-
PDF
- Split View
-
Views
-
Cite
Cite
Mingxuan Ma, Qunli Wei, Min Meng, Jiale Yin, Lei Du, Xia Zhu, Mengjun Min, Xueyan Zhou, Xiaoxing Yin, Yinhan Gong, Application of Partially Substituted 3,5-Dimethylphenylcarbamate-(3-(2-O-β-Cyclodextrin)-2-Hydroxypropoxy)-Propylsilyl-Appended Silica Particles as Chiral Stationary Phase for Multi-mode High-performance Liquid Chromatography, Journal of Chromatographic Science, Volume 55, Issue 8, September 2017, Pages 839–845, https://doi.org/10.1093/chromsci/bmx044
Close - Share Icon Share
Abstract
A new type of partially substituted 3,5-dimethylphenylcarbamate-(3-(2-O-β-cyclodextrin)-2-hydroxypropoxy)-propylsilyl-appended silica particles (MP-CD-HPS) have been prepared by a convenient post-immobilization derivazition procedure. The MP-CD-HPS has been successfully used as chiral stationary phase (CSP) for high-performance liquid chromatography (HPLC) under normal phase, reversed phase and polar organic mobile phase conditions. The chromatographic evaluation results show that the MP-CD-HPS has excellent selectivity for the separation of aromatic positional isomers and enantiomers of some chiral compounds. The multi-mode HPLC separation results also indicate that both the stable ether spacer linking to the wider torus rim of β-cyclodextrin in the MP-CD-HPS phase and the hydroxyl residues in the partially substituted β-cyclodextrin have important contributions to chiral recognitions and chromatographic separations.
Introduction
Development of new chiral stationary phases (CSPs) with different enantioselectivities for separation of different chiral molecules is important in many fields including natural product research, stereo-specific organic synthesis, pharmaceutical industry, food science and environmental studies (1, 2). A number of CPSs are now commercially available and their number is steadily increasing since one single CSP cannot separate all chiral compounds (2). Since the β-cyclodextrin and its derivatives exhibited excellent chiral recognition, there are many chiral separations achieved on β-cyclodextrin and its derivative-based CSPs (3). Most of the β-cyclodextrins in those CSPs including commercial CSPs, e.g., Cyclobond I DMP (4, 5), etc., are immobilized through the spacer linking to the narrow torus rim by reaction with the primary hydroxyl group (6) since the primary hydroxyl groups on the narrow torus rim are more reactive than the secondary hydroxyl groups on the wider torus rim of the β-cyclodextrin (7, 8). However, in most situations, chiral solutes are included into the β-cyclodextrin cavity through the wider torus side (5). Ng and co-worker (9) have reported a completely substituted β-cyclodextrin-bonded CSP which is immobilized through the less reactive secondary hydroxyl group. This CSP exhibits excellent enantioselectivity for some chiral compounds. However, the spacer in the CSP (9) contains nitrogen which may easily undergo hydrolysis under some chromatographic conditions.
Previously, we reported (10) a new type of (3-(2-O-β-cyclodextrin)-2-hydroxypropoxy)-propylsilyl-appended silica particles (CD-HPS) in which the β-cyclodextrin was immobilized via a stable ether spacer through the less reactive secondary hydroxyl group on the wider torus rim. By using CD-HPS as starting materials, two new types of partially substituted β-cyclodextrin-bonded CSPs, naphthylcarbamate-substituted β-cyclodextrin-bonded phase NCCD-HPS (11) and bromoacetate-substituted β-cyclodextrin-bonded phase BACD-HPS (12), were prepared and applied as CSPs in HPLC. The NCCD-HPS and BACD-HPS phases exhibited excellent chromatographic performance since the spacer in those bonded phases provided extra steric hindrance interaction and hydrophobic interaction for solute to enter the β-cyclodextrin cavity through the wider torus rim to form inclusion complex (11). The hydroxyl residues in the partially substituted β-cyclodextrin not only contributed to chromatographic separations, but also allowed the NCCD-HPS and BACD-HPS to be able to work under both normal and reversed-phase conditions (12).
Interestingly, the completely substituted 3,5-dimethylphenylcarbamate-β-cyclodextrin-based CSPs exhibited excellent chiral recognition for wide range of chiral compounds (5, 13). Therefore, it is our interest to prepare a new type of partially substituted 3,5-dimethylphenylcarbamate β-cyclodextrin-bonded phase via a stable ether spacer linking to the wider torus rim of the β-cyclodextrin and to evaluate the chromatographic performance for use as CSP in multi-mode HPLC.
In this article, we describe a convenient post-immobilization derivation procedure to prepare the new type of partially substituted 3,5-dimethylphenylcarbamate-(3-(2-O-β-cyclodextrin)-2-hydroxypropoxy)-propylsilyl-appended silica particles (MP-CD-HPS) via reaction of CD-HPS with excessive 3,5-dimethylphenyl isocyanate in anhydrous pyridine. The chromatographic behavior of the new bonded phase MP-CD-HPS is evaluated with several disubstituted benzenes and some chiral compounds under multi-mode HPLC conditions including normal phase, reversed phase and polar organic mobile phase conditions.
