain measures, or geometric measures such as Poincaré plots,17 EntropyX can be calculated using very short time series.
Outcomes
The primary endpoint was the first adjudicated appropriate ICD shock for VT/VF. The secondary endpoint was composite of the first appropriate ICD shock and all-cause mortality. After ICD shock or death, all available data including intracardiac electrograms prior to the event were reviewed by a committee of board-certified clinical cardiac electrophysiologists blinded to the EntropyXQT analysis. The committee adjudicated the type of arrhythmia eliciting the shock. Deaths were ascertained by phone interviews with the next of kin and by searches of the National Death Index.
Statistical analysis
Statistical analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX, USA). Continuous variables were compared between two groups using t-test and between ≥3 groups using one-way analysis of variance. Categorical variables were compared using a χ2 test.
To evaluate the association of EntropyXQT with the study endpoints of appropriate ICD shock and composite events, we used Cox proportional hazards analyses adjusted for the SHFS model15 or for a base model comprising demographics (age at implant, gender, race), medical history [paroxysmal atrial fibrillation (AF), smoking, diabetes mellitus, ischaemic cardiomyopathy, NYHA class], prescribed medications (aspirin, β-blocker, ACE inhibitor or angiotensin receptor blocker, aldosterone antagonist, statin, antiarrhythmic, loop diuretic), physical exam [body mass index, mean arterial pressure (MAP)], laboratory results (sodium, potassium, BUN), biomarkers (hsCRP, NT-proBNP), EF, and time and frequency domain measures of HRV, and QT variability [heart rate, PVC burden, SDNN, low frequency, high frequency, low:high frequency ratio, QT-heart rate coherence, QTVI]. The proportional hazards assumption was verified using Schoenfeld's residuals.
The added discrimination of EntropyXQT was evaluated by comparing multivariable models with and without EntropyXQT using the differences in Harrell's C-statistic. Continuous and categorical net reclassification improvement (NRI) and integrated discrimination improvement (IDI) values were calculated as previously described.23 Ten-fold cross validation was performed to avoid overestimating the incremental value. All 95% confidence intervals for the receiver operating characteristic (ROC) area under the curve, NRI, and IDI were obtained using 1000 bootstrap samples. NRI and IDI were calculated using STATA add-ons from the Uppsala Clinical Research Center (http://www.ucr.uu.se/en/index.php/epistat/program-code/306-nri-and-idi) and the Fred Hutchinson Cancer Research Center (http://labs.fhcrc.org/pepe/dabs/ppsoft.html). Statistical significance was set at P < 0.05.
Results
Patient characteristics
The cohort of 816 patients had a mean age of 61 years; 28% of patients were women, 36% were African American, 20% had a history of paroxysmal AF, 33% had diabetes, and 56% ischaemic cardiomyopathy (Table 1). This profile is similar to prior clinical ICD trials except for a higher percentage of African Americans in the present study.
Patient and ECG characteristics by events
| . | Appropriate ICD shock (n = 134) . | All-cause mortality (166) . | Composite events (300) . | Alive (516) . | Total cohort (816) . |
|---|---|---|---|---|---|
| Age, years | 60.6 ± 11.9 | 65.6 ± 11.3‡ | 63.4 ± 11.9‡ | 58.5 ± 12.4 | 60.3 ± 12.5 |
| Male, % | 79.9* | 73.8 | 76.3* | 69.4 | 71.9 |
| Caucasian, % | 67.2 | 60.7 | 63.3 | 60.7 | 61.6 |
| African American, % | 30.6 | 36.9 | 34.3 | 36.2 | 35.5 |
| Asian or American Indian, % | 2.2 | 2.4 | 2.3 | 3.1 | 2.8 |
| Cardiac resynchronization, % | 23.1 | 30.4 | 27.3 | 27.1 | 27.2 |
| History of paroxysmal AF, % | 19.4 | 31.5† | 26.3* | 16.3 | 20.0 |
| Smoking, % | 74.6* | 75.0* | 74.7* | 62.6 | 67.0 |
| Hypertension, % | 54.5 | 67.9 | 62.0 | 57.9 | 59.4 |
| Hyperlipidaemia, % | 43.3 | 50.0 | 47.3 | 41.9 | 43.9 |
| Diabetes mellitus, % | 35.8 | 46.4‡ | 41.7‡ | 27.3 | 32.6 |
| Ischaemic cardiomyopathy, % | 55.2 | 65.5 | 60.7 | 53.5 | 56.1 |
| Body mass index, kg/m2 | 29.6 ± 5.7 | 28.5 ± 5.7* | 29.0 ± 5.7* | 30.0 ± 6.5 | 29.6 ± 6.2 |
| Mean arterial pressure, mmHg | 89.2 ± 13.2* | 89.9 ± 12.9* | 89.5 ± 12.9* | 92.6 ± 13.8 | 91.4 ± 13.5 |
| Aspirin, % | 69.4 | 72.6 | 71.0 | 65.1 | 67.3 |
| Beta blocker, % | 86.6 | 89.9 | 88.7 | 91.1 | 90.2 |
| ACE inhibitor or ARB, % | 85.8 | 87.4 | 87.0 | 89.9 | 88.8 |
| Aldosterone antagonist, % | 19.4 | 29.2 | 25.0 | 25.2 | 25.1 |
| Statin, % | 70.1 | 72.6 | 71.3 | 70.2 | 70.6 |
| Antiarrhythmic, % | 11.9* | 12.5* | 12.0 | 6.6 | 8.6 |
| Loop diuretic, % | 64.2 | 76.2 | 71.0 | 62.0 | 65.3 |
| NYHA Class I | 26.9* | 13.1 | 19.3 | 19.4 | 19.4 |
| NYHA Class II | 39.6 | 31.5 | 35.0 | 43.0 | 40.1 |
| NYHA Class III | 33.6 | 55.4 | 45.7 | 37.6 | 40.6 |
