-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Gaudino, Francesco Alessandrini, Franco Glieca, Nicola Luciani, Carlo Cellini, Claudio Pragliola, Mauro Morelli, Carlo Canosa, Giuseppe Nasso, Gianfederico Possati, Survival after aortic valve replacement for aortic stenosis: does left ventricular mass regression have a clinical correlate?, European Heart Journal, Volume 26, Issue 1, January 2005, Pages 51–57, https://doi.org/10.1093/eurheartj/ehi012
Close - Share Icon Share
Aim The effects of post-operative left ventricular mass regression (LVMR) on clinical outcome after aortic valve surgery remains to be established. This study was intended to establish the impact of patient characteristics on post-operative survival in patients referred for aortic valve replacement (AVR), with particular regard to LVMR.
Methods and results Two hundred and sixty consecutive cases submitted to aortic valve replacement for valvular stenosis were prospectively followed for a mean of 28±9 months. Baseline, characteristics and extent of LVMR were tested for association with survival by uni- and multivariable analysis. Ten deaths occurred during hospital stay and 52 during out-of-hospital follow-up. Mean left ventricular mass decreased from 190±43 to 158±70 g/m2 (P<0.001). Older age, advanced functional class, hypertension, reduced left ventricle ejection fraction, and high pre-operative left ventricular mass index were associated with reduced survival. Overall the extent of LVMR did not influence the clinical results, while only early (<6 months) LVMR was weakly associated with mid-term outcome.
Conclusion Survival after aortic valve surgery is mainly determined by the pre-operative functional cardiac and systemic status. The extent of LVMR does not correlate with clinical outcome, whereas aggressive treatment of hypertension may improve post-operative survival.
Introduction
Aortic stenosis is rapidly becoming the most frequent indication for surgery on the aortic valve in the western world.1
Traditionally it has been considered that, in patients submitted to aortic valve replacement (AVR) for valvular stenosis, the post-operative regression of left ventricular hypertrophy (LVH) should correlate with patient outcome. However, to date this assumption has not received rigorous objective verification.
This study was intended to establish the impact of pre-operative patient characteristics on post-operative survival in patients submitted to AVR for isolated valvular stenosis, with particular attention to the potential correlation between post-operative LVH regression and clinical outcome and to the determinants of left ventricular mass reduction after surgery.
Patients and methods
From March 1998 all patients submitted to primary isolated elective AVR for valvular stenosis were prospectively enrolled. To December 2002, a total of 260 cases were included. All patients had undergone pre-operative transthoracic echocardiography, coronary angiography, and left ventricular angiography. The main pre-operative characteristics of the 260 patients are summarized in Table 1.
Surgical technique
All operations were performed by the same surgical team using a standard technique. Median sternotomy, normothermic cardiopulmonary bypass, and isothermic ante-retrograde blood cardioplegia were always used. The majority of patients (72%) received a bileaflet mechanical valve. In the remaining 28%, stented porcine bioprostheses were used. Fifty patients (19%) received a valvular prosthesis of ≤21 mm in diameter.
Follow-up procedure
All patients were re-evaluated at 1 and 6 months after surgery and every year thereafter. At each follow-up visit an electrocardiogram and transthoracic echocardiogram were performed. Medical therapy was reassessed and modified at each follow-up visit as needed. For hypertensive patients medical therapy was instituted and eventually implemented at each follow-up visit, in order to achieve a systemic blood pressure of less than 140/90 or 130/80 in the presence of compelling indications to anti-hypertensive therapy. ACE-inhibitors were prescribed at discharge and continued indefinitely in all hypertensive patients, in the absence of contraindications.
Echocardiography
All echocardiograms were performed by the same operator using the same equipment both pre-operatively and during follow-up. End-diastolic measurements of left ventricle internal dimension (LVID), interventricular septal thickness (IVST), and posterior wall thickness (PWT) were obtained according to the guidelines of the American Society of Echocardiography.2 The left ventricular mass index (LVMI) was calculated according to the formula described by Devereux and co-authors.3
All echocardiographic data were then reviewed by two blinded external reviewers; major discrepancies were resolved after common re-evaluation.
Data regarding the late follow-up were derived from the latest available echocardiogram.
Statistical analysis
Values are expressed as mean±standard deviation for continuous variables or as percentages for discrete variables.
In accordance with the published literature,4–7 baseline patient variables tested for association with survival were: age, sex, body surface area, New York Heart Association (NYHA) functional class, hypertension, diabetes, serum creatinine (mg/100 mL), left ventricular (LV) ejection fraction, LV end diastolic diameter (mm), mean pulmonary artery pressure (mmHg), LV mass index, native aortic valve gradient, year of operation, prosthetic valve type (mechanical vs. bioprosthesis), prosthetic valve size, post-operative aortic jet velocity, mean trans-prosthetic gradient, and effective prosthetic orifice area index.
All haemodynamic parameters and LV dimensions were determined at pre-operative transthoracic echocardiography.
