-
PDF
- Split View
-
Views
-
Cite
Cite
N. Sharma, S. Srivastava, F. Kern, W. Xian, K. G. Yeoh, T. Ming, F. McKeon, K. Y. Ho, CEACAM 6, a novel marker for the diagnosis of Barrett's esophagus, Diseases of the Esophagus, Volume 30, Issue 9, September 2017, Pages 1–5, https://doi.org/10.1093/dote/dox026
Close - Share Icon Share
Summary
Barrett's esophagus (BE) is a premalignant condition associated with the development of esophageal adenocarcinoma (EAC). Despite the low risk of progression to EAC, evidence highlights the notably poor survival rates of this malignancy. The mainstay form of diagnosis of BE is endoscopy and biopsy sampling. However, research emphasizes limitations with regards to the histological detection of BE and associated dysplasia. The aim of this study is to evaluate the clinical significance of CEACAM6 as a potential biomarker for the diagnosis of BE and beyond. Retrospective tissue samples were obtained from columnar lined esophagus without goblet cells (n = 27), BE (n = 18), BE associated dysplasia (n = 16), and EAC (n = 24). Standardized immunohistochemistry for CEACAM6 was performed followed by quantitative staining analysis. Statistical analysis across the BE spectrum for CEACAM6 was undertaken and a P value <0.05 was considered significant. CEACAM6 expression increased from columnar lined epithelium (CLE) to BE with a subsequent decrease to dysplasia and adenocarcinoma. The expression of CEACAM6 was significant from CLE to BE at p 0.001, CLE to dysplasia at p 0.001, BE to dysplasia at p 0.006, CLE to adenocarcinoma at p 0.001 and BE to adenocarcinoma at p 0.001. There was no significant difference in expression between dysplasia and adenocarcinoma (P = 0.15). Our findings highlight the increasing expression of CEACAM6 from CLE to BE with a subsequent decrease to dysplasia and adenocarcinoma. In view of this, we advocate the utilization of this marker for the enhanced diagnosis of BE and for the distinction of BE and dysplasia.
INTRODUCTION
Barrett's esophagus (BE) is a premalignant condition associated with the development of esophageal adenocarcinoma (EAC). It arises following long standing gastroesophageal reflux with histological transformation of the distal esophagus, from stratified squamous epithelium to one that is columnar lined in nature. The US definition adds a slight nuance with the requirement for columnar lined epithelium (CLE) in addition to goblet cells, namely intestinal metaplasia (IM) in view of the increased malignant risk.1 Currently, the risk of progression to EAC is decreasing, estimated at 0.12%.2 Having said this however, patients with this form of malignancy are associated with poor survival rates estimated at 10%–15%. Hence it is imperative, that patients with BE are diagnosed accordingly and followed up as needed.
Currently, the gold standard method of diagnosis is white light endoscopy and biopsy sampling in a quadrantic approach every 1–2 cm3. From a histological standpoint, evidence highlights concerns regarding the inter observer disagreement for Barrett's dysplasia.3 This is primarily due to long standing gastroesophageal reflux, which can lead to regenerative changes hence overlapping the morphological features of dysplasia. For the diagnosis of IM, alcian blue is sometimes utilized. However, this approach leads to the staining of pseudogoblet cells, which are not classically true goblet cells leading to the occurrence of false positives.4 Furthermore, the literature highlights the fact that general pathologists can also interpret the presence of CLE with varying degrees of accuracy.5 As medicine is becoming more specialized, such a condition often relies on expert endoscopists and gastrointestinal (GI) pathologists to make a diagnosis. However, this resource is limited and typically only available at specialist centers, which are not easily accessible by all patients. As a result, there is a gap in the field to ensure uniform standards are reached in the pathological diagnosis of BE and beyond. This has been highlighted by both US and UK societies, which suggest the need to develop markers for the histological assessment of Barrett's associated dysplasia.6
CEACAM 6 (carcinoembryonic antigen related cell adhesion molecule 6) is a gene located on chromosome 19 encoding a protein that is part of the CEA family whose members are GPI anchored cell surface glycoproteins responsible for cellular adhesion.7 The gene is known to affect the sensitivity of tumor cells to the adenovirus with the protein acting as a receptor for Escherichia coli adhesion to ileal cells in patients with Crohn's disease.7 CEACAM expression has been noted to increase from a gastric perspective from normal gastric mucosa, to atrophic gastritis, intestinal metaplasia, dysplasia and cancer.8 Recently, CEACAM6 expression has been examined in cloned Barrett's stem cells in comparison with patient-matched gastric epithelial stem cells.9 In this study, we aimed to examine the expression pattern of this marker gene in Barrett's esophagus, associated dysplasia and cancer with the goal to determine its value as a diagnostic biomarker.