| Ejection fraction, % | 22.4 ± 7.8 | 21.2 ± 7.9* | 21.7 ± 7.9* | 22.9 ± 7.9 | 22.4 ± 7.9 |
| Sodium, mEq/L | 138.5 ± 3.0 | 138.7 ± 3.4 | 138.6 ± 3.2 | 139.0 ± 3.0 | 138.8 ± 3.1 |
| Potassium, mEq/L | 4.22 ± 0.44 | 4.19 ± 0.45 | 4.20 ± 0.44 | 4.22 ± 0.43 | 4.22 ± 0.44 |
| BUN, mg/dL | 20.5 [16–26] | 25.0 [18–34]‡ | 22.0 [17–30]‡ | 18.0 [15–24] | 20.0 [15–26] |
| Creatinine, mg/dL | 1.0 [0.9–1.2] | 1.3 [1.0–1.6]‡ | 1.1 [0.9–1.4]† | 1.0 [0.9–1.3] | 1.1 [0.9–1.3] |
| hsCRP, μg/mL | 4.6 [2.4–11.3] | 7.2 [2.8–6.7]† | 5.7 [2.4–3.9]* | 3.4 [1.5–8.6] | 4.4 [1.6–11] |
| NT-ProBNP, ng/mL | 2.5 [1.7–3.9] | 3.7 [2.5–7.2]‡ | 3.1 [2.0–5.2]† | 2.2 [1.6–3.5] | 2.5 [1.7–4.0] |
| Troponin T, ng/mL | 0.01 [0.0–0.04] | 0.03 [0.0–0.07] | 0.02 [0.0–0.06] | 0.00 [0.0–0.02] | 0.01 [0.0–0.03] |
| SHFS 5-year predicted mortality, % | 30.6 ± 16.0‡ | 42.8 ± 18.1‡ | 37.5 ± 18.2‡ | 24.5 ± 12.7 | 29.3 ± 16.2 |
| Heart rate, bpm | 71.7 ± 14.7 | 74.7 ± 15.7* | 73.4 ± 15.3 | 71.8 ± 13.0 | 72.4 ± 13.9 |
| PVC, % | 3.7 ± 4.8† | 2.8 ± 3.7 | 3.2 ± 4.2* | 2.3 ± 4.0 | 2.6 ± 4.1 |
| QRSD, ms | 121.0 ± 33.0 | 127.9 ± 31.2 | 125.0 ± 32.2 | 122.5 ± 34.1 | 123.5 ± 33.4 |
| HFQRSD, ms | 122.1 ± 34.9 | 124.1 ± 32.5 | 123.4 ± 33.6 | 121.0 ± 32.9 | 121.9 ± 33.2 |
| RMS40, μV | 25.6 ± 21.3 | 22.1 ± 21.6* | 23.8 ± 21.6* | 35.0 ± 65.0 | 30.8 ± 53.1 |
| LAS40, ms | 53.1 ± 38.4 | 55.6 ± 33.8* | 54.3 ± 36.0* | 47.7 ± 32.1 | 50.2 ± 33.8 |
| JT interval, ms | 288.9 ± 56.9 | 293.7 ± 55.2 | 291.4 ± 56.1 | 290.2 ± 52.2 | 290.7 ± 53.6 |
| JT variance, ms2 | 15.6 ± 17.1 | 15.1 ± 20.8 | 15.2 ± 19.2 | 13.2 ± 25.2 | 13.9 ± 23.2 |
| SDNN, ms | 75.8 ± 72.3† | 57.8 ± 73.9 | 65.5 ± 73.7* | 52.3 ± 47.5 | 57.1 ± 58.8 |
| LF : HF, ratio | 1.66 ± 1.84 | 1.39 ± 1.69 | 1.52 ± 1.76 | 1.74 ± 2.63 | 1.66 ± 2.35 |
| QT interval, ms | 439.0 ± 56.7 | 436.9 ± 60.2 | 437.5 ± 58.3 | 433.7 ± 65.0 | 435.1 ± 62.8 |
| QTc, ms | 456.6 ± 43.7 | 474.3 ± 49.9† | 466.2 ± 47.9* | 457.9 ± 40.8 | 461.2 ± 43.9 |
| QT-heart rate coherence | 0.415 ± 0.166 | 0.407 ± 0.186 | 0.410 ± 0.177 | 0.436 ± 0.192 | 0.427 ± 0.187 |
| QTVI | −0.81 ± 0.72 | −0.68 ± 0.65† | −0.73 ± 0.68* | −0.88 ± 0.62 | −0.82 ± 0.65 |
| EntropyXQT | 2.77 ± 0.69‡ | 2.69 ± 0.65‡ | 2.72 ± 0.67‡ | 2.43 ± 0.58 | 2.54 ± 0.63 |
| . | Appropriate ICD shock (n = 134) . | All-cause mortality (166) . | Composite events (300) . | Alive (516) . | Total cohort (816) . |
|---|---|---|---|---|---|
| Age, years | 60.6 ± 11.9 | 65.6 ± 11.3‡ | 63.4 ± 11.9‡ | 58.5 ± 12.4 | 60.3 ± 12.5 |
| Male, % | 79.9* | 73.8 | 76.3* | 69.4 | 71.9 |
| Caucasian, % | 67.2 | 60.7 | 63.3 | 60.7 | 61.6 |
| African American, % | 30.6 | 36.9 | 34.3 | 36.2 | 35.5 |
| Asian or American Indian, % | 2.2 | 2.4 | 2.3 | 3.1 | 2.8 |
| Cardiac resynchronization, % | 23.1 | 30.4 | 27.3 | 27.1 | 27.2 |
| History of paroxysmal AF, % | 19.4 | 31.5† | 26.3* | 16.3 | 20.0 |
| Smoking, % | 74.6* | 75.0* | 74.7* | 62.6 | 67.0 |
| Hypertension, % | 54.5 | 67.9 | 62.0 | 57.9 | 59.4 |
| Hyperlipidaemia, % | 43.3 | 50.0 | 47.3 | 41.9 | 43.9 |
| Diabetes mellitus, % | 35.8 | 46.4‡ | 41.7‡ | 27.3 | 32.6 |
| Ischaemic cardiomyopathy, % | 55.2 | 65.5 | 60.7 | 53.5 | 56.1 |
| Body mass index, kg/m2 | 29.6 ± 5.7 | 28.5 ± 5.7* | 29.0 ± 5.7* | 30.0 ± 6.5 | 29.6 ± 6.2 |
| Mean arterial pressure, mmHg | 89.2 ± 13.2* | 89.9 ± 12.9* | 89.5 ± 12.9* | 92.6 ± 13.8 | 91.4 ± 13.5 |
| Aspirin, % | 69.4 | 72.6 | 71.0 | 65.1 | 67.3 |
| Beta blocker, % | 86.6 | 89.9 | 88.7 | 91.1 | 90.2 |
| ACE inhibitor or ARB, % | 85.8 | 87.4 | 87.0 | 89.9 | 88.8 |
| Aldosterone antagonist, % | 19.4 | 29.2 | 25.0 | 25.2 | 25.1 |
| Statin, % | 70.1 | 72.6 | 71.3 | 70.2 | 70.6 |
| Antiarrhythmic, % | 11.9* | 12.5* | 12.0 | 6.6 | 8.6 |
| Loop diuretic, % | 64.2 | 76.2 | 71.0 | 62.0 | 65.3 |
| NYHA Class I | 26.9* | 13.1 | 19.3 | 19.4 | 19.4 |
| NYHA Class II | 39.6 | 31.5 | 35.0 | 43.0 | 40.1 |
| NYHA Class III | 33.6 | 55.4 | 45.7 | 37.6 | 40.6 |
| Ejection fraction, % | 22.4 ± 7.8 | 21.2 ± 7.9* | 21.7 ± 7.9* | 22.9 ± 7.9 | 22.4 ± 7.9 |
| Sodium, mEq/L | 138.5 ± 3.0 | 138.7 ± 3.4 | 138.6 ± 3.2 | 139.0 ± 3.0 | 138.8 ± 3.1 |
| Potassium, mEq/L | 4.22 ± 0.44 | 4.19 ± 0.45 | 4.20 ± 0.44 | 4.22 ± 0.43 | 4.22 ± 0.44 |
| BUN, mg/dL | 20.5 [16–26] | 25.0 [18–34]‡ | 22.0 [17–30]‡ | 18.0 [15–24] | 20.0 [15–26] |
| Creatinine, mg/dL | 1.0 [0.9–1.2] | 1.3 [1.0–1.6]‡ | 1.1 [0.9–1.4]† | 1.0 [0.9–1.3] | 1.1 [0.9–1.3] |
| hsCRP, μg/mL | 4.6 [2.4–11.3] | 7.2 [2.8–6.7]† | 5.7 [2.4–3.9]* | 3.4 [1.5–8.6] | 4.4 [1.6–11] |
| NT-ProBNP, ng/mL | 2.5 [1.7–3.9] | 3.7 [2.5–7.2]‡ | 3.1 [2.0–5.2]† | 2.2 [1.6–3.5] | 2.5 [1.7–4.0] |
| Troponin T, ng/mL | 0.01 [0.0–0.04] | 0.03 [0.0–0.07] | 0.02 [0.0–0.06] | 0.00 [0.0–0.02] | 0.01 [0.0–0.03] |
| SHFS 5-year predicted mortality, % | 30.6 ± 16.0‡ | 42.8 ± 18.1‡ | 37.5 ± 18.2‡ | 24.5 ± 12.7 | 29.3 ± 16.2 |
| Heart rate, bpm | 71.7 ± 14.7 | 74.7 ± 15.7* | 73.4 ± 15.3 | 71.8 ± 13.0 | 72.4 ± 13.9 |
| PVC, % | 3.7 ± 4.8† | 2.8 ± 3.7 | 3.2 ± 4.2* | 2.3 ± 4.0 | 2.6 ± 4.1 |