Experimental
Instrumentation
Chromatographic separations were carried out on an Agilent Technologies (Waldbronn, Germany) Model 1260 HPLC system consisting of an autosampler, a binary pump, a degasser and a variable wave length UV detector or on a Shimadzu (Tokyo, Japan) LC20 HPLC system with two high-pressure pumps, an autosampler, a variable wave length UV detector. The Agilent HPLC data were recorded and processed by using ChemStation software (Rev.C.01.04). The Shimadzu HPLC data were processed by LCsolution software (Rev.1.24 SP1.). A Perkin Elmer (Norwalk, CT, USA) 2400 elemental analyzer was used for elemental analysis of the synthetic silica particles.
Chemicals
Premium Rf spherical silica gel (3 μm, 100Å) was purchased from Sorbent Technologies (Atlanta, GA, USA) and were dried in 0.1-mmHg (1 mmHg = 133.322 Pa) vacuum at 120°C for 12 h before chemical reaction. β-cyclodextrin (β-CD) was obtained from Merck (Schuchardt, Hohenbrunn, Germany). The disubstituted benzene derivatives were purchased from Fluka (Buchs, Switzerland). Anhydrous pyridine and analytical-grade 3,5-dimethylphenyl isocyanate, formic acid (FA), acetic acid (AA), triethylamine (TEA) and chiral compounds were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade solvents acetonitrile (ACN), hexane and methanol (MeOH) were purchased from Mreda (Charlotte, NC, USA) and isopropanol (IPA) was obtained from Fisher (Fair Lawn, NJ. USA). Ultrapure water was obtained by using a Milli-Q water purification system (Bedford, MA, USA).
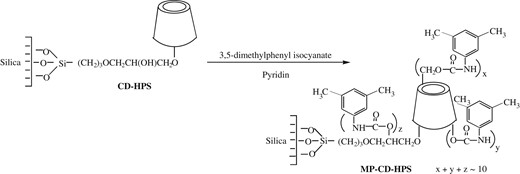
Preparation of the new bonded silica particles MP-CD-HPS
The starting material CD-HPS for preparing MP-CD-HPS was synthesized according to our previously reported procedure (10, 12) by anchoring the β-CD onto silica particles at C(2) position. The amount of anchored β-CD in CD-HPS was 142 μmol g−1. The new phase MP-CD-HPS was then prepared by reaction of the obtained CD-HPS with excessive 3,5-dimethylphenyl isocyanate in anhydrous pyridine. Figure 1 shows the synthetic routine. Typically, after 2 g of dry CD-HPS particles were suspended into 80 mL anhydrous pyridine, excessive 3,5-dimethylphenyl isocyanate was added to the reaction mixture. Then, the reaction was continued for 24 h at 90°C under protection of dry nitrogen gas. Finally, the obtained MP-CD-HPS was filtered with G4 glass filter, successively washed with pyridine, IPA, acetone, water and methanol, and then purified by Soxhlet extraction with acetone overnight. After drying for 6 h under vacuum at 60°C, elemental analysis was performed for the bonded phase MP-CD-HPS. The result of C, 19.76%; H, 2.61%; indicated the amount of the substituted 3,5-dimethylphenylcarbamate moieties in the new bonded phase as 1,405 μmol g−1. Accordingly, the degree of substitution was calculated to be around 10 in the new MP-CD-HPS particles.
Chromatographic procedures
The new phase MP-CD-HPS was packed into a 150 mm × 2.0 mm I.D. stainless steel column (Phenomenex, Torrance, CA, USA) for use as CSP in HPLC by using a previously reported procedure (14). A series of mixtures of hexane/IPA, ACN/water, ACN/water/FA, methanol/water and methanol/ACN/AA/TEA by different volume ratios were used as mobile phases for the multi-mode HPLC separations. The samples (0.5–5 mmol/L) were prepared in the mobile phases and filtered through 0.2 μm membrane before injection. The injection volume is about 0.5–10 μL. The void volume marker (t0) was determined by the baseline perturbation resulting from injection of the mobile phase. All the HPLC separations were carried out at room temperature.
Results
Evaluation of the MP-CD-HPS-packed column using disubstituted benzenes
The MP-CD-HPS-packed column was firstly evaluated by separating positional isomers of nitroaniline and nitrophenol using mixtures of methanol/water as mobile phases under isocratic conditions. The influence of the methanol content in the mobile phase on the retention of the solutes on the MP-CD-HPS is shown in Table I. It was observed that the retention factors of all the solutes gradually increased when the content of methanol decreased. This implied that the MP-CD-HPS had hydrophobic interactions with the solutes. These observations were similar to the retention trend of the disubstituted benzenes on the CD-HPS-packed column (11). Compared to the starting material CD-HPS (11), the new bonded phase MP-CD-HPS exhibited generally higher retention for the same solutes under the similar conditions using mixture of methanol/water as mobile phases (except for 20% methanol conditions). The MP-CD-HPS also exhibited higher separation resolutions under the similar chromatographic conditions. For example, when using the same mixture of methanol/water (20:80, v/v) as mobile phase, better separation resolutions for isomers of nitroaniline (RS(m,o) = 3.53 and RS(o,p) = 3.36 on MP-CD-HPS, and RS(m,o) = 1.07 and RS(o,p) = 2.81 on CD-HPS) were achieved on the MP-CD-HPS-packed column. This suggests that some extra interactions, e.g., pi–pi interaction and dipolar interaction, etc., are imparted after some hydroxyl groups of β-CD were substituted by 3,5-dimethylphenylcarbamate groups. This enhances the chromatographic separation and retention of the solutes.