MATERIAL AND METHODS
Case selection
Retrospective analysis of formalin fixed paraffin embedded (FFPE) distal esophageal biopsies from patients who had presented for an upper GI endoscopy from the year 2004 to 2015 (n = 85) at the National University Hospital, Singapore was performed. We selected two groups of patients based on their endoscopic findings:
Group 1: 45 patients who had at least one biopsy sample from endoscopically visible salmon colored columnar epithelia above the gastroesophageal junction (defined as the proximal ends of the gastric folds). Histologically, 18 patients were diagnosed as BE (with goblet cells) and 27 were diagnosed as non-goblet CLE.
Group 2: 40 patients who had an endoscopically visible lesion (mucosal irregularity or nodular lesion) above the gastroesophageal junction, indicative of low to high grade dysplasia and/or adenocarcinoma (n = 16, LGD/HGD; n = 24, EAC) within the distal esophagus.
In the group of non-goblet CLE, each case showed salmon colored discoloration of the esophageal mucosa above the gastroesophageal junction, by endoscopy. A hiatus hernia was absent and the length of columnar metaplasia varied from 2 to 5 cm. In the BE group, endoscopically, all the patients showed tongues of salmon colored columnar mucosa above the gastroesophageal junction and the length of BE ranged from 3 to 6 cm. Clinicopathological information was obtained from medical records, which included age, gender, ethnicity, and endoscopic operator findings. Follow-up history of the patients was obtained wherever possible. Ethical approval for this study was obtained from the Domain Specific Review Board, National Healthcare Group.
Histopathology and immunohistochemistry
Consecutive sections of 4 microns were cut from each FFPE tissue block. One section was stained with hematoxylin and eosin (H&E) using standard techniques, to confirm the histological diagnosis. Further, unstained sections were placed on coated slides for immunohistochemical analysis.
On light microscopy, CLE was identified as columnar epithelium without goblet cells from the distal esophagus (n = 27). Barrett's esophagus was diagnosed as distal esophageal columnar epithelium with goblet cells (n = 18). Dysplasia was diagnosed by the updated Vienna classification (n = 16) and the histomorphological diagnosis of EAC (n = 24) was based on the WHO classification of tumors.10,11
Immunohistochemistry was performed using the commercially available antibody Anti CEACAM6 (Abcam, Cambridge, UK). We also undertook a comparative analysis with p53 (DAKO, USA) in view of its currently recognized potential in a diagnosis of dysplasia. Briefly, slides were deparaffinized in Histoclear followed by rehydration in ethanol. Pressure antigen retrieval was performed in citrate buffer pH 6.0 (Dako, Denmark). Next, slides were stained using a standard automated immunohistochemistry protocol (Bond-Max, Leica Biosystems, USA) and detected using a polymer HRP detection system (Bond Polymer Refine Detection, Leica Biosystems, USA). Visualization of CEACAM6 was undertaken by DAB. Slides were incubated for 15 minutes with hematoxylin to serve as a counterstain.
Immunoscoring
All slides were evaluated by the use of a standard light microscope. Immunohistochemical staining was assessed by a trained pathologist (SS) and confirmed by a second pathologist (MT). Interobserver concordance was set at 90% and wherever there was disagreement between the observers, an average score was taken. Immunoreactivity was assessed evaluating the percentage of immunopositive cells versus the total number of epithelial cells from the area of interest and grouped as, 0% (0),1–10% (1), 11–50% (2), 51–80% (3), and 81–100% (4). Grades 0 and 1 were considered negative. Intensity of the marker was also scored on a four-point scale (negative, weak, moderate, and strong). The immunoreactivity score (IRS) was recorded and calculated as the percentage of positive cells times the intensity of the marker and the final scores ranged from 0 to 12.12 While scores 0–3 were considered negative, scores 4–12 were considered positive.