| QRSD, ms | 121.0 ± 33.0 | 127.9 ± 31.2 | 125.0 ± 32.2 | 122.5 ± 34.1 | 123.5 ± 33.4 |
| HFQRSD, ms | 122.1 ± 34.9 | 124.1 ± 32.5 | 123.4 ± 33.6 | 121.0 ± 32.9 | 121.9 ± 33.2 |
| RMS40, μV | 25.6 ± 21.3 | 22.1 ± 21.6* | 23.8 ± 21.6* | 35.0 ± 65.0 | 30.8 ± 53.1 |
| LAS40, ms | 53.1 ± 38.4 | 55.6 ± 33.8* | 54.3 ± 36.0* | 47.7 ± 32.1 | 50.2 ± 33.8 |
| JT interval, ms | 288.9 ± 56.9 | 293.7 ± 55.2 | 291.4 ± 56.1 | 290.2 ± 52.2 | 290.7 ± 53.6 |
| JT variance, ms2 | 15.6 ± 17.1 | 15.1 ± 20.8 | 15.2 ± 19.2 | 13.2 ± 25.2 | 13.9 ± 23.2 |
| SDNN, ms | 75.8 ± 72.3† | 57.8 ± 73.9 | 65.5 ± 73.7* | 52.3 ± 47.5 | 57.1 ± 58.8 |
| LF : HF, ratio | 1.66 ± 1.84 | 1.39 ± 1.69 | 1.52 ± 1.76 | 1.74 ± 2.63 | 1.66 ± 2.35 |
| QT interval, ms | 439.0 ± 56.7 | 436.9 ± 60.2 | 437.5 ± 58.3 | 433.7 ± 65.0 | 435.1 ± 62.8 |
| QTc, ms | 456.6 ± 43.7 | 474.3 ± 49.9† | 466.2 ± 47.9* | 457.9 ± 40.8 | 461.2 ± 43.9 |
| QT-heart rate coherence | 0.415 ± 0.166 | 0.407 ± 0.186 | 0.410 ± 0.177 | 0.436 ± 0.192 | 0.427 ± 0.187 |
| QTVI | −0.81 ± 0.72 | −0.68 ± 0.65† | −0.73 ± 0.68* | −0.88 ± 0.62 | −0.82 ± 0.65 |
| EntropyXQT | 2.77 ± 0.69‡ | 2.69 ± 0.65‡ | 2.72 ± 0.67‡ | 2.43 ± 0.58 | 2.54 ± 0.63 |
Values are mean ± SD, median [IQR], or percent. Statistical comparisons vs. Alive: *P < 0.05, †P < 0.001, ‡P < 0.00001.
Patient and ECG characteristics by events
| . | Appropriate ICD shock (n = 134) . | All-cause mortality (166) . | Composite events (300) . | Alive (516) . | Total cohort (816) . |
|---|---|---|---|---|---|
| Age, years | 60.6 ± 11.9 | 65.6 ± 11.3‡ | 63.4 ± 11.9‡ | 58.5 ± 12.4 | 60.3 ± 12.5 |
| Male, % | 79.9* | 73.8 | 76.3* | 69.4 | 71.9 |
| Caucasian, % | 67.2 | 60.7 | 63.3 | 60.7 | 61.6 |
| African American, % | 30.6 | 36.9 | 34.3 | 36.2 | 35.5 |
| Asian or American Indian, % | 2.2 | 2.4 | 2.3 | 3.1 | 2.8 |
| Cardiac resynchronization, % | 23.1 | 30.4 | 27.3 | 27.1 | 27.2 |
| History of paroxysmal AF, % | 19.4 | 31.5† | 26.3* | 16.3 | 20.0 |
| Smoking, % | 74.6* | 75.0* | 74.7* | 62.6 | 67.0 |
| Hypertension, % | 54.5 | 67.9 | 62.0 | 57.9 | 59.4 |
| Hyperlipidaemia, % | 43.3 | 50.0 | 47.3 | 41.9 | 43.9 |
| Diabetes mellitus, % | 35.8 | 46.4‡ | 41.7‡ | 27.3 | 32.6 |
| Ischaemic cardiomyopathy, % | 55.2 | 65.5 | 60.7 | 53.5 | 56.1 |
| Body mass index, kg/m2 | 29.6 ± 5.7 | 28.5 ± 5.7* | 29.0 ± 5.7* | 30.0 ± 6.5 | 29.6 ± 6.2 |
| Mean arterial pressure, mmHg | 89.2 ± 13.2* | 89.9 ± 12.9* | 89.5 ± 12.9* | 92.6 ± 13.8 | 91.4 ± 13.5 |
| Aspirin, % | 69.4 | 72.6 | 71.0 | 65.1 | 67.3 |
| Beta blocker, % | 86.6 | 89.9 | 88.7 | 91.1 | 90.2 |
| ACE inhibitor or ARB, % | 85.8 | 87.4 | 87.0 | 89.9 | 88.8 |
| Aldosterone antagonist, % | 19.4 | 29.2 | 25.0 | 25.2 | 25.1 |
| Statin, % | 70.1 | 72.6 | 71.3 | 70.2 | 70.6 |
| Antiarrhythmic, % | 11.9* | 12.5* | 12.0 | 6.6 | 8.6 |
| Loop diuretic, % | 64.2 | 76.2 | 71.0 | 62.0 | 65.3 |
| NYHA Class I | 26.9* | 13.1 | 19.3 | 19.4 | 19.4 |
| NYHA Class II | 39.6 | 31.5 | 35.0 | 43.0 | 40.1 |
| NYHA Class III | 33.6 | 55.4 | 45.7 | 37.6 | 40.6 |
| Ejection fraction, % | 22.4 ± 7.8 | 21.2 ± 7.9* | 21.7 ± 7.9* | 22.9 ± 7.9 | 22.4 ± 7.9 |
| Sodium, mEq/L | 138.5 ± 3.0 | 138.7 ± 3.4 | 138.6 ± 3.2 | 139.0 ± 3.0 | 138.8 ± 3.1 |
| Potassium, mEq/L | 4.22 ± 0.44 | 4.19 ± 0.45 | 4.20 ± 0.44 | 4.22 ± 0.43 | 4.22 ± 0.44 |
| BUN, mg/dL | 20.5 [16–26] | 25.0 [18–34]‡ | 22.0 [17–30]‡ | 18.0 [15–24] | 20.0 [15–26] |
| Creatinine, mg/dL | 1.0 [0.9–1.2] | 1.3 [1.0–1.6]‡ | 1.1 [0.9–1.4]† | 1.0 [0.9–1.3] | 1.1 [0.9–1.3] |
| hsCRP, μg/mL | 4.6 [2.4–11.3] | 7.2 [2.8–6.7]† | 5.7 [2.4–3.9]* | 3.4 [1.5–8.6] | 4.4 [1.6–11] |
| NT-ProBNP, ng/mL | 2.5 [1.7–3.9] | 3.7 [2.5–7.2]‡ | 3.1 [2.0–5.2]† | 2.2 [1.6–3.5] | 2.5 [1.7–4.0] |
| Troponin T, ng/mL | 0.01 [0.0–0.04] | 0.03 [0.0–0.07] | 0.02 [0.0–0.06] | 0.00 [0.0–0.02] | 0.01 [0.0–0.03] |
| SHFS 5-year predicted mortality, % | 30.6 ± 16.0‡ | 42.8 ± 18.1‡ | 37.5 ± 18.2‡ | 24.5 ± 12.7 | 29.3 ± 16.2 |
| Heart rate, bpm | 71.7 ± 14.7 | 74.7 ± 15.7* | 73.4 ± 15.3 | 71.8 ± 13.0 | 72.4 ± 13.9 |
| PVC, % | 3.7 ± 4.8† | 2.8 ± 3.7 | 3.2 ± 4.2* | 2.3 ± 4.0 | 2.6 ± 4.1 |
| QRSD, ms | 121.0 ± 33.0 | 127.9 ± 31.2 | 125.0 ± 32.2 | 122.5 ± 34.1 | 123.5 ± 33.4 |
| HFQRSD, ms | 122.1 ± 34.9 | 124.1 ± 32.5 | 123.4 ± 33.6 | 121.0 ± 32.9 | 121.9 ± 33.2 |
| RMS40, μV | 25.6 ± 21.3 | 22.1 ± 21.6* | 23.8 ± 21.6* | 35.0 ± 65.0 | 30.8 ± 53.1 |
| LAS40, ms | 53.1 ± 38.4 | 55.6 ± 33.8* | 54.3 ± 36.0* | 47.7 ± 32.1 | 50.2 ± 33.8 |
| JT interval, ms | 288.9 ± 56.9 | 293.7 ± 55.2 | 291.4 ± 56.1 | 290.2 ± 52.2 | 290.7 ± 53.6 |
| JT variance, ms2 | 15.6 ± 17.1 | 15.1 ± 20.8 | 15.2 ± 19.2 | 13.2 ± 25.2 | 13.9 ± 23.2 |
| SDNN, ms | 75.8 ± 72.3† | 57.8 ± 73.9 | 65.5 ± 73.7* | 52.3 ± 47.5 | 57.1 ± 58.8 |
| LF : HF, ratio | 1.66 ± 1.84 | 1.39 ± 1.69 | 1.52 ± 1.76 | 1.74 ± 2.63 | 1.66 ± 2.35 |
| QT interval, ms | 439.0 ± 56.7 | 436.9 ± 60.2 | 437.5 ± 58.3 | 433.7 ± 65.0 | 435.1 ± 62.8 |
| QTc, ms | 456.6 ± 43.7 | 474.3 ± 49.9† | 466.2 ± 47.9* | 457.9 ± 40.8 | 461.2 ± 43.9 |