Retention Factors (k) for Disubstitued Benzenes on the MP-CD-HPS-Packed Column Using Mixture of Methanol and Water as Mobile Phase
| Solutes . | Methanol:water (v/v)a . | ||||
|---|---|---|---|---|---|
| 100:0 . | 80:20 . | 60:40 . | 40:60 . | 20:80 . | |
| o-Nitrophenol | 0.55 | 1.17 | 1.73 | 6.48 | 9.30 |
| m-Nitrophenol | 0.17 | 0.48 | 0.81 | 3.61 | 7.19 |
| p-Nitrophenol | 0.79 | 1.49 | 2.27 | 9.35 | 12.14 |
| o-Nitroaniline | 0.28 | 0.55 | 0.78 | 3.93 | 10.12 |
| m-Nitroaniline | 0.25 | 0.51 | 0.65 | 3.02 | 7.05 |
| p-Nitroaniline | 0.54 | 0.99 | 1.45 | 6.13 | 12.39 |
| Solutes . | Methanol:water (v/v)a . | ||||
|---|---|---|---|---|---|
| 100:0 . | 80:20 . | 60:40 . | 40:60 . | 20:80 . | |
| o-Nitrophenol | 0.55 | 1.17 | 1.73 | 6.48 | 9.30 |
| m-Nitrophenol | 0.17 | 0.48 | 0.81 | 3.61 | 7.19 |
| p-Nitrophenol | 0.79 | 1.49 | 2.27 | 9.35 | 12.14 |
| o-Nitroaniline | 0.28 | 0.55 | 0.78 | 3.93 | 10.12 |
| m-Nitroaniline | 0.25 | 0.51 | 0.65 | 3.02 | 7.05 |
| p-Nitroaniline | 0.54 | 0.99 | 1.45 | 6.13 | 12.39 |
aConditions: 150 mm × 2.0 mm I.D stainless steel column packed with 3 μm MP-CD-HPS particles; 0.15 mL min−1 mobile phase flow rate; 220 nm UV detection.
Retention Factors (k) for Disubstitued Benzenes on the MP-CD-HPS-Packed Column Using Mixture of Methanol and Water as Mobile Phase
| Solutes . | Methanol:water (v/v)a . | ||||
|---|---|---|---|---|---|
| 100:0 . | 80:20 . | 60:40 . | 40:60 . | 20:80 . | |
| o-Nitrophenol | 0.55 | 1.17 | 1.73 | 6.48 | 9.30 |
| m-Nitrophenol | 0.17 | 0.48 | 0.81 | 3.61 | 7.19 |
| p-Nitrophenol | 0.79 | 1.49 | 2.27 | 9.35 | 12.14 |
| o-Nitroaniline | 0.28 | 0.55 | 0.78 | 3.93 | 10.12 |
| m-Nitroaniline | 0.25 | 0.51 | 0.65 | 3.02 | 7.05 |
| p-Nitroaniline | 0.54 | 0.99 | 1.45 | 6.13 | 12.39 |
| Solutes . | Methanol:water (v/v)a . | ||||
|---|---|---|---|---|---|
| 100:0 . | 80:20 . | 60:40 . | 40:60 . | 20:80 . | |
| o-Nitrophenol | 0.55 | 1.17 | 1.73 | 6.48 | 9.30 |
| m-Nitrophenol | 0.17 | 0.48 | 0.81 | 3.61 | 7.19 |
| p-Nitrophenol | 0.79 | 1.49 | 2.27 | 9.35 | 12.14 |
| o-Nitroaniline | 0.28 | 0.55 | 0.78 | 3.93 | 10.12 |
| m-Nitroaniline | 0.25 | 0.51 | 0.65 | 3.02 | 7.05 |
| p-Nitroaniline | 0.54 | 0.99 | 1.45 | 6.13 | 12.39 |
aConditions: 150 mm × 2.0 mm I.D stainless steel column packed with 3 μm MP-CD-HPS particles; 0.15 mL min−1 mobile phase flow rate; 220 nm UV detection.
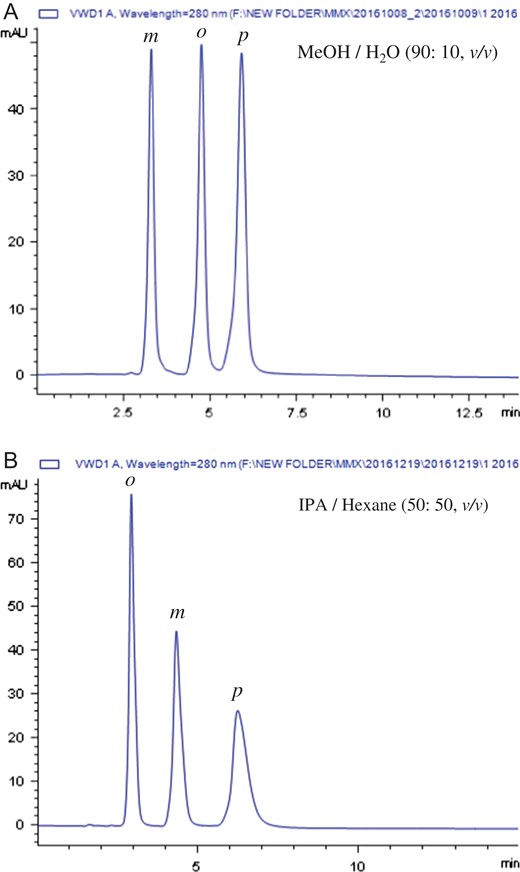
Baseline separations of the positional isomers of nitroaniline and nitrophenol were achieved on the MP-CD-HPS-packed columns under the reversed-phase conditions. Interestingly, we found that the MP-CD-HPS also could be used for separation of all the positional isomers under normal phase conditions by using of mixture of IPA/hexane as mobile phase. Figure 2 shows chromatograms for separation of the isomers of o-, m-, p-nitrophenol under reversed-phase condition and o-, m-, p-nitroaniline under normal phase condition on the MP-CD-HPS-packed column.