Since upper limits for increased LVMIs adopted in the general population apply poorly to patients with surgical aortic valve stenosis8 we analysed all the pre-operative LVMI of our series and defined the cut-off for the definition of increased LVMI at the superior decile, according to a previously described method.8 Following this approach, pre-operative LVMI was considered increased if ≥240 g/m2 in men and ≥200 g/m2 in females.
Moreover, left ventricular mass regression (LVMR, defined as the difference in LVMI between the pre-operative echocardiogram and the last available exam) and early left ventricular mass regression (ELVMR, defined as the difference in LVMI between the pre-operative echocardiogram and the one performed at the 6 month visit) were tested for association with late survival. LVMR and ELVMR were studied in the multivariable analysis as continuous variables. In addition the same baseline patient variables tested for association with risk were also tested for association with LVMR and ELVMR.
For group comparisons, parametric (unpaired Student's t-test) and non-parametric tests (Kruskal–Wallis for unpaired data, when deviation for normal distribution of the data was assumed at the Kolmogorov–Smirnoff test) for continuous variables, and the χ2 test for nominal variables were adopted. Univariate analysis was employed for identification of explanatory variables (significance level: 0.20) to be included in the Cox proportional hazards regression analysis. Before inclusion, these were assessed for association (if showing parallel regression lines, i.e. co-linearity) and tested by a stepwise forward selective procedure, and significance was set at the 0.05 level. If two or more variables were associated with each other, only the variable showing the strongest relationship with the response variable was included and the others discarded. The assumptions of linearity were assessed and satisfied using a general linear model (GLM) univariate method. Given the relatively small size of the sample, the model was validated by a ‘jack-knife’ procedure. The proportional hazards assumptions were verified by formal checks (hypothesis test), and no adjustments to meet the assumptions were needed for any variable.
The Kaplan–Meier method for survival analysis was employed for generating a tabular summary of estimates (not shown) and the corresponding step-function survival and hazard curves. The paired Student's t-test and Wilcoxon signed-rank test were adopted for matched data analysis. Two-tailed tests were adopted for better accuracy. The day of surgery is considered as day 1 for survival analyses.
Statistical analysis was performed using MINITAB release 13 statistical software (MINITAB Inc, State College, PA, USA).
Major endpoints for risk analysis were post-operative death at any time point during the follow-up, LVMR, and ELVMR. Statistical significance was assumed for a P-value of <0.05.
Results
Clinical results
The main intra- and post-operative characteristics of the 260 patients are summarized in Table 2. There were 10 in-hospital deaths (3.8%); three patients (1.1%) had a fatal post-operative acute myocardial infarction, two (0.7%) died due to intractable ventricular arrhythmia, two (0.7%) due to multi-organ failure, two (0.7%) had fatal peri-operative stroke, and one (0.3%) died due to respiratory failure.
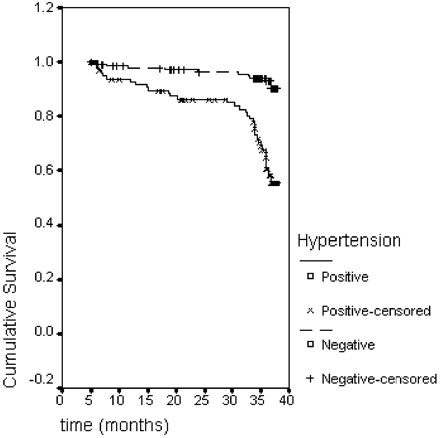
Mid-term follow-up in survivors was available in all cases (100%) and median follow-up time was 34 months (interquartile range: 26–45 months). During this period, 34 patients died from non-valve-related causes and 18 due to valve-related complications (heart failure in 10, stroke in four, sudden death in three, and myocardial infarction in one case). Figure 1, conversely, reports survival estimates as calculated for hypertensive vs. non-hypertensive patients. At 35 months, the former had a survival estimate of ∼60%, while the latter had an estimate of >95%.
The predictors of post-operative mortality at univariate and/or multivariable analysis are reported in Table 3. All variables that did not correlate with mortality at univariate analysis (such as sex, diabetes, body surface area, serum creatinine, LV end diastolic diameter, mean pulmonary artery pressure, native aortic valve gradient, year of operation, prosthetic valve type and size, post-operative aortic jet velocity, mean trans-prosthetic gradient, and effective prosthetic orifice area index) were not considered in the multivariable analysis.