| QT-heart rate coherence | 0.415 ± 0.166 | 0.407 ± 0.186 | 0.410 ± 0.177 | 0.436 ± 0.192 | 0.427 ± 0.187 |
| QTVI | −0.81 ± 0.72 | −0.68 ± 0.65† | −0.73 ± 0.68* | −0.88 ± 0.62 | −0.82 ± 0.65 |
| EntropyXQT | 2.77 ± 0.69‡ | 2.69 ± 0.65‡ | 2.72 ± 0.67‡ | 2.43 ± 0.58 | 2.54 ± 0.63 |
| . | Appropriate ICD shock (n = 134) . | All-cause mortality (166) . | Composite events (300) . | Alive (516) . | Total cohort (816) . |
|---|---|---|---|---|---|
| Age, years | 60.6 ± 11.9 | 65.6 ± 11.3‡ | 63.4 ± 11.9‡ | 58.5 ± 12.4 | 60.3 ± 12.5 |
| Male, % | 79.9* | 73.8 | 76.3* | 69.4 | 71.9 |
| Caucasian, % | 67.2 | 60.7 | 63.3 | 60.7 | 61.6 |
| African American, % | 30.6 | 36.9 | 34.3 | 36.2 | 35.5 |
| Asian or American Indian, % | 2.2 | 2.4 | 2.3 | 3.1 | 2.8 |
| Cardiac resynchronization, % | 23.1 | 30.4 | 27.3 | 27.1 | 27.2 |
| History of paroxysmal AF, % | 19.4 | 31.5† | 26.3* | 16.3 | 20.0 |
| Smoking, % | 74.6* | 75.0* | 74.7* | 62.6 | 67.0 |
| Hypertension, % | 54.5 | 67.9 | 62.0 | 57.9 | 59.4 |
| Hyperlipidaemia, % | 43.3 | 50.0 | 47.3 | 41.9 | 43.9 |
| Diabetes mellitus, % | 35.8 | 46.4‡ | 41.7‡ | 27.3 | 32.6 |
| Ischaemic cardiomyopathy, % | 55.2 | 65.5 | 60.7 | 53.5 | 56.1 |
| Body mass index, kg/m2 | 29.6 ± 5.7 | 28.5 ± 5.7* | 29.0 ± 5.7* | 30.0 ± 6.5 | 29.6 ± 6.2 |
| Mean arterial pressure, mmHg | 89.2 ± 13.2* | 89.9 ± 12.9* | 89.5 ± 12.9* | 92.6 ± 13.8 | 91.4 ± 13.5 |
| Aspirin, % | 69.4 | 72.6 | 71.0 | 65.1 | 67.3 |
| Beta blocker, % | 86.6 | 89.9 | 88.7 | 91.1 | 90.2 |
| ACE inhibitor or ARB, % | 85.8 | 87.4 | 87.0 | 89.9 | 88.8 |
| Aldosterone antagonist, % | 19.4 | 29.2 | 25.0 | 25.2 | 25.1 |
| Statin, % | 70.1 | 72.6 | 71.3 | 70.2 | 70.6 |
| Antiarrhythmic, % | 11.9* | 12.5* | 12.0 | 6.6 | 8.6 |
| Loop diuretic, % | 64.2 | 76.2 | 71.0 | 62.0 | 65.3 |
| NYHA Class I | 26.9* | 13.1 | 19.3 | 19.4 | 19.4 |
| NYHA Class II | 39.6 | 31.5 | 35.0 | 43.0 | 40.1 |
| NYHA Class III | 33.6 | 55.4 | 45.7 | 37.6 | 40.6 |
| Ejection fraction, % | 22.4 ± 7.8 | 21.2 ± 7.9* | 21.7 ± 7.9* | 22.9 ± 7.9 | 22.4 ± 7.9 |
| Sodium, mEq/L | 138.5 ± 3.0 | 138.7 ± 3.4 | 138.6 ± 3.2 | 139.0 ± 3.0 | 138.8 ± 3.1 |
| Potassium, mEq/L | 4.22 ± 0.44 | 4.19 ± 0.45 | 4.20 ± 0.44 | 4.22 ± 0.43 | 4.22 ± 0.44 |
| BUN, mg/dL | 20.5 [16–26] | 25.0 [18–34]‡ | 22.0 [17–30]‡ | 18.0 [15–24] | 20.0 [15–26] |
| Creatinine, mg/dL | 1.0 [0.9–1.2] | 1.3 [1.0–1.6]‡ | 1.1 [0.9–1.4]† | 1.0 [0.9–1.3] | 1.1 [0.9–1.3] |
| hsCRP, μg/mL | 4.6 [2.4–11.3] | 7.2 [2.8–6.7]† | 5.7 [2.4–3.9]* | 3.4 [1.5–8.6] | 4.4 [1.6–11] |
| NT-ProBNP, ng/mL | 2.5 [1.7–3.9] | 3.7 [2.5–7.2]‡ | 3.1 [2.0–5.2]† | 2.2 [1.6–3.5] | 2.5 [1.7–4.0] |
| Troponin T, ng/mL | 0.01 [0.0–0.04] | 0.03 [0.0–0.07] | 0.02 [0.0–0.06] | 0.00 [0.0–0.02] | 0.01 [0.0–0.03] |
| SHFS 5-year predicted mortality, % | 30.6 ± 16.0‡ | 42.8 ± 18.1‡ | 37.5 ± 18.2‡ | 24.5 ± 12.7 | 29.3 ± 16.2 |
| Heart rate, bpm | 71.7 ± 14.7 | 74.7 ± 15.7* | 73.4 ± 15.3 | 71.8 ± 13.0 | 72.4 ± 13.9 |
| PVC, % | 3.7 ± 4.8† | 2.8 ± 3.7 | 3.2 ± 4.2* | 2.3 ± 4.0 | 2.6 ± 4.1 |
| QRSD, ms | 121.0 ± 33.0 | 127.9 ± 31.2 | 125.0 ± 32.2 | 122.5 ± 34.1 | 123.5 ± 33.4 |
| HFQRSD, ms | 122.1 ± 34.9 | 124.1 ± 32.5 | 123.4 ± 33.6 | 121.0 ± 32.9 | 121.9 ± 33.2 |
| RMS40, μV | 25.6 ± 21.3 | 22.1 ± 21.6* | 23.8 ± 21.6* | 35.0 ± 65.0 | 30.8 ± 53.1 |
| LAS40, ms | 53.1 ± 38.4 | 55.6 ± 33.8* | 54.3 ± 36.0* | 47.7 ± 32.1 | 50.2 ± 33.8 |
| JT interval, ms | 288.9 ± 56.9 | 293.7 ± 55.2 | 291.4 ± 56.1 | 290.2 ± 52.2 | 290.7 ± 53.6 |
| JT variance, ms2 | 15.6 ± 17.1 | 15.1 ± 20.8 | 15.2 ± 19.2 | 13.2 ± 25.2 | 13.9 ± 23.2 |
| SDNN, ms | 75.8 ± 72.3† | 57.8 ± 73.9 | 65.5 ± 73.7* | 52.3 ± 47.5 | 57.1 ± 58.8 |
| LF : HF, ratio | 1.66 ± 1.84 | 1.39 ± 1.69 | 1.52 ± 1.76 | 1.74 ± 2.63 | 1.66 ± 2.35 |
| QT interval, ms | 439.0 ± 56.7 | 436.9 ± 60.2 | 437.5 ± 58.3 | 433.7 ± 65.0 | 435.1 ± 62.8 |
| QTc, ms | 456.6 ± 43.7 | 474.3 ± 49.9† | 466.2 ± 47.9* | 457.9 ± 40.8 | 461.2 ± 43.9 |
| QT-heart rate coherence | 0.415 ± 0.166 | 0.407 ± 0.186 | 0.410 ± 0.177 | 0.436 ± 0.192 | 0.427 ± 0.187 |
| QTVI | −0.81 ± 0.72 | −0.68 ± 0.65† | −0.73 ± 0.68* | −0.88 ± 0.62 | −0.82 ± 0.65 |
| EntropyXQT | 2.77 ± 0.69‡ | 2.69 ± 0.65‡ | 2.72 ± 0.67‡ | 2.43 ± 0.58 | 2.54 ± 0.63 |
Values are mean ± SD, median [IQR], or percent. Statistical comparisons vs. Alive: *P < 0.05, †P < 0.001, ‡P < 0.00001.
The median 5-year predicted mortality based on the SHFS was 25%. Over an average of 45 ± 24 months, there were 300 composite events; 134 patients received at least one appropriate ICD shock and 166 patients died without receiving any appropriate ICD shocks. The deaths were from heart failure (n = 38), PEA/asystole/sudden death (n = 18), septic shock (n = 17), cancer (n = 13), respiratory failure (n = 8), gastrointestinal disease (n = 3), acute neurological event (n = 8), abdominal aortic aneurysm (n = 1), appendicitis (n = 1), and unknown (n = 59). Patients without events (n = 516) had a lower SHFS 5-year predicted mortality risk; were more likely to be younger and female; to have higher MAP, EF, QTVI, and EntropyXQT; to have lower values of serum BUN, creatinine, NT-proBNP, and hsCRP; and were less likely to have a history of paroxysmal AF, smoking, diabetes, and taking antiarrhythmic medications.