Typical chromatogram for separation of positional isomers of (A) o-, m-, p-nitrophenol under reversed-phase condition and (B) o-, m-, p-nitroaniline under normal phase condition on the MP-CD-HPS-packed column.
Enantioseparation of the chiral compounds
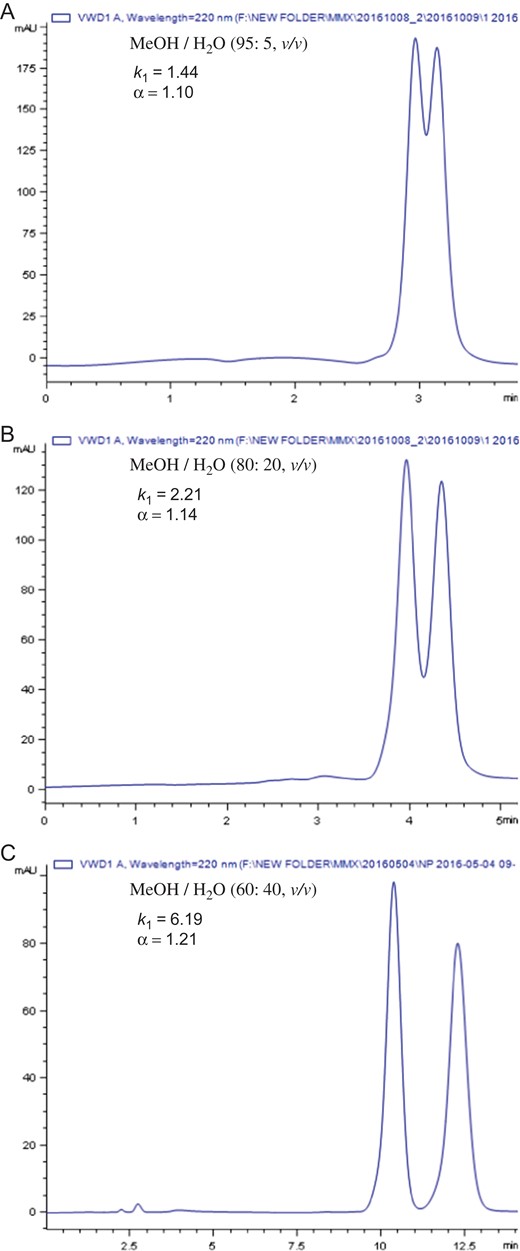
In this study, the MP-CD-HPS-packed column was further evaluated under multi-mode HPLC conditions, including normal phase, reversed phase and polar organic mobile phase conditions, for enantioseparation of chiral compounds. A typical progressive separation of the enantiomers of 4-bromo-alpha-methylbenzyl alcohol on MP-CD-HPS-packed column was shown in Figure 3 when mobile phase composition of methanol/water was varied. It was observed that decreasing the methanol content in the mobile phase resulted in longer retention, higher enantioselectivity and higher enantioseparation resolution as shown in Figure 3. This suggests that the main chiral recognition mechanism is the formation of inclusion complex in which the hydrophobic portion of the solute is included in the β-CD cavity (11, 12), and the 3,5-dimethylphenylcarbamate units provide further other interactions (5, 11), e.g., hydrogen-bonding interaction and dipolar interaction, etc., with the solute. Since the spacer is connected at the wider torus rim of β-CD in MP-CD-HPS as shown in Figure 1, it can provide extra steric interaction and hydrophobic interaction for the solute enantiomer to enter the β-CD cavity via the wider torus rim to form inclusion complex. This enhances the chiral recognition and enantioselectivity. Complete separation of enantiomers of the 4-bromo-alpha-methylbenzyl alcohol was easily obtained when methanol content was reduced to 60% (or less) on the MP-CD-HPS-packed column when using mixture of methanol/water as mobile phase.
Progressive enantioseparation of 4-bromo-alpha-methylbenzyl alcohol on the MP-CD-HPS-packed column as composition of mobile phase is varied.
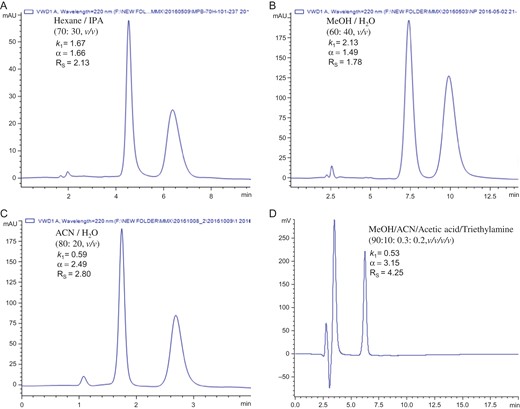
Table II lists the enantioseparation data for the chiral compounds resolved on the MP-CD-HPS-packed column under polar organic mobile phase, normal phase and reversed-phase conditions, including the addition of FA or AA into the mobile phases. Figure 4 shows some typical chromatograms of the enantioseparation of the chiral compounds on the MP-CD-HPS-packed column under the multi-mode HPLC conditions. Those results indicated that the new MP-CD-HPS phase exhibited excellent chromatographic performance for separation of a wide range of chiral compounds.