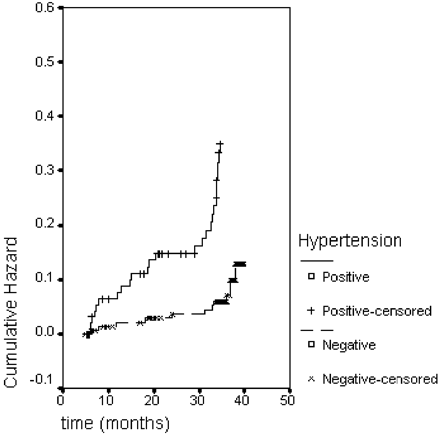
The factors associated with reduced survival were advanced age, higher NYHA class, presence of hypertension, low LV ejection fraction, and high pre-operative LVMI. Of note, the extent of LVMR did not influence the clinical outcome and ELVMR was only weakly associated with survival. Hypertensive patients had significantly worse survival than non-hypertensive cases, as shown in Figure 2. The importance of successful pressure control on survival is outlined by the fact that the proportion of death was significantly higher among those patients in whom medical anti-hypertensive therapy was unable to effectively control blood pressure values (11/78 controlled hypertension vs. 23/36 uncontrolled hypertension; P<0.001).
Echocardiographic data
Detailed results of the echocardiographic follow-up are given in Table 4.
There was a significant reduction of LVMI and LV dimensions during the follow-up period, with the most significant reduction occurring during the first 6 months after surgery.
Age, sex, NYHA class, diabetes, body surface area, serum creatinine, LV ejection fraction, LV end-diastolic diameter, mean pulmonary artery pressure, native aortic valve gradient, year of operation, prosthetic valve type and size, post-operative aortic jet velocity, mean trans-prosthetic gradient, and effective prosthetic orifice area index were not associated with LVMR and ELVMR at univariate analysis and were therefore not included in the multivariable analysis.
Only pre-operative LVMI and absence of hypertension were associated with ELVMR [P=0.02 at univariate analysis with RR (95% CI) of 3.73 (2.5–5.08) and P=0.04 in multivariable tests; and P=0.018 at univariate analysis with RR (95% CI) of 2.88 (1.0–6.51) and P=0.02 at multivariable analysis, respectively]. Both parameters were also associated with LVMR [P=0.013 at univariate analysis, with RR (95% CI) of 4.29 (2.12–7.56) and P=0.03 at multivariable tests and P=0.02 at univariate analysis with RR (95% CI) of 3.84 (2.67–5.39) and P=0.04 at multivariable tests respectively].
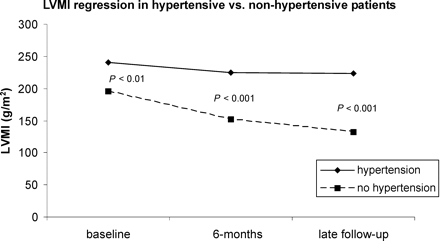
Hypertension appeared to have a particularly significant negative effect on LVMI reduction: hypertensive patients had higher pre-operative LVMI, showed only slight LVMR during the first 6 months after surgery and tended to return to pre-operative level, or even increase, at mid-term follow-up (see Figure 3). Hypertension also had a detrimental impact on survival (see Figure 1) and increased risk of death during the follow-up period (see Figure 2).
Discussion
The dogma: LVMR after aortic valve surgery influences post-operative survival
Traditionally, it has been considered that regression of LVH after AVR should correlate with patient outcome in terms of survival and quality of life benefits.9 On this basis, several technical alternatives and valvular substitutes aimed at maximizing ventricular mass regression have been proposed.10–13
However, this assumption is mainly based on the proven clinical benefits of LVMR among medical patients submitted to anti-hypertensive treatment.14,15 As the histological and structural basis of LVH due to chronic hypertension are known to be different from those of LVH due to aortic valve pathology,16,17 the value of applying the observations performed in hypertensive cases to surgical patients with aortic valve pathology appears to be at least questionable.
Of note, in the cardiac surgery literature there is no conclusive evidence of this supposed beneficial clinical effect of post-operative LVMI reduction. Although animal and clinical studies have suggested a detrimental effect of hypertrophy on LV performance, arrhythmia incidence, and susceptibility to ischaemia,18,19 the correlation between hypertrophy regression and clinical outcome has been not thoroughly investigated. In a review of the literature we identified only two studies which directly correlated the extent of LVMI regression after surgery with the post-operative outcome: a small retrospective study which reported some clinical benefits,20 and a large multi-centre prospective series which gave the opposite results.21 However, both investigations had several methodological flaws and cannot be regarded as conclusive in this regard.
Of note, very recently even some of the presumed pathophysiological benefits related to post-operative hypertrophy regression (such as the relief of ischaemia or improved diastolic function) have been questioned.22,23
Challenging the dogma
In this study we have attempted to determine the impact of LVMR after surgery on survival and the main finding is the lack of association between LVMR and mid-term survival after AVR.
In our population of 260 patients submitted to AVR for aortic stenosis, LVMI decreased significantly after surgery (from 190±43 to 158±70 g/m2: P<0.001). However, the amount of mass regression did not influence clinical results in the years after the operation; the only factors associated with post-operative outcome were those that reflect pre-operative cardiac and systemic functional status of the patients (age, NYHA class, hypertension, LV ejection fraction, and pre-operative LVMI).