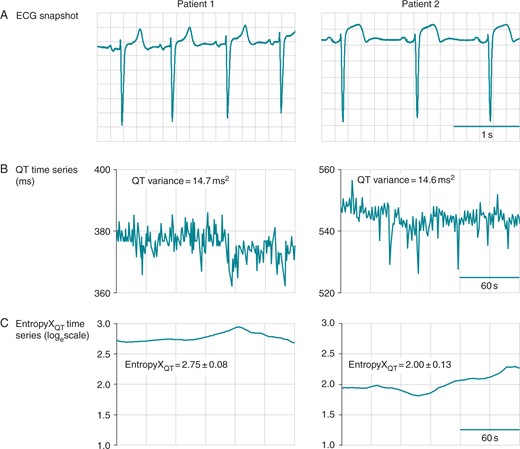
Sample tracings of EntropyXQT. Baseline data from two patients are shown, including a 3-s ECG segment (A), horizontal time axis 0.2 s/box, and corresponding time series (30 s/box) for the QT interval (B) and EntropyXQT (C). The QT variances were 14.7 and 14.6 ms2 in the left and right panels, respectively. EntropyXQT was used to quantify the distinct patterns underlying the variability of the QT time series and yielded values (mean ± SD) of 2.75 ± 0.080 (left panel) and 2.00 ± 0.131 (right).
EntropyXQT and outcomes
The adjusted hazard ratio comparing the 5th to the 1st quintile of EntropyXQT was 3.29 (95% CI 1.7–6.2), 2.3 (1.5–3.4), and 2.2 (1.5–3.3) for the first appropriate ICD shock, all-cause mortality, and composite events, respectively (Table 2). These results were not affected by the substitution of one or more of the 30 predictors in the base model with other covariates from Table 1.
Effect of EntropyXQT by quintiles on incrementally adjusted proportional hazards ratio in Models 1–4
| . | Quintile 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Appropriate ICD shock | |||||
| Cases/non-cases (n) | 16/153 | 23/140 | 23/136 | 33/130 | 39/123 |
| Hazard ratio, model 1 | 1, reference | 1.76 [0.93–3.36] | 1.56 [0.82–3.00] | 2.16 [1.17–3.97] | 2.88 [1.59–5.22] |
| Hazard ratio, model 2 | 1, reference | 1.76 [0.92–3.36] | 1.41 [0.73–2.73] | 2.02 [1.09–3.74] | 2.81 [1.54–5.13] |
| Hazard ratio, model 3 | 1, reference | 1.73 [0.90–3.31] | 1.42 [0.74–2.75] | 2.03 [1.09–3.77] | 2.82 [1.53–5.19] |
| Hazard ratio, model 4 | 1, reference | 1.79 [0.94–3.44] | 1.47 [0.76–2.85] | 2.23 [1.19–4.19] | 3.29 [1.74–6.21] |
| All-cause mortality | |||||
| Cases/non-cases (n) | 25/142 | 30/131 | 28/135 | 34/128 | 49/112 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.58 [1.05–2.38] | 1.14 [0.75–1.73] | 1.46 [0.98–2.16] | 2.07 [1.42–3.04] |
| Hazard ratio, model 3 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.33] | 2.20 [1.49–3.26] |
| Hazard ratio, model 4 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.35] | 2.21 [1.47–3.32] |
| Composite events | |||||
| Cases/non-cases (n) | 40/127 | 54/107 | 51/112 | 68/95 | 87/75 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.61 [1.06–2.43] | 1.18 [0.77–1.81] | 1.56 [1.05–2.32] | 2.27 [1.54–3.34] |
| Hazard ratio, model 3 | 1, reference | 1.59 [1.05–2.42] | 1.28 [0.83–1.96] | 1.66 [1.11–2.48] | 2.39 [1.61–3.56] |
| Hazard ratio, model 4 | 1, reference | 1.56 [1.03–2.37] | 1.26 [0.82–1.93] | 1.65 [1.10–2.48] | 2.28 [1.53–3.41] |
| . | Quintile 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Appropriate ICD shock | |||||
| Cases/non-cases (n) | 16/153 | 23/140 | 23/136 | 33/130 | 39/123 |
| Hazard ratio, model 1 | 1, reference | 1.76 [0.93–3.36] | 1.56 [0.82–3.00] | 2.16 [1.17–3.97] | 2.88 [1.59–5.22] |
| Hazard ratio, model 2 | 1, reference | 1.76 [0.92–3.36] | 1.41 [0.73–2.73] | 2.02 [1.09–3.74] | 2.81 [1.54–5.13] |
| Hazard ratio, model 3 | 1, reference | 1.73 [0.90–3.31] | 1.42 [0.74–2.75] | 2.03 [1.09–3.77] | 2.82 [1.53–5.19] |
| Hazard ratio, model 4 | 1, reference | 1.79 [0.94–3.44] | 1.47 [0.76–2.85] | 2.23 [1.19–4.19] | 3.29 [1.74–6.21] |
| All-cause mortality | |||||
| Cases/non-cases (n) | 25/142 | 30/131 | 28/135 | 34/128 | 49/112 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.58 [1.05–2.38] | 1.14 [0.75–1.73] | 1.46 [0.98–2.16] | 2.07 [1.42–3.04] |
| Hazard ratio, model 3 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.33] | 2.20 [1.49–3.26] |
| Hazard ratio, model 4 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.35] | 2.21 [1.47–3.32] |
| Composite events | |||||
| Cases/non-cases (n) | 40/127 | 54/107 | 51/112 | 68/95 | 87/75 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.61 [1.06–2.43] | 1.18 [0.77–1.81] | 1.56 [1.05–2.32] | 2.27 [1.54–3.34] |
| Hazard ratio, model 3 | 1, reference | 1.59 [1.05–2.42] | 1.28 [0.83–1.96] | 1.66 [1.11–2.48] | 2.39 [1.61–3.56] |
| Hazard ratio, model 4 | 1, reference | 1.56 [1.03–2.37] | 1.26 [0.82–1.93] | 1.65 [1.10–2.48] | 2.28 [1.53–3.41] |
The proportional hazards ratios [95% confidence interval] corresponding to hierarchical models 1–4 are shown by quintiles of EntropyXQT. The absolute risk is indicated as the number of cases (bold) and non-cases. This analysis was based on 45 ± 24 months of follow-up of 816 patients; there were 300 composite events, including 134 events for the first appropriate ICD shock (primary endpoint) and 166 events for all-cause mortality. Competing risks regression analysis yielded similar results.