Typical Enantioseparation Data for the Chiral Compounds Resolved on the MP-CD-HPS-Packed Column
| Solutes . | Mobile phase . | Separation dataa . | ||
|---|---|---|---|---|
| k1 . | α . | RS . | ||
| Flavanone | IPA/hexane (30:70, v/v) | 1.67 | 1.66 | 2.13 |
| 1-Phenyl-1,2-ethanediol | IPA/hexane (3:97, v/v) | 1.67 | 1.48 | 0.55 |
| Stilbene Oxide | IPA/hexane (3:97, v/v) | 0.84 | 1.19 | 0.73 |
| 4-Chloro-alpha-methylbenzyl Alcohol | ACN/water (20:80, v/v) | 12.50 | 1.18 | 2.12 |
| Benzoin | ACN/water (20:80, v/v) | 2.25 | 1.10 | 1.00 |
| Anisoin | ACN/water (20:80, v/v) | 8.25 | 1.20 | 0.79 |
| Thalidomide | ACN/water (20:80, v/v) | 2.81 | 1.21 | 0.91 |
| Omeprazole | ACN/water (40:60, v/v) | 3.25 | 1.19 | 1.33 |
| Lansoprazole | ACN/water (80:20, v/v) | 0.59 | 2.49 | 2.80 |
| Ketoconazole | ACN/water (80:20, v/v) | 0.90 | 4.56 | 4.92 |
| Racecadotril | ACN/water (80:20, v/v) | 0.30 | 1.67 | 1.00 |
| Aminoglutethimide | ACN/water (20:80, v/v) | 0.65 | 1.34 | 1.08 |
| Indapamide | ACN/water (20:80, v/v) | 2.04 | 1.18 | 1.06 |
| Flavanone | MeOH/water (80:20, v/v) | 2.85 | 1.60 | 3.09 |
| 4-Phenyl-2-butanol | MeOH/water (60:40, v/v) | 3.49 | 1.26 | 1.83 |
| Naringenin | MeOH/water (80:20, v/v) | 2.74 | 1.33 | 0.98 |
| 4′-Hydroxyflavanone | MeOH/water (80:20, v/v) | 2.13 | 1.49 | 1.78 |
| 6-Methoxyflavanone | MeOH/water (80:20, v/v) | 2.32 | 2.06 | 4.17 |
| 4-Chromanol | MeOH/water (40:60, v/v) | 3.71 | 1.19 | 2.17 |
| 4-Bromo-alpha-methylbenzyl Alcohol | MeOH/water (60:40, v/v) | 6.19 | 1.21 | 1.73 |
| 3-Phenylphthalide | MeOH/water (60:40, v/v) | 4.42 | 1.18 | 1.15 |
| Stilbene Oxide | MeOH/water (80:20, v/v) | 2.25 | 1.18 | 1.14 |
| Econazole | MeOH/water (80:20, v/v) | 4.18 | 1.18 | 0.63 |
| Miconazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.45 | 3.29 | 4.86 |
| Carbinoxamine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.53 | 3.15 | 4.25 |
| Amlodipine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 1.00 | 2.50 | 4.00 |
| Econazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.43 | 3.33 | 5.00 |
| Nifedipine | ACN/water/FA (40:60:0.1, v/v/v) | 1.13 | 1.62 | 1.93 |
| Promethazine | ACN/water/FA (40:60:0.1, v/v/v) | 1.00 | 4.13 | 2.69 |
| Nisoldipine | ACN/water/FA (60:40:0.1, v/v/v) | 2.44 | 1.23 | 1.05 |
| Solutes . | Mobile phase . | Separation dataa . | ||
|---|---|---|---|---|
| k1 . | α . | RS . | ||
| Flavanone | IPA/hexane (30:70, v/v) | 1.67 | 1.66 | 2.13 |
| 1-Phenyl-1,2-ethanediol | IPA/hexane (3:97, v/v) | 1.67 | 1.48 | 0.55 |
| Stilbene Oxide | IPA/hexane (3:97, v/v) | 0.84 | 1.19 | 0.73 |
| 4-Chloro-alpha-methylbenzyl Alcohol | ACN/water (20:80, v/v) | 12.50 | 1.18 | 2.12 |
| Benzoin | ACN/water (20:80, v/v) | 2.25 | 1.10 | 1.00 |
| Anisoin | ACN/water (20:80, v/v) | 8.25 | 1.20 | 0.79 |
| Thalidomide | ACN/water (20:80, v/v) | 2.81 | 1.21 | 0.91 |
| Omeprazole | ACN/water (40:60, v/v) | 3.25 | 1.19 | 1.33 |
| Lansoprazole | ACN/water (80:20, v/v) | 0.59 | 2.49 | 2.80 |
| Ketoconazole | ACN/water (80:20, v/v) | 0.90 | 4.56 | 4.92 |
| Racecadotril | ACN/water (80:20, v/v) | 0.30 | 1.67 | 1.00 |
| Aminoglutethimide | ACN/water (20:80, v/v) | 0.65 | 1.34 | 1.08 |
| Indapamide | ACN/water (20:80, v/v) | 2.04 | 1.18 | 1.06 |
| Flavanone | MeOH/water (80:20, v/v) | 2.85 | 1.60 | 3.09 |
| 4-Phenyl-2-butanol | MeOH/water (60:40, v/v) | 3.49 | 1.26 | 1.83 |
| Naringenin | MeOH/water (80:20, v/v) | 2.74 | 1.33 | 0.98 |