Of note, LVMR, ELVMR, and survival were all strictly dependent on the severity of pre-operative hypertrophy.
We acknowledge the existence of possible bias due to missing echocardiographic data. In any case, given the limited number of patients which were lost at echocardiographic follow-up among survivors at each time interval (one at 6 months and three at 36 months), we believe that the deriving bias is negligible. Moreover, the adoption of the Kaplan–Meier method accounts for censored data in the survival analysis with reduction of bias. Thereafter, given the multiple testing nature of the present analysis, we acknowledge the risk of increase in type I error. Studies with larger patient cohorts should be performed in order to definitively confirm our findings. It seems then that there is a threshold of myocardial hypertrophy beyond which histological and functional normalization become impossible, even in the presence of echocardiographic evidence of regression. Before the achievement of this limit, echocardiographic LVMR reflects real hypertrophy reduction; once this value has been reached, LVMR is possible only in part and is probably mainly the resultant of reversal of hypertrophy in the less damaged cells and remodelling of the fibrous component of the left ventricle; the main pathological alterations of the myocardium and their ominous clinical effects remain in this case unmodified and LV mass reduction becomes a mere echocardiographic finding, with no clinical implications.
Anyhow, the importance of the pre-operative cardiac and systemic functional status of the patient in determining survival by far outweighs that of echocardiographic LVMR.
On the basis of these observations, the dogma on the prognostic value of post-operative hypertrophy reduction after AVR should probably be questioned and further extensive investigation on this subject appears to be necessary.
The role of hypertension
Based on our data, hypertension had a strong negative impact on LVMI reduction and survival after surgery. Hypertensive patients tended to have higher pre-operative LVMI, lower hypertrophy reduction, and worse clinical outcome in the years after surgery, especially when medical therapy was unable to achieve optimal blood pressure levels. This concept is also supported by the finding of a higher incidence of heart failure and bleeding as cause of death among hypertensive patients (Table 5). This strong influence of hypertension on the post-operative outcome of patients submitted to AVR has already been noted by others24 and it is interesting to note that in cases with uncontrolled hypertension LVMI increases in the years after the operation.
The observation that the main determinants of LVMI reduction after surgery are medical (hypertension, pre-operative LVMI), and that surgical variables (prosthesis type and size) are of limited importance, clearly outlines the importance of taking these factors into account in all the investigations on the effect of surgical treatments or devices on ventricular mass regression. It is tempting to speculate that failure to do this could have greatly contributed to the heterogeneity of the results reported in the studies on this subject.10–13,25,26
Conclusions and practical implications
In conclusion, in a large cohort of patients submitted to isolated AVR for pure aortic stenosis we found that age, NYHA class, hypertension, LV ejection fraction, and severity of pre-operative hypertrophy influence post-operative survival. The extent of post-operative LVMR does not affect clinical outcome. Mass regression is not influenced by surgical factors and appears to be mainly determined by the pre-operative LVMI and the presence or absence of hypertension. These observations underscore the important role of strict post-operative medical control of blood pressure to improve survival in patients submitted to AVR.
We acknowledge that potential limitations of the present work include the relatively low number of patients available at the end of the follow-up and the limited follow-up period. Studies with larger sample size and increased follow-up period are necessary to confirm our conclusions.
In addition, from a surgical perspective, our findings also open the question of the significance of surgical techniques or valvular substitutes intended to maximize LVMR at the expense of more complex operations, higher surgical risk, and increased in-hospital costs.
Finally, although speculative, it is possible that earlier intervention (before the achievement of the critical irreversible ventricular hypertrophy threshold and taking into account the functional cardiac and systemic conditions) may improve post-operative survival in patients undergoing surgery for aortic stenosis.
Figure 1 Kaplan–Meier curve showing survival at 38 months for hypertensive vs. non-hypertensive patients.
Figure 2 Hazard function for hypertensive vs. non-hypertensive patients.
Figure 3 Wilcoxon signed-rank test was employed for evaluation of significance.
Baseline patient characteristics
| Age (years)a | 66.3±18.2 |
| Maleb | 186 (71.5%) |
| NYHA classb | |
| I-II | 117 (45%) |
| III | 91 (35%) |
| IV | 52 (20%) |
| Hypertensionb | 120 (46.2%) |
| Diabetesb | 29 (11.1%) |
| Chronic obstructive pulmonary diseaseb | 21 (8%) |
| Renal insufficiencyb | 12 (4.6%) |
| Peripheral vascular diseaseb | 29 (11.1%) |
| Smokingb | 50 (19.2%) |
| LV ejection fractiona | 0.55±0.21 |
| LVMI (g/m2)a | 190±43 |
| Age (years)a | 66.3±18.2 |
| Maleb | 186 (71.5%) |
| NYHA classb | |
| I-II | 117 (45%) |
| III | 91 (35%) |
| IV | 52 (20%) |
| Hypertensionb | 120 (46.2%) |
| Diabetesb | 29 (11.1%) |
| Chronic obstructive pulmonary diseaseb | 21 (8%) |
| Renal insufficiencyb | 12 (4.6%) |
| Peripheral vascular diseaseb | 29 (11.1%) |
| Smokingb | 50 (19.2%) |
| LV ejection fractiona | 0.55±0.21 |
| LVMI (g/m2)a | 190±43 |
aMean±SD.
bn(%). LVMI, left ventricular mass index; NYHA, New York Heart Association.