Model 1 consisted of only EntropyXQT. Model 2 consisted of EntropyXQT, demographics (age at implant, gender, race), medical history (paroxysmal AF, smoking, diabetes mellitus, ischaemic cardiomyopathy, NYHA class) and prescribed medications (aspirin, β-blocker, ACE inhibitor and/or angiotensin receptor blocker, aldosterone antagonist, statin, antiarrhythmic, loop diuretic). Model 3 consisted of all covariates in Model 2 as well as physical exam (body mass index, MAP), laboratory results (sodium, potassium, BUN), biomarkers (hsCRP, NT-proBNP) and EF. Model 4 consisted of all covariates in Model 3 as well as 5-min ECG time and frequency domain measures of HRV and QT variability (heart rate, per cent PVC, SDNN, low:high frequency ratio, QT-heart rate coherence, QTVI).
Effect of EntropyXQT by quintiles on incrementally adjusted proportional hazards ratio in Models 1–4
| . | Quintile 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Appropriate ICD shock | |||||
| Cases/non-cases (n) | 16/153 | 23/140 | 23/136 | 33/130 | 39/123 |
| Hazard ratio, model 1 | 1, reference | 1.76 [0.93–3.36] | 1.56 [0.82–3.00] | 2.16 [1.17–3.97] | 2.88 [1.59–5.22] |
| Hazard ratio, model 2 | 1, reference | 1.76 [0.92–3.36] | 1.41 [0.73–2.73] | 2.02 [1.09–3.74] | 2.81 [1.54–5.13] |
| Hazard ratio, model 3 | 1, reference | 1.73 [0.90–3.31] | 1.42 [0.74–2.75] | 2.03 [1.09–3.77] | 2.82 [1.53–5.19] |
| Hazard ratio, model 4 | 1, reference | 1.79 [0.94–3.44] | 1.47 [0.76–2.85] | 2.23 [1.19–4.19] | 3.29 [1.74–6.21] |
| All-cause mortality | |||||
| Cases/non-cases (n) | 25/142 | 30/131 | 28/135 | 34/128 | 49/112 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.58 [1.05–2.38] | 1.14 [0.75–1.73] | 1.46 [0.98–2.16] | 2.07 [1.42–3.04] |
| Hazard ratio, model 3 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.33] | 2.20 [1.49–3.26] |
| Hazard ratio, model 4 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.35] | 2.21 [1.47–3.32] |
| Composite events | |||||
| Cases/non-cases (n) | 40/127 | 54/107 | 51/112 | 68/95 | 87/75 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.61 [1.06–2.43] | 1.18 [0.77–1.81] | 1.56 [1.05–2.32] | 2.27 [1.54–3.34] |
| Hazard ratio, model 3 | 1, reference | 1.59 [1.05–2.42] | 1.28 [0.83–1.96] | 1.66 [1.11–2.48] | 2.39 [1.61–3.56] |
| Hazard ratio, model 4 | 1, reference | 1.56 [1.03–2.37] | 1.26 [0.82–1.93] | 1.65 [1.10–2.48] | 2.28 [1.53–3.41] |
| . | Quintile 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Appropriate ICD shock | |||||
| Cases/non-cases (n) | 16/153 | 23/140 | 23/136 | 33/130 | 39/123 |
| Hazard ratio, model 1 | 1, reference | 1.76 [0.93–3.36] | 1.56 [0.82–3.00] | 2.16 [1.17–3.97] | 2.88 [1.59–5.22] |
| Hazard ratio, model 2 | 1, reference | 1.76 [0.92–3.36] | 1.41 [0.73–2.73] | 2.02 [1.09–3.74] | 2.81 [1.54–5.13] |
| Hazard ratio, model 3 | 1, reference | 1.73 [0.90–3.31] | 1.42 [0.74–2.75] | 2.03 [1.09–3.77] | 2.82 [1.53–5.19] |
| Hazard ratio, model 4 | 1, reference | 1.79 [0.94–3.44] | 1.47 [0.76–2.85] | 2.23 [1.19–4.19] | 3.29 [1.74–6.21] |
| All-cause mortality | |||||
| Cases/non-cases (n) | 25/142 | 30/131 | 28/135 | 34/128 | 49/112 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.58 [1.05–2.38] | 1.14 [0.75–1.73] | 1.46 [0.98–2.16] | 2.07 [1.42–3.04] |
| Hazard ratio, model 3 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.33] | 2.20 [1.49–3.26] |
| Hazard ratio, model 4 | 1, reference | 1.54 [1.02–2.34] | 1.20 [0.78–1.84] | 1.56 [1.04–2.35] | 2.21 [1.47–3.32] |
| Composite events | |||||
| Cases/non-cases (n) | 40/127 | 54/107 | 51/112 | 68/95 | 87/75 |
| Hazard ratio, model 1 | 1, reference | 1.50 [1.00–2.26] | 1.28 [0.85–1.94] | 1.63 [1.10–2.41] | 2.36 [1.63–3.44] |
| Hazard ratio, model 2 | 1, reference | 1.61 [1.06–2.43] | 1.18 [0.77–1.81] | 1.56 [1.05–2.32] | 2.27 [1.54–3.34] |
| Hazard ratio, model 3 | 1, reference | 1.59 [1.05–2.42] | 1.28 [0.83–1.96] | 1.66 [1.11–2.48] | 2.39 [1.61–3.56] |
| Hazard ratio, model 4 | 1, reference | 1.56 [1.03–2.37] | 1.26 [0.82–1.93] | 1.65 [1.10–2.48] | 2.28 [1.53–3.41] |
The proportional hazards ratios [95% confidence interval] corresponding to hierarchical models 1–4 are shown by quintiles of EntropyXQT. The absolute risk is indicated as the number of cases (bold) and non-cases. This analysis was based on 45 ± 24 months of follow-up of 816 patients; there were 300 composite events, including 134 events for the first appropriate ICD shock (primary endpoint) and 166 events for all-cause mortality. Competing risks regression analysis yielded similar results.