| 4′-Hydroxyflavanone | MeOH/water (80:20, v/v) | 2.13 | 1.49 | 1.78 |
| 6-Methoxyflavanone | MeOH/water (80:20, v/v) | 2.32 | 2.06 | 4.17 |
| 4-Chromanol | MeOH/water (40:60, v/v) | 3.71 | 1.19 | 2.17 |
| 4-Bromo-alpha-methylbenzyl Alcohol | MeOH/water (60:40, v/v) | 6.19 | 1.21 | 1.73 |
| 3-Phenylphthalide | MeOH/water (60:40, v/v) | 4.42 | 1.18 | 1.15 |
| Stilbene Oxide | MeOH/water (80:20, v/v) | 2.25 | 1.18 | 1.14 |
| Econazole | MeOH/water (80:20, v/v) | 4.18 | 1.18 | 0.63 |
| Miconazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.45 | 3.29 | 4.86 |
| Carbinoxamine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.53 | 3.15 | 4.25 |
| Amlodipine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 1.00 | 2.50 | 4.00 |
| Econazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.43 | 3.33 | 5.00 |
| Nifedipine | ACN/water/FA (40:60:0.1, v/v/v) | 1.13 | 1.62 | 1.93 |
| Promethazine | ACN/water/FA (40:60:0.1, v/v/v) | 1.00 | 4.13 | 2.69 |
| Nisoldipine | ACN/water/FA (60:40:0.1, v/v/v) | 2.44 | 1.23 | 1.05 |
ak1 is the retention factor for the enantiomer eluted out first; α is the selectivity; RS is the resolution.
Typical Enantioseparation Data for the Chiral Compounds Resolved on the MP-CD-HPS-Packed Column
| Solutes . | Mobile phase . | Separation dataa . | ||
|---|---|---|---|---|
| k1 . | α . | RS . | ||
| Flavanone | IPA/hexane (30:70, v/v) | 1.67 | 1.66 | 2.13 |
| 1-Phenyl-1,2-ethanediol | IPA/hexane (3:97, v/v) | 1.67 | 1.48 | 0.55 |
| Stilbene Oxide | IPA/hexane (3:97, v/v) | 0.84 | 1.19 | 0.73 |
| 4-Chloro-alpha-methylbenzyl Alcohol | ACN/water (20:80, v/v) | 12.50 | 1.18 | 2.12 |
| Benzoin | ACN/water (20:80, v/v) | 2.25 | 1.10 | 1.00 |
| Anisoin | ACN/water (20:80, v/v) | 8.25 | 1.20 | 0.79 |
| Thalidomide | ACN/water (20:80, v/v) | 2.81 | 1.21 | 0.91 |
| Omeprazole | ACN/water (40:60, v/v) | 3.25 | 1.19 | 1.33 |
| Lansoprazole | ACN/water (80:20, v/v) | 0.59 | 2.49 | 2.80 |
| Ketoconazole | ACN/water (80:20, v/v) | 0.90 | 4.56 | 4.92 |
| Racecadotril | ACN/water (80:20, v/v) | 0.30 | 1.67 | 1.00 |
| Aminoglutethimide | ACN/water (20:80, v/v) | 0.65 | 1.34 | 1.08 |
| Indapamide | ACN/water (20:80, v/v) | 2.04 | 1.18 | 1.06 |
| Flavanone | MeOH/water (80:20, v/v) | 2.85 | 1.60 | 3.09 |
| 4-Phenyl-2-butanol | MeOH/water (60:40, v/v) | 3.49 | 1.26 | 1.83 |
| Naringenin | MeOH/water (80:20, v/v) | 2.74 | 1.33 | 0.98 |
| 4′-Hydroxyflavanone | MeOH/water (80:20, v/v) | 2.13 | 1.49 | 1.78 |
| 6-Methoxyflavanone | MeOH/water (80:20, v/v) | 2.32 | 2.06 | 4.17 |
| 4-Chromanol | MeOH/water (40:60, v/v) | 3.71 | 1.19 | 2.17 |
| 4-Bromo-alpha-methylbenzyl Alcohol | MeOH/water (60:40, v/v) | 6.19 | 1.21 | 1.73 |
| 3-Phenylphthalide | MeOH/water (60:40, v/v) | 4.42 | 1.18 | 1.15 |
| Stilbene Oxide | MeOH/water (80:20, v/v) | 2.25 | 1.18 | 1.14 |
| Econazole | MeOH/water (80:20, v/v) | 4.18 | 1.18 | 0.63 |
| Miconazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.45 | 3.29 | 4.86 |
| Carbinoxamine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.53 | 3.15 | 4.25 |
| Amlodipine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 1.00 | 2.50 | 4.00 |
| Econazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.43 | 3.33 | 5.00 |
| Nifedipine | ACN/water/FA (40:60:0.1, v/v/v) | 1.13 | 1.62 | 1.93 |
| Promethazine | ACN/water/FA (40:60:0.1, v/v/v) | 1.00 | 4.13 | 2.69 |