Baseline patient characteristics
| Age (years)a | 66.3±18.2 |
| Maleb | 186 (71.5%) |
| NYHA classb | |
| I-II | 117 (45%) |
| III | 91 (35%) |
| IV | 52 (20%) |
| Hypertensionb | 120 (46.2%) |
| Diabetesb | 29 (11.1%) |
| Chronic obstructive pulmonary diseaseb | 21 (8%) |
| Renal insufficiencyb | 12 (4.6%) |
| Peripheral vascular diseaseb | 29 (11.1%) |
| Smokingb | 50 (19.2%) |
| LV ejection fractiona | 0.55±0.21 |
| LVMI (g/m2)a | 190±43 |
| Age (years)a | 66.3±18.2 |
| Maleb | 186 (71.5%) |
| NYHA classb | |
| I-II | 117 (45%) |
| III | 91 (35%) |
| IV | 52 (20%) |
| Hypertensionb | 120 (46.2%) |
| Diabetesb | 29 (11.1%) |
| Chronic obstructive pulmonary diseaseb | 21 (8%) |
| Renal insufficiencyb | 12 (4.6%) |
| Peripheral vascular diseaseb | 29 (11.1%) |
| Smokingb | 50 (19.2%) |
| LV ejection fractiona | 0.55±0.21 |
| LVMI (g/m2)a | 190±43 |
aMean±SD.
bn(%). LVMI, left ventricular mass index; NYHA, New York Heart Association.
In-hospital data
| Event . | Number and percentage of patients or mean±SD . |
|---|---|
| Deatha | 10 (3.8) |
| Cause of deatha | |
| Myocardial infarction | 3 (1.1) |
| Arrhythmia | 2 (0.7) |
| Multiorgan failure | 2 (0.7) |
| Stroke | 2 (0.7) |
| Respiratory failure | 1 (0.3) |
| Post-operative complicationsa | |
| Myocardial infarction | 5 (1.9) |
| Arrhythmia | 4 (1.4) |
| Need for IABP | 4 (1.4) |
| Stroke | 5 (1.9) |
| Renal failure | 6 (2.3) |
| Revision for bleeding | 5 (1.9) |
| Mediastinitis | 2 (0.7) |
| Mean ICU stay (h)b | 38.3±6.1 |
| Mean post-operative in-hospital stay (days)b | 5.1±1.2 |
| Event . | Number and percentage of patients or mean±SD . |
|---|---|
| Deatha | 10 (3.8) |
| Cause of deatha | |
| Myocardial infarction | 3 (1.1) |
| Arrhythmia | 2 (0.7) |
| Multiorgan failure | 2 (0.7) |
| Stroke | 2 (0.7) |
| Respiratory failure | 1 (0.3) |
| Post-operative complicationsa | |
| Myocardial infarction | 5 (1.9) |
| Arrhythmia | 4 (1.4) |
| Need for IABP | 4 (1.4) |
| Stroke | 5 (1.9) |
| Renal failure | 6 (2.3) |
| Revision for bleeding | 5 (1.9) |
| Mediastinitis | 2 (0.7) |
| Mean ICU stay (h)b | 38.3±6.1 |
| Mean post-operative in-hospital stay (days)b | 5.1±1.2 |
an (%).
bMean±SD. IABP, intra-aortic balloon pumping; ICU, intensive care unit.
In-hospital data
| Event . | Number and percentage of patients or mean±SD . |
|---|---|
| Deatha | 10 (3.8) |
| Cause of deatha | |
| Myocardial infarction | 3 (1.1) |
| Arrhythmia | 2 (0.7) |
| Multiorgan failure | 2 (0.7) |
| Stroke | 2 (0.7) |
| Respiratory failure | 1 (0.3) |
| Post-operative complicationsa | |
| Myocardial infarction | 5 (1.9) |
| Arrhythmia | 4 (1.4) |
| Need for IABP | 4 (1.4) |
| Stroke | 5 (1.9) |
| Renal failure | 6 (2.3) |
| Revision for bleeding | 5 (1.9) |
| Mediastinitis | 2 (0.7) |
| Mean ICU stay (h)b | 38.3±6.1 |
| Mean post-operative in-hospital stay (days)b | 5.1±1.2 |
| Event . | Number and percentage of patients or mean±SD . |
|---|---|
| Deatha | 10 (3.8) |
| Cause of deatha | |
| Myocardial infarction | 3 (1.1) |
| Arrhythmia | 2 (0.7) |
| Multiorgan failure | 2 (0.7) |
| Stroke | 2 (0.7) |
| Respiratory failure | 1 (0.3) |
| Post-operative complicationsa | |
| Myocardial infarction | 5 (1.9) |
| Arrhythmia | 4 (1.4) |
| Need for IABP | 4 (1.4) |
| Stroke | 5 (1.9) |
| Renal failure | 6 (2.3) |
| Revision for bleeding | 5 (1.9) |
| Mediastinitis | 2 (0.7) |
| Mean ICU stay (h)b | 38.3±6.1 |
| Mean post-operative in-hospital stay (days)b | 5.1±1.2 |
an (%).