Model 1 consisted of only EntropyXQT. Model 2 consisted of EntropyXQT, demographics (age at implant, gender, race), medical history (paroxysmal AF, smoking, diabetes mellitus, ischaemic cardiomyopathy, NYHA class) and prescribed medications (aspirin, β-blocker, ACE inhibitor and/or angiotensin receptor blocker, aldosterone antagonist, statin, antiarrhythmic, loop diuretic). Model 3 consisted of all covariates in Model 2 as well as physical exam (body mass index, MAP), laboratory results (sodium, potassium, BUN), biomarkers (hsCRP, NT-proBNP) and EF. Model 4 consisted of all covariates in Model 3 as well as 5-min ECG time and frequency domain measures of HRV and QT variability (heart rate, per cent PVC, SDNN, low:high frequency ratio, QT-heart rate coherence, QTVI).
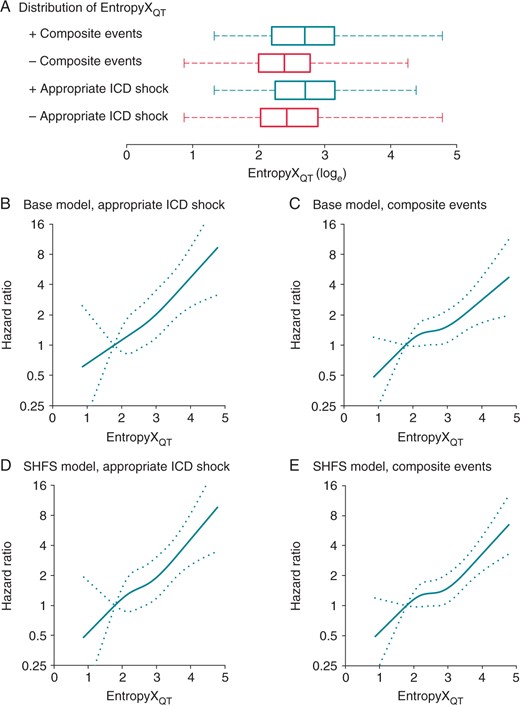
Multivariate-adjusted hazard ratios from EntropyXQT. (A) The frequency distribution of EntropyXQT in cases (deep) and non-cases (red) is represented in the box and whisker plot, including inter-quartile range (box), median (vertical band), and maximum and minimum values (dashed whiskers). (B) The curves represent multivariate-adjusted hazard ratios (solid line) with 95% confidence intervals (dotted lines) based on restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of EntropyXQT distribution. The reference value (hazard ratio = 1) was set at 1.79, the 10th percentile of the EntropyXQT distribution. The hazard ratios for the primary endpoint of the first appropriate ICD shock were adjusted for the base model, which was composed of demographics (age at implant, gender, race), medical history (paroxysmal AF, smoking, diabetes mellitus, ischaemic cardiomyopathy, NYHA class), medications (aspirin, β- blocker, ACE inhibitor or angiotensin receptor blocker, aldosterone antagonist, statin, antiarrhythmic, loop diuretic), physical exam (body mass index, MAP), laboratory results (sodium, potassium, BUN), biomarkers (hsCRP, NT-proBNP), EF, and time and frequency domain measures of HRV and QT variability (heart rate, percent PVC, SDNN, low:high frequency ratio, QT-heart rate coherence, QTVI). (C) The hazard ratios for the secondary endpoint of composite events (appropriate ICD shock and all-cause mortality) were adjusted for the base model. (D) The hazard ratios for appropriate ICD shock were adjusted for the Seattle heart failure score (SHFS) model. (E) The hazard ratios for composite events were adjusted for the SHFS model.
The incrementally adjusted proportional hazards ratio for EntropyXQT demonstrated that the predictive value of EntropyXQT was independent from all covariates evaluated in this study (Table 2), including other measures of repolarization variability (Supplementary material online, Figure S1). Varying the sequence of incremental adjustments for the covariates did not affect the results.
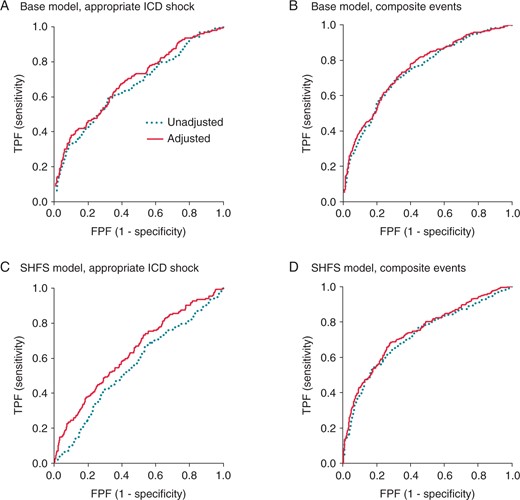
Effect on receiver operating characteristic (ROC) curves after the addition of EntropyXQT. The ROC curves were compared for the unadjusted base or SHFS models before (dotted line) and after the addition of EntropyXQT. (A) For the primary endpoint of the first appropriate ICD shock, the ROC area under curve (AUC; mean ± SE) after adding EntropyXQT (0.634 ± 0.0290) was larger than that of the base model alone (0.594 ± 0.0311; ΔAUC = 0.0396 ± 0.0159; P < 0.05). (B) For composite events, the AUC after adding EntropyXQT (0.702 ± 0.0211) was larger than that of the base model alone (0.682 ± 0.0219; ΔAUC = 0.0197 ± 0.00894; P < 0.05). (C) For appropriate ICD shock, the AUC after adding EntropyXQT (0.631 ± 0.0268) was larger than that of the SHFS model alone (0.552 ± 0.0382; ΔAUC = 0.0791 ± 0.0394; P < 0.01). (D) For composite events, the AUC after adding EntropyXQT (0.740 ± 0.0186) was larger than that of the SHFS model alone (0.719 ± 0.0190; ΔAUC = 0.0208 ± 0.00923; P < 0.05).
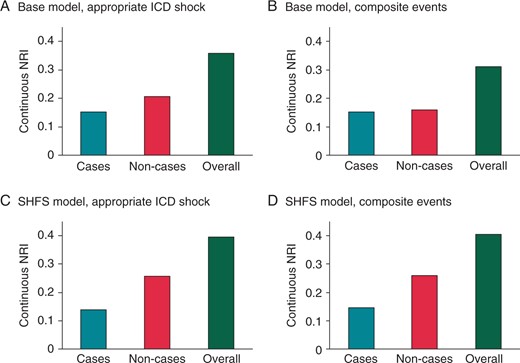
NRI after the addition of EntropyXQT. The continuous NRI is plotted for cases (turquoise), non-cases (red), and overall (green) for the base and SHFS models. (A) For the primary endpoint of the first appropriate ICD shock, continuous NRI of the base model with EntropyXQT was 0.152 (95% CI 0.035–0.311), 0.206 (0.102–0.283), and 0.358 (0.161–0.568) for cases, non-cases, and overall, respectively. The IDI was 0.020 (0.005–0.047), 0.005 (0.001–0.011), and 0.026 (0.006–0.057) for cases, non-cases, and overall, respectively. (B) For composite events, continuous NRI of the base model with EntropyXQT was 0.153 (0.025–0.225), 0.159 (0.054–0.225), and 0.312 (0.108–0.422) for cases, non-cases, and overall, respectively. The IDI was 0.014 (0.003–0.028), 0.008 (0.002–0.017), and 0.022 (0.006–0.045) for cases, non-cases, and overall, respectively. (C) For appropriate ICD shock, continuous NRI of the SHFS model with EntropyXQT was 0.139 (0.020–0.257), 0.257 (0.178–0.320), and 0.396 (0.227–0.550) for cases, non-cases, and overall, respectively. The IDI was 0.032 (0.011–0.059), 0.007 (0.002–0.014), and 0.039 (0.014–0.073) for cases, non-cases, and overall, respectively. (D) For composite events, the continuous NRI of the SHFS model with EntropyXQT was 0.147 (0.045–0.223), 0.260 (0.173–0.326), and 0.406 (0.246–0.526) for cases, non-cases, and overall, respectively. The IDI was 0.021 (0.009–0.040), 0.012 (0.005–0.023), and 0.034 (0.013–0.063) for cases, non-cases, and overall, respectively.