Statistical analysis
Statistical analysis was performed using IBM SPSS v 20.0 for Windows (IBM, USA). To evaluate the significance between paired/unpaired groups of cases for different types of lesions, the Kruskal–Wallis test was used. To analyze the difference between two groups, the Mann–Whitney test was applied. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Overall there were 24 females and 61 males in this cohort and the median ages were 60, 58.6, 70.6, and 67.2 years in the CLE, BE, dysplasia, and EAC groups, respectively. There were no significant differences between the four groups regarding age and gender. In the dysplasia group, there were 11 patients with high grade dysplasia (68.7%) and 5 with low grade dysplasia (31.3%). In the adenocarcinoma group, 8.3% were well differentiated, 37.5% were moderately differentiated, and 54.2% were poorly differentiated types of adenocarcinoma. All the EAC patients were followed up and 18 patients were alive at the time of the study and five patients had died because of EAC complications while in 1 there was no follow up information available.
Immunohistochemical results
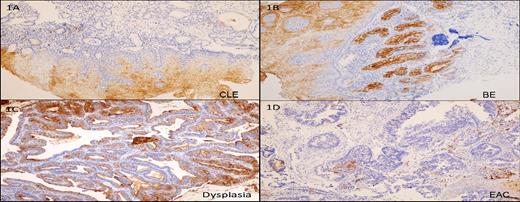
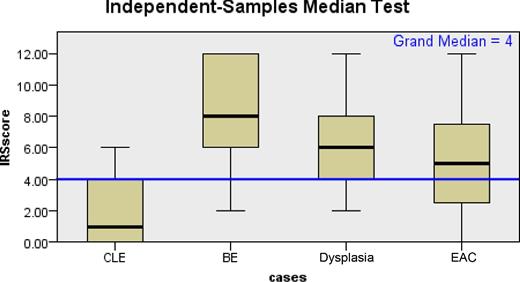
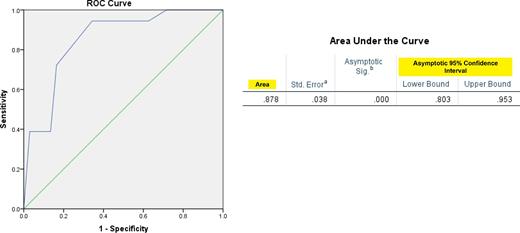
CEACAM6 expression was observed predominantly in the cytoplasm of the columnar cells (Fig. 1a). It was also observed as membranous expression, which was accentuated on the luminal side of the epithelium. 33.3% of cases in the CLE group showed mild to moderate expression (median H score 10.0, range: 0–120, IRS range: 0–6). While 100% of BE (Fig. 1b) (median H score 200, range: 50–300, IRS range: 2–12) and 93.7% of dysplasia cases (Fig. 1c) (median H score 140, range: 20–300, IRS range: 2–12) showed moderate to strong expression, 66.6% of adenocarcinoma cases (Fig. 1d) showed positive expression ranging from mild to moderate to strong (median H score 90, range: 0–300 and IRS range: 0–12). The overall difference in the mean H-score between the four groups was statistically significant. The AUC for CEACAM6 in BE was 0.88 (Fig. 3). Within the different groups, CEACAM6 expression in the CLE group was significantly lower than the BE, dysplasia, and adenocarcinoma groups (P < 0.001) and the expression in the BE group was significantly higher than the dysplasia and adenocarcinoma groups (P < 0.05) (Fig. 2). However, no significant difference was observed between the dysplasia and adenocarcinoma groups (P = 0.15). Within the EAC group, CEACAM6 expression was higher in poorly differentiated carcinomas as compared to well and moderately differentiated carcinomas and this proved significant (68.8% vs. 31.3%; P = 0.04). All the five patients who had died because of EAC complications showed a positive IRS score and it was mildly significant (P = 0.06). The superficial squamous epithelium served as the internal positive control and was consistently CEACAM6 positive.
The immunohistochemical expression of CEACAM6. 1 (A), Focal positive expression in CLE. 1 (B); Strong membranous and cytoplasmic expression in BE. 1 (C) Strong membranous and cytoplasmic expression in dysplasia. 1 (D) Negative expression in adenocarcinoma.
Box plot showing median distribution of CEACAM6 in the CLE, BE, dysplasia, and adenocarcinoma groups.