bMean±SD. IABP, intra-aortic balloon pumping; ICU, intensive care unit.
Predictors of mortality at univariate and multivariable analysis
| . | P (univariate) . | Coefficient (β) . | RR (95% CI) . | P (Cox proportional hazards regression) . |
|---|---|---|---|---|
| Age | <0.001 | 3.045 | 2.86 (1.73–4.73) | 0.002 |
| NYHA class III-IV (n=143; 55%) | <0.001 | 4.529 | 6.11 (3.51–10.62) | <0.001 |
| Hypertension (n=120; 46.2%) | 0.002 | 2.005 | 2.25 (1.32–3.85) | 0.04 |
| LV ejection fraction | <0.001 | 3.71 | 4.29 (2.5–7.15) | <0.001 |
| Pre-operative LVMI | ||||
| Males | 0.002 | 1.5 | 2.2 (1.41–5.09) | 0.018 |
| Females | 0.004 | 1.2 | 1.5 (0.70–7.0) | 0.035 |
| ELVMR | 0.002 | 1.1 | 1.0 (0.28–1.25) | 0.047 |
| LVMR | 0.049 | −0.86 | 1.0 (0.40–1.22) | 0.068 |
| . | P (univariate) . | Coefficient (β) . | RR (95% CI) . | P (Cox proportional hazards regression) . |
|---|---|---|---|---|
| Age | <0.001 | 3.045 | 2.86 (1.73–4.73) | 0.002 |
| NYHA class III-IV (n=143; 55%) | <0.001 | 4.529 | 6.11 (3.51–10.62) | <0.001 |
| Hypertension (n=120; 46.2%) | 0.002 | 2.005 | 2.25 (1.32–3.85) | 0.04 |
| LV ejection fraction | <0.001 | 3.71 | 4.29 (2.5–7.15) | <0.001 |
| Pre-operative LVMI | ||||
| Males | 0.002 | 1.5 | 2.2 (1.41–5.09) | 0.018 |
| Females | 0.004 | 1.2 | 1.5 (0.70–7.0) | 0.035 |
| ELVMR | 0.002 | 1.1 | 1.0 (0.28–1.25) | 0.047 |
| LVMR | 0.049 | −0.86 | 1.0 (0.40–1.22) | 0.068 |
RR and CI relate to the Cox regression analysis. The last column includes the results of the multivariable analysis.
Predictors of mortality at univariate and multivariable analysis
| . | P (univariate) . | Coefficient (β) . | RR (95% CI) . | P (Cox proportional hazards regression) . |
|---|---|---|---|---|
| Age | <0.001 | 3.045 | 2.86 (1.73–4.73) | 0.002 |
| NYHA class III-IV (n=143; 55%) | <0.001 | 4.529 | 6.11 (3.51–10.62) | <0.001 |
| Hypertension (n=120; 46.2%) | 0.002 | 2.005 | 2.25 (1.32–3.85) | 0.04 |
| LV ejection fraction | <0.001 | 3.71 | 4.29 (2.5–7.15) | <0.001 |
| Pre-operative LVMI | ||||
| Males | 0.002 | 1.5 | 2.2 (1.41–5.09) | 0.018 |
| Females | 0.004 | 1.2 | 1.5 (0.70–7.0) | 0.035 |
| ELVMR | 0.002 | 1.1 | 1.0 (0.28–1.25) | 0.047 |
| LVMR | 0.049 | −0.86 | 1.0 (0.40–1.22) | 0.068 |
| . | P (univariate) . | Coefficient (β) . | RR (95% CI) . | P (Cox proportional hazards regression) . |
|---|---|---|---|---|
| Age | <0.001 | 3.045 | 2.86 (1.73–4.73) | 0.002 |
| NYHA class III-IV (n=143; 55%) | <0.001 | 4.529 | 6.11 (3.51–10.62) | <0.001 |
| Hypertension (n=120; 46.2%) | 0.002 | 2.005 | 2.25 (1.32–3.85) | 0.04 |
| LV ejection fraction | <0.001 | 3.71 | 4.29 (2.5–7.15) | <0.001 |
| Pre-operative LVMI | ||||
| Males | 0.002 | 1.5 | 2.2 (1.41–5.09) | 0.018 |
| Females | 0.004 | 1.2 | 1.5 (0.70–7.0) | 0.035 |
| ELVMR | 0.002 | 1.1 | 1.0 (0.28–1.25) | 0.047 |
| LVMR | 0.049 | −0.86 | 1.0 (0.40–1.22) | 0.068 |
RR and CI relate to the Cox regression analysis. The last column includes the results of the multivariable analysis.