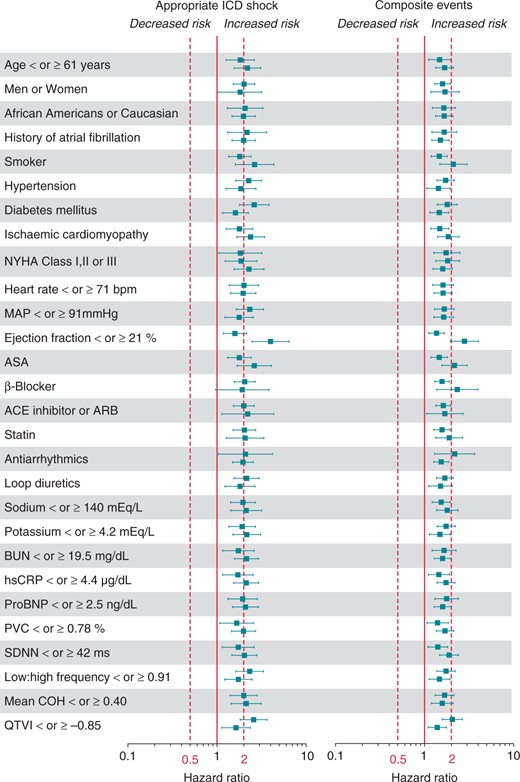
Multivariable-adjusted hazard ratios for EntropyXQT by subgroups. The adjusted hazard ratios with 95% confidence intervals are plotted for EntropyXQT as a continuous variate for the presence (top) and absence (bottom) of each categorical covariate and for the lower (top) and upper (bottom) median of each continuous covariate.
Discussion
Non-linear dynamic analyses for quantifying intrinsic patterns underlying the variability of physiological signals enhance contemporary strategies for risk prediction and may provide insight into altered homeostatic signalling5,21,24 that cannot be identified by conventional analyses.3–7,9–12 By applying these principles, we found that EntropyXQT, a novel non-linear measure of the degree to which fluctuation patterns in cardiac repolarization repeat themselves, strongly predicted appropriate ICD shock for VT/VF and all-cause mortality independent of other established risk factors in a cohort of primary prevention ICD patients. More importantly, these results demonstrate that EntropyXQT may further improve our discrimination of individuals who may stand to benefit from primary prevention ICDs. The robustness of EntropyXQT and ease of its measurement from ambulatory ECGs and implantable devices have important implications for risk prediction at the individual and population levels.
More than half a million patients per year in the USA are eligible for device implantation because of a low EF.25 However, there is significant heterogeneity in the patient population defined as eligible for ICD implantation based on the EF and many patients in this group would be considered relatively low risk except for the presence of a low EF.26 Even in high-risk patients, the older and sicker may not receive the same mortality benefit from primary prevention ICDs after accounting for co-morbidities and competing risks.27,28 Indeed, most patients with primary prevention ICDs do not receive shocks for VT/VF1,29 and those who receive shocks have an increased mortality risk despite the presence of the ICD.29
Current strategies for risk stratification based on deterministic linear measures have demonstrated limited clinical utility, including left ventricular EF,2 heart rate-corrected QT interval, QTVI, microvolt T-wave alternans (MTWA), SAECG, HRV, baroreflex sensitivity, heart rate profile during and after exercise, and biomarkers such as serum BNP level. For example, preserved HRV appears to predict low risk,30 but not universally.16,31,32 Large patient datasets have facilitated the derivation and validation of risk-prediction models, which integrate multiple deterministic clinical variables into a single cohesive measure, e.g. SHFS,15 albeit with limited clinical application. For example, the SHFS has limited accuracy for predicting outcomes in HF patients with implanted devices.33
Our findings are consistent with a body of evidence suggesting that cardiac entropy provides important mechanistic and clinical insight above and beyond that available from conventional analyses rooted in deterministic linear principles.3–7,9–12 Heart rate entropy has proved important in the early detection of sepsis and reducing mortality in premature infants6 and for improved discrimination of lethal from non-lethal arrhythmias in adult patients with primary prevention ICDs.7 In a prior study of 47 HF patients followed over 1 year after ICD implantation,8 higher approximate entropy of cardiac repolarization was independently associated with mortality whereas conventional measures of HRV and QT variability were not.8
The present study was conducted for up to 7 years of follow-up in patients with left ventricular dysfunction, a challenging cohort to manage with high rates of readmission for HF, myocardial infarction, or other non-cardiac co-morbidities. In this high-risk population, the mechanism by which EntropyXQT predicted lethal ventricular arrhythmias and all-cause mortality remains speculative. The processes that influence repetition of distinct patterns of ventricular repolarization likely differ between failing and non-failing hearts. For example, the expression and function of ion channels that contribute to cardiac repolarization are altered in HF patients. Autonomic dysfunction, a hallmark of HF, contributes to the increased temporal complexity of ventricular repolarization, particularly from increased cholinergic signalling.34 Further, this complexity may reflect dynamics of immune responses and progression of inflammatory disease via the cholinergic anti-inflammatory pathway.35 Our findings are consistent with the concept of progressive uncoupling of homeostatic signalling between organ systems5,21 and divergent autonomic, humoral and cytokine modulation35 of cardiac repolarization dynamics related to lethal ventricular arrhythmias and all-cause mortality.24 These effects may be indistinguishable in conventional measures of variability.4,7 The ability to recognize and quantify these subclinical non-linear signatures of advanced disease before clinical deterioration explains the complementary role of EntropyXQT with conventional methods of risk stratification and warrants prospective evaluation.
Conclusions
The limitations of risk stratification of individuals for primary prevention ICD implantation impose a large socioeconomic burden. The need for a readily available, inexpensive, non-invasive method for risk stratification is a clinical and public health priority. The present study has demonstrated that EntropyXQT is a fundamentally distinct and independent measure from conventional risk predictors. EntropyXQT may be used not only to identify individuals who would most likely benefit from ICDs but this paradigm of characterizing short-term physiological dynamics by repetition of distinct underlying patterns also has the potential for broad clinical application. The benefit of this novel strategy may be further enhanced when applied to a low-risk population with marginal or no indication for ICDs in the presence of less advanced cardiovascular disease.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
We gratefully acknowledge Barry Fetics for providing the MATLAB code for QT variability analysis, and Wendy Post and Roger Blumenthal for thoughtful comments. We gratefully acknowledge Barbara Butcher, Sanaz Norgard, Deborah Disilvestre, and Solmaz Masoudi for managing the PROSE-ICD study.
Conflict of interest: A.C. has received honoraria from Biotronik, Boston Scientific, Medtronic, and St. Jude Medical. All other authors have no disclosures.
Funding
This work was supported by the Donald W. Reynolds Cardiovascular Clinical Center at the Johns Hopkins University, NIH HL R01 091062 (G.F.T.) and NIH HL R01 103946 (A.C.).
References
- primary prevention
- electrocardiogram
- sudden death
- heart rate
- implantable defibrillators
- heart failure
- ischemic cardiomyopathy
- physical examination
- exposure
- biological markers
- demography
- entropy
- follow-up
- roc curve
- shock
- heart
- mortality
- patient prognosis
- sinus rhythm
- ventricular arrhythmia
- ejection fraction
- implantable defibrillator insertion
- inappropriate shocks from implanted defibrillator
- stratification
- african american