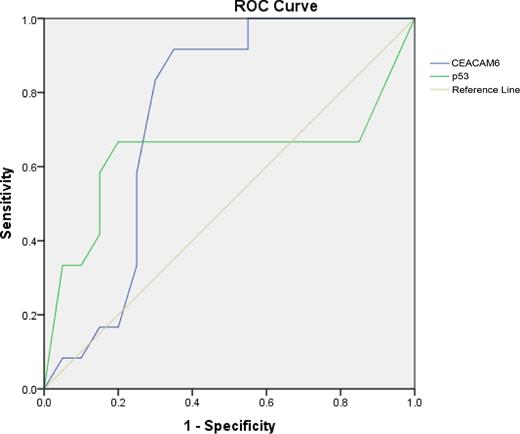
p53 was observed as a mild nuclear expression in 44.4% of CLE cases, a strong nuclear expression in 66.6% of BE and dysplasia cases and in 87.5% of EAC cases. On plotting a receiver operating characteristic curve (ROC) for the dysplasia group, we noted an area under the curve (AUC) for CEACAM 6 of 0.746 and for p53 of 0.633 and the difference was significant (P = 0.022) (Fig. 4).
DISCUSSION
Our study analyzed the expression of CEACAM6 in BE patients and associated malignancy within an Asian setting. In view of the increasing incidence of EAC globally, astute diagnosis of the premalignant phase is essential in order to capture at risk patients. Endoscopy and biopsy sampling is the current gold standard. Yet problems exist with regards to histological assessment, including interobserver disagreement for the condition in particular for associated dysplasia. Furthermore, the utilization of alcian blue for the diagnosis of intestinal metaplasia is associated with the detection of pseudogoblet cells and hence false positive results. In this regard, there has been a push for the need for adjunct biomarkers to aid in the diagnosis of BE and beyond in order to better enhance pathological accuracy.
In our study, we observed an increasing trend in the expression of CEACAM6 from CLE to BE. The expression then subsequently decreased from BE to dysplasia and adenocarcinoma. This has two implications from a clinical perspective. First, it will allow pathologists to distinguish more accurately the presence of intestinal metaplasia and hence ensure concerns in relation to alcian blue use are minimized. Second, the decreasing expression between BE and dysplasia will allow for a more accurate distinction between these two tissue types and hence better agreement on what is truly dysplasia. This is particularly useful in view of the fact that long standing GERD can lead to regenerative changes and mask the dysplastic presence. Furthermore, there is strong evidence to suggest that early dysplasia is typically downgraded to nondysplastic BE following re- review by expert pathologists. This adds further weight to the utilization of CEACAM 6.
CEACAM6 expression has recently been studied in gastric, colorectal, breast, and ovarian cancer.13,–16 Overexpression of CEACAM6 is associated with poor prognosis in these carcinomas. In our study, we observed that CEACAM6 expression decreased from BE to dysplasia to EAC. However, within the EAC group, poorly differentiated carcinomas were associated with a positive IRS score and were associated with poor prognosis. Zhou et al. investigated the expression of CEACAM5 in nongastric cancer and gastric cancer tissue.8 Their findings highlighted an increasing progression from normal gastric mucosa to atrophic gastritis, intestinal metaplasia, dysplasia, and cancer (P < 0.05). We therefore hypothesized that in view of the existence of intestinal metaplasia in Barrett's esophagus, the CEACAM family would also be favorably expressed. Furthermore, the comparison of CEACAM 6 as a dysplastic marker particularly in reference to p53 proves promising and further work is needed for validation.
We recognize the limited sample size in an Asian setting. From an endoscopic perspective, the membranous expression of CEACAM6 warrants further investigation. Autofluorescence imaging is an endoscopic modality aimed at enhancing lesion detection. Yet work thus far has been disappointing in terms of wide spread adoption, primarily due to its limited sensitivities. The utilization of cell surface markers for enhanced endoscopic imaging is aimed at overcoming this. Bird-Lieberman et al. enhanced the endoscopic detection of Barrett's dysplasia with fluorescent lectins.17 More recently, Jeyasekharan et al. utilized fluorescently conjugated CEACAM6 in the detection of gastric cancer ex vivo.18 There is a need therefore to explore this avenue in the field of BE. In addition, further research would prove useful in analyzing the expression of CEACAM 6 in a larger patient cohort of CLE, BE, and associated neoplasia.
References
Author notes
Specific author contributions: All authors contributed equally to the conception and design, and analysis and interpretation of data, drafting the article and revising it critically for important intellectual content; and final approval of the version to be published. Supriya Srivastava and Teh Ming were the primary pathologists involved in the histological analysis.
Conflicts of interest: The authors declare that they have no conflict of interest.