| Nisoldipine | ACN/water/FA (60:40:0.1, v/v/v) | 2.44 | 1.23 | 1.05 |
| Solutes . | Mobile phase . | Separation dataa . | ||
|---|---|---|---|---|
| k1 . | α . | RS . | ||
| Flavanone | IPA/hexane (30:70, v/v) | 1.67 | 1.66 | 2.13 |
| 1-Phenyl-1,2-ethanediol | IPA/hexane (3:97, v/v) | 1.67 | 1.48 | 0.55 |
| Stilbene Oxide | IPA/hexane (3:97, v/v) | 0.84 | 1.19 | 0.73 |
| 4-Chloro-alpha-methylbenzyl Alcohol | ACN/water (20:80, v/v) | 12.50 | 1.18 | 2.12 |
| Benzoin | ACN/water (20:80, v/v) | 2.25 | 1.10 | 1.00 |
| Anisoin | ACN/water (20:80, v/v) | 8.25 | 1.20 | 0.79 |
| Thalidomide | ACN/water (20:80, v/v) | 2.81 | 1.21 | 0.91 |
| Omeprazole | ACN/water (40:60, v/v) | 3.25 | 1.19 | 1.33 |
| Lansoprazole | ACN/water (80:20, v/v) | 0.59 | 2.49 | 2.80 |
| Ketoconazole | ACN/water (80:20, v/v) | 0.90 | 4.56 | 4.92 |
| Racecadotril | ACN/water (80:20, v/v) | 0.30 | 1.67 | 1.00 |
| Aminoglutethimide | ACN/water (20:80, v/v) | 0.65 | 1.34 | 1.08 |
| Indapamide | ACN/water (20:80, v/v) | 2.04 | 1.18 | 1.06 |
| Flavanone | MeOH/water (80:20, v/v) | 2.85 | 1.60 | 3.09 |
| 4-Phenyl-2-butanol | MeOH/water (60:40, v/v) | 3.49 | 1.26 | 1.83 |
| Naringenin | MeOH/water (80:20, v/v) | 2.74 | 1.33 | 0.98 |
| 4′-Hydroxyflavanone | MeOH/water (80:20, v/v) | 2.13 | 1.49 | 1.78 |
| 6-Methoxyflavanone | MeOH/water (80:20, v/v) | 2.32 | 2.06 | 4.17 |
| 4-Chromanol | MeOH/water (40:60, v/v) | 3.71 | 1.19 | 2.17 |
| 4-Bromo-alpha-methylbenzyl Alcohol | MeOH/water (60:40, v/v) | 6.19 | 1.21 | 1.73 |
| 3-Phenylphthalide | MeOH/water (60:40, v/v) | 4.42 | 1.18 | 1.15 |
| Stilbene Oxide | MeOH/water (80:20, v/v) | 2.25 | 1.18 | 1.14 |
| Econazole | MeOH/water (80:20, v/v) | 4.18 | 1.18 | 0.63 |
| Miconazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.45 | 3.29 | 4.86 |
| Carbinoxamine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.53 | 3.15 | 4.25 |
| Amlodipine | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 1.00 | 2.50 | 4.00 |
| Econazole | MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v) | 0.43 | 3.33 | 5.00 |
| Nifedipine | ACN/water/FA (40:60:0.1, v/v/v) | 1.13 | 1.62 | 1.93 |
| Promethazine | ACN/water/FA (40:60:0.1, v/v/v) | 1.00 | 4.13 | 2.69 |
| Nisoldipine | ACN/water/FA (60:40:0.1, v/v/v) | 2.44 | 1.23 | 1.05 |
ak1 is the retention factor for the enantiomer eluted out first; α is the selectivity; RS is the resolution.
Typical chromatograms for enantioseparations of chiral compounds on MP-CD-HPS-packed column. (A) flavanone, mobile phase: Hexane/IPA (70:30, v/v); (B) 4′-hydroxyflavanone, mobile phase: MeOH/water (80:20, v/v); (C) lansoprazole, mobile phase: ACN/water (80:20, v/v); and (D) carbinoxamine, mobile phase: MeOH/ACN/AA/TEA (90:10:0.3:0.2, v/v/v/v).
Discussion
The MP-CD-HPS phase exhibited excellent chromatographic performance for separation of the positional isomers of the disubstituted benzenes under both normal and reversed-phase conditions. Interestingly, the elution order of nitroaniline isomers under the normal phase o < m < p (as shown in Figure 2B) is different from the order m < o < p under the reversed-phase conditions (as shown in Table I). This implies that the separation mechanisms (6, 14) under normal and reversed-phase conditions are different for the MP-CD-HPS-packed column and the new stationary phase can provide different chromatographic separations for the solutes under the two different modes of HPLC.