Echocardiographic data
| . | LVMI (g/m2) . | LVEDDI (mm/m2) . | LVESDI (mm/m2) . | EF . | IVST (mm) . | PWT (mm) . |
|---|---|---|---|---|---|---|
| Pre-operative (n=260) | 190±43 | 29±3 | 19±4 | 0.55±0.21 | 16±2 | 13±1 |
| Six month follow-up (n=248) | 162±69* | 27±2* | 18±3* | 0.54±0.18 | 14±2* | 10±2* |
| Late follow-up (n=205) | 158±70* | 26±2*,** | 17±2*,** | 0.57±0.16 | 14 ±1* | 10±1* |
| . | LVMI (g/m2) . | LVEDDI (mm/m2) . | LVESDI (mm/m2) . | EF . | IVST (mm) . | PWT (mm) . |
|---|---|---|---|---|---|---|
| Pre-operative (n=260) | 190±43 | 29±3 | 19±4 | 0.55±0.21 | 16±2 | 13±1 |
| Six month follow-up (n=248) | 162±69* | 27±2* | 18±3* | 0.54±0.18 | 14±2* | 10±2* |
| Late follow-up (n=205) | 158±70* | 26±2*,** | 17±2*,** | 0.57±0.16 | 14 ±1* | 10±1* |
*P≤0.05 compared to baseline.
**P≤0.05 compared to 6 months follow-up.
Data regarding the late follow-up were derived from the latest available echocardiogram; ‘Late follow-up’ means >36 months. EF, left ventricular ejection fraction; IVST, interventricular septal thickness; LVEDDI, left ventricular end-diastolic diameter index; LVESDI, left ventricular end-systolic diameter index.
Echocardiographic data
| . | LVMI (g/m2) . | LVEDDI (mm/m2) . | LVESDI (mm/m2) . | EF . | IVST (mm) . | PWT (mm) . |
|---|---|---|---|---|---|---|
| Pre-operative (n=260) | 190±43 | 29±3 | 19±4 | 0.55±0.21 | 16±2 | 13±1 |
| Six month follow-up (n=248) | 162±69* | 27±2* | 18±3* | 0.54±0.18 | 14±2* | 10±2* |
| Late follow-up (n=205) | 158±70* | 26±2*,** | 17±2*,** | 0.57±0.16 | 14 ±1* | 10±1* |
| . | LVMI (g/m2) . | LVEDDI (mm/m2) . | LVESDI (mm/m2) . | EF . | IVST (mm) . | PWT (mm) . |
|---|---|---|---|---|---|---|
| Pre-operative (n=260) | 190±43 | 29±3 | 19±4 | 0.55±0.21 | 16±2 | 13±1 |
| Six month follow-up (n=248) | 162±69* | 27±2* | 18±3* | 0.54±0.18 | 14±2* | 10±2* |
| Late follow-up (n=205) | 158±70* | 26±2*,** | 17±2*,** | 0.57±0.16 | 14 ±1* | 10±1* |
*P≤0.05 compared to baseline.
**P≤0.05 compared to 6 months follow-up.
Data regarding the late follow-up were derived from the latest available echocardiogram; ‘Late follow-up’ means >36 months. EF, left ventricular ejection fraction; IVST, interventricular septal thickness; LVEDDI, left ventricular end-diastolic diameter index; LVESDI, left ventricular end-systolic diameter index.