Development of new CSP for multi-mode HPLC is a useful practical strategy (2). This allows a single CSP can separate more chiral compounds under different modes of mobile phase conditions (1, 2, 5). When used as CSP in multi-mode HPLC, the new phase MP-CD-HPS showed excellent enantioselectivities for a wide range of chiral compounds including flavonoids, beta blockers and anti-inflammatory drugs as listed in Table II. The column efficiency for the MP-CD-HPS-packed column was determined as 34,400 plates m−1 under reversed-phase condition by using p-nitrophenol as solute and methanol/water (60:40, v/v) as the mobile phase. This column efficiency is much higher than most of other similar β-CD-based CSP-packed columns (5, 14). For example, under the same conditions, IPC4CD-HPS-packed column efficiency was reported as 26,600 plates m−1 (14). Under the similar conditions, column efficiencies for the highly substituted 3,5-dimethylphenylcarbamate-β-CD-bonded CSP Material IC2-packed column (5) and Material IA2-packed column (5) were only 12,000 plates m−1. The higher column efficiency allows the MP-CD-HPS-packed column to be able to achieve faster and better enantioseparation.
The MP-CD-HPS-packed column exhibited better enantioseparation and improved chromatographic performance than some commercial and previously reported β-CD-based columns. Compared to commercial cyclobond I DMP column (13) in which the spacer was connected to the primary hydroxyl group on the narrow torus rim of β-CD, the MP-CD-HPS-packed column exhibited better enantioselectivities for some chiral compounds. For example, better enantioselectivities for stilbene oxide (α ~1 on cyclobond I DMP (13) and α = 1.19 on MP-CD-HPS under normal phase condition; α ~ 1 on cyclobond I DMP (13) and α = 1.18 on MP-CD-HPS under reversed-phase condition) and flavanone (α = 1.12 on cyclobond I DMP (13) and α = 1.66 on MP-CD-HPS under normal phase condition; α = 1.05 on cyclobond I DMP (13) and α = 1.60 on MP-CD-HPS under reversed-phase condition) were obtained on the MP-CD-HPS-packed column. This suggests that the spacer which was connected to the secondary hydroxyl group on the wider torus rim of β-cyclodextrin in the MP-CD-HPS had important contribution to the enantioseparation of those chiral compounds. Compared to the previously reported IPC4CD-HPS-packed column (14), better enantioseparations for some chiral compounds, eg., ketoconazole (no enantioseparation on IPC4CD-HPS, however, baseline enantioseparation (α = 4.56, RS = 4.92) on MP-CD-HPS) and promethazine (α = 2.10, RS = 1.45 on IPC4CD-HPS and α = 4.13, RS = 2.69 on MP-CD-HPS), etc., were achieved on the new MP-CD-HPS-packed column. Compared to the highly substituted (substitution degree ~18 (5)) 3,5-dimethylphenylcarbamate-β-CD-bonded CSP Material IC2 (5) and Material IA2 (5) in which the β-CDs were immobilized via secondary hydroxyl groups, the better enantioselectivities for some chiral compounds, e.g., benzoin (α ~1 on Material IC2 and Material IA2 (5, 13) and α = 1.10 on MP-CD-HPS) and stilbene oxide (α = 1.03 Material IC2 and α = 1.07 on Material IA2 (5, 13) and α = 1.18 on MP-CD-HPS), etc., were obtained under the same reversed-phase conditions on the MP-CD-HPS phase. Compared to another type of completely substituted perphenylcarbamoylated β-CD-based phase mono-2A-azido-2A-deoxyperphenylcarbamoylated β-cyclodextrin-bonded CSP in which the β-CD was immobilized via a secondary hydroxyl group on the wider torus rim of the β-CD (9), the new phase MP-CD-HPS also exhibited shorter retention and better enantioselectivities for some chiral compounds. For example, under the same reversed-phase conditions, shorter retention and better enantioselectivities and separation resolution for indapamide (k1 = 3.01, α = 1.16, RS = 0.94 on the completely substituted perphenylcarbamoylated β-CD-bonded CSP (9) and k1 = 2.04, α = 1.18, RS = 1.06 on MP-CD-HPS) and 6-methoxyflavanone (k1 = 3.52, α = 1.50, RS = 3.67 on the completely substituted perphenylcarbamoylated β-CD-bonded CSP (9) and k1 = 2.32, α = 2.06, RS = 4.17 on MP-CD-HPS) were achieved on MP-CD-HPS. This indicates that the hydroxyl residues (unsubstituted hydroxyl groups) of the β-CD in the MP-CD-HPS also played important contribution in the chiral recognitions for some chiral compounds. All those results also suggest that the new type of partially substituted β-CD-bonded phase MP-CD-HPS has some advantages for use as CSP for chiral separation.
Conclusion
A new type of partially substituted 3,5-dimethylphenylcarbamate-β-CD-bonded phase MP-CD-HPS has been conveniently prepared from CD-HPS and successfully used as CSP in multi-mode HPLC. The MP-CD-HPS phase has shown excellent selectivity for the separation of aromatic positional isomers and enantiomers of some chiral compounds. Besides of the main β-CD backbone, both the stable spacer connecting at the wider torus rim of β-CD in the MP-CD-HPS and the hydroxyl residues in the partially substituted β-CD have important contributions to chiral recognitions and chromatographic separations. The MP-CD-HPS phase has also exhibited fairly robust chromatographic performance in multi-mode HPLC.
Acknowledgments
The authors thank Xuzhou Medical University for financial support of this work (Grant No.: 531150).
References
Author notes
Joint first author.