Cause of post-operative death in hypertensive and non-hypertensive patients at the end of follow-up
| Cause of death . | Hypertensive . | Non-hypertensive . | P- value . |
|---|---|---|---|
| Valve-related | |||
| Myocardial infarction | 1 | 0 | 0.98 |
| Stroke | 3 | 1 | 0.46 |
| Sudden death or malignant arrhythmia | 2 | 1 | 0.78 |
| Heart failure | 9 | 1 | 0.031 |
| Non-valve-related | |||
| Multiorgan failure | 8 | 9 | 0.27 |
| Respiratory failure | 7 | 4 | 0.65 |
| Untractable bleeding | 5 | 0 | 0.046 |
| Pancreatitis | 1 | 0 | 0.38 |
| Cause of death . | Hypertensive . | Non-hypertensive . | P- value . |
|---|---|---|---|
| Valve-related | |||
| Myocardial infarction | 1 | 0 | 0.98 |
| Stroke | 3 | 1 | 0.46 |
| Sudden death or malignant arrhythmia | 2 | 1 | 0.78 |
| Heart failure | 9 | 1 | 0.031 |
| Non-valve-related | |||
| Multiorgan failure | 8 | 9 | 0.27 |
| Respiratory failure | 7 | 4 | 0.65 |
| Untractable bleeding | 5 | 0 | 0.046 |
| Pancreatitis | 1 | 0 | 0.38 |
Cause of post-operative death in hypertensive and non-hypertensive patients at the end of follow-up
| Cause of death . | Hypertensive . | Non-hypertensive . | P- value . |
|---|---|---|---|
| Valve-related | |||
| Myocardial infarction | 1 | 0 | 0.98 |
| Stroke | 3 | 1 | 0.46 |
| Sudden death or malignant arrhythmia | 2 | 1 | 0.78 |
| Heart failure | 9 | 1 | 0.031 |
| Non-valve-related | |||
| Multiorgan failure | 8 | 9 | 0.27 |
| Respiratory failure | 7 | 4 | 0.65 |
| Untractable bleeding | 5 | 0 | 0.046 |
| Pancreatitis | 1 | 0 | 0.38 |
| Cause of death . | Hypertensive . | Non-hypertensive . | P- value . |
|---|---|---|---|
| Valve-related | |||
| Myocardial infarction | 1 | 0 | 0.98 |
| Stroke | 3 | 1 | 0.46 |
| Sudden death or malignant arrhythmia | 2 | 1 | 0.78 |
| Heart failure | 9 | 1 | 0.031 |
| Non-valve-related | |||
| Multiorgan failure | 8 | 9 | 0.27 |
| Respiratory failure | 7 | 4 | 0.65 |
| Untractable bleeding | 5 | 0 | 0.046 |
| Pancreatitis | 1 | 0 | 0.38 |
Lund O, Chandrasekaran V, Grocott-Mason R et al. Primary aortic valve replacement with allografts over twenty-five years: valve-related and procedure-related determinants of outcome.
Sahn DJ, Demaria A, Kisslo J et al. Recommendation regarding quantitation in M-mode echocardiography: results of survey of echocardiographic measurements.
Devereux RB, Alonso DR, Lutas EM et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings.
Lytle BW, Cosgrove DM, Taylor PC et al. Primary isolated aortic valve replacement. Early and late results.
Lund O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention.
Lund O, Pilegaard H, Nielsen TT et al. Thirty-day mortality after valve replacement for aortic stenosis over the last 22 years. A multivariate risk stratification.
Mehta RH, Bruckman D, Das S et al. Implication of increased left ventricular mass index on in-hospital outcomes in patients undergoing aortic valve surgery.
Garcia Fuster R, Montero Arguto JA, Gil Albarova O et al. Left ventricular mass index in aortic valve surgery: a new index for early valve replacement?
Panidis IP, Kotler MN, Ren JF et al. Development and regression of left ventricular hypertrophy.
Jin XY, Zhang ZM, Gibson DG et al. Effects of valve substitute on changes in left ventricular function and hypertrophy after aortic valve replacement.
De Paulis R, Sommariva L, Colagrande L et al. Regression of left ventricular hypertrophy after aortic valve replacement for aortic stenosis with different valve substitutes.
Bech-Hanssen O, Caidahl K, Wall B et al. Influence of aortic valve replacement, prosthesis type, and size on functional outcome and ventricular mass in patients with aortic stenosis.
Walther T, Falk V, Langebartels G et al. Prospectively randomized evaluation of stentless versus conventional biological aortic valves. Impact on early regression of left ventricular hypertrophy.
Levy D, Garrison RJ, Savage DD et al. Prognostic implication of echocardiographically determined left ventricular mass in the Framingham heart study.
Koren MJ, Devereux RB, Casale PN et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncompleted essential hypertension.
Krayenbuehl HP, Hess OM, Monrad ES et al. Left ventricular myocardial structure in aortic valve disease early, intermediate, and late after aortic valve replacement.
Villari B, Chiriello M. Interstitial changes and ventricular hypertrophy in man.
Messerli FH, Soria F. Ventricular dysrhythmias, left ventricular hypertrophy, and sudden death.
Hamasaki S, Al Suwaidi J, Higano ST et al. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy.
Lessick J, Mutlak D, Markiewicz W et al. Failure of left ventricular hypertrophy to regress after surgery for aortic valve stenosis.
Eichinger WB, Botzenhardt F, Gunzinger R et al. Left ventricluar mass regression after aortic valve replacement with the mosaic bioprosthesis.
Gonzales-Juanatey JR, Vega FM, Gude F et al. Influence of prosthesis size and left ventricular mass on left ventricular diastolic reserve in patients with aortic valve prostheses.
Rajappan K, Rimoldi OE, Camici PG et al. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis.
Lund O, Emmertsen K, Dorup I et al. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile.
Blackstone EH, Cosgrove DM, Jamieson WRE et al. Prosthesis size and long-term survival after aortic valve replacement.
De Carlo M, Milano AD, Nardi C et al. Serial doppler echocardiographic evaluation of small-sized Sorin Bicarbon prostheses.