-
PDF
- Split View
-
Views
-
Cite
Cite
Shelli R. Kesler, Vinod Menon, Allan L. Reiss, Neurofunctional Differences Associated with Arithmetic Processing in Turner Syndrome, Cerebral Cortex, Volume 16, Issue 6, June 2006, Pages 849–856, https://doi.org/10.1093/cercor/bhj028
Close - Share Icon Share
Abstract
Turner syndrome (TS) is a neurogenetic disorder characterized by the absence of one X chromosome in a phenotypic female. Individuals with TS are at risk for impairments in mathematics. We investigated the neural mechanisms underlying arithmetic processing in TS. Fifteen subjects with TS and 15 age-matched typically developing controls were scanned using functional MRI while they performed easy (two-operand) and difficult (three-operand) versions of an arithmetic processing task. Both groups activated fronto-parietal regions involved in arithmetic processing during the math tasks. Compared with controls, the TS group recruited additional neural resources in frontal and parietal regions during the easier, two-operand math task. During the more difficult three-operand task, individuals with TS demonstrated significantly less activation in frontal, parietal and subcortical regions than controls. However, the TS group's performance on both math tasks was comparable to controls. Individuals with TS demonstrate activation differences in fronto-parietal areas during arithmetic tasks compared with controls. They must recruit additional brain regions during a relatively easy task and demonstrate a potentially inefficient response to increased task difficulty compared with controls.
Introduction
Turner syndrome (TS) is a genetic disorder characterized by monosomy X in a phenotypic female (Zinn et al., 1993). Within the cognitive domain, math is an area where deficits are commonly noted among individuals with TS. Girls with TS tend to demonstrate lower math achievement scores compared with controls, often beginning at an early age (Rovet et al., 1994; Mazzocco, 1998). Several studies indicate that arithmetic deficits in TS are specific to calculation procedures and retrieval of number facts rather than to number processing, or number production and comprehension (Temple and Marriott, 1998; Temple and Sherwood, 2002; Bruandet et al., 2004). Compared with controls, girls with TS tend to be slower in performing arithmetic tasks and demonstrate greater impairment as task difficulty increases (Temple and Marriott, 1998; Temple and Sherwood, 2002; Molko et al., 2003).
Most models of mathematical ability include two distinct components: number processing, or the comprehension and production of numbers, and calculation, which includes number facts and procedural knowledge (Dehaene et al., 1999, 2003; Temple and Sherwood, 2002). These arithmetic skills have been shown to rely on fronto-parietal systems. Specifically, number processing is thought to rely on language systems in the frontal and temporal regions whereas calculation relies primarily on parietal cortex (Dehaene et al., 1999, 2003; Menon et al., 2000b; Zago et al., 2001). TS tends to impact upon both parietal and frontal systems in particular, evidenced both by neuropsychological (Romans et al., 1997; Ross et al., 2002; Nijhuis-van der Sanden et al., 2003) and neuroimaging studies (Murphy et al., 1993, 1997; Reiss et al., 1995; Haberecht et al., 2001; Brown et al., 2002, 2004; Tamm et al., 2003; Kesler et al., 2004).
Some investigators who have studied TS assert that arithmetic deficits are related to visuo-spatial impairments (Buchanan et al., 1997; Mazzocco, 1998), while others have shown evidence that poor math strategies in combination with inefficient fact retrieval, particularly in timed situations, are responsible (Rovet et al., 1994). Given that previous studies demonstrate impairment in calculation relative to number processing in TS, increased abnormalities in parietal compared with frontal systems would be expected. To date there has been one investigation of the neural correlates of math difficulty in TS (Molko et al., 2003). While the results of this study did indicate parietal deficits in TS, concomitant activation abnormalities in the anterior cingulate in these same participants were not discussed. Other limitations of this study included use of TS participants with mosaic karyotypes, and lack of information on participant IQ or group differences in general cognitive ability. Additionally this study employed a number processing task involving addition only and the dimension of difficulty was limited to number size (e.g. 4 versus 10); all stimuli consisted of two operands. Previous studies from our laboratory have indicated that the combination of addition and subtraction involves arithmetic computation as opposed to the fact retrieval and rote memory capacities tested by addition only (Menon et al., 2000b). We therefore sought to further clarify the neural correlates of arithmetic processing in TS by examining brain activation patterns associated with an arithmetic task comprised of both addition and subtraction with two levels of mathematical complexity; a two-operand task and a more difficult three-operand task.
Materials and Methods
Participants
All potential participants were interviewed and screened by telephone for assessment of medical and psychiatric history. Fifteen right-handed subjects with TS (mean age = 14.4 ± 4.8; range = 7.1–24.4) and 15 right-handed typically developing control subjects (mean age = 14.6 ± 3.8, range = 7.7–21) received cognitive assessments and underwent functional magnetic resonance imaging (fMRI) scanning after giving written informed consent. Documentation of X monosomy on standardized karyotype was obtained from the diagnosing physician or facility. Ten girls with TS had retained a maternal X chromosome and five had retained the paternal X chromosome. This ratio of participants with a maternally derived to paternally derived X chromosome is consistent with what is typically found in the TS population (Jacobs et al., 1990, 1997). Only girls with a monosomic 45,X genotype (non-mosaic) were included in this study given that mosaicism tends to moderate outcome in TS (Jones, 1997; Zinn et al., 1998). The possibility of occult mosaicism in our subjects cannot be completely ruled out by karyotype alone. However, even when sophisticated methods for detecting additional X or Y chromosome material are utilized in individuals with 45, X karyotype (Wiktor and Van Dyke, 2004), the majority of such individuals are confirmed as having X monosomy. Control and TS participants were excluded for any history of neurological or psychiatric disorder, as well as for MRI contraindications such as metal implants and orthodontia. The human subjects committee at Stanford University School of Medicine approved the protocols used in this study.
Cognitive Assessment
Intellectual functioning (IQ) was measured using the Wechsler Intelligence Scale for Children Third Edition (Wechsler, 1991) for participants age 16 and younger. For those 17 and older, IQ was measured using the Wechsler Adult Intelligence Scale Revised Edition (Wechsler, 1981).
Functional Image Acquisition
Images were acquired on a 1.5T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI) with Echospeed gradients using a custom-built whole head coil. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 2 s using a T2*-weighted gradient echo spiral pulse sequence (TR = 2000 ms, TE = 40 ms, flip angle = 89° and 1 interleave) (Glover and Lai, 1998). The field of view was 240 mm and the effective in-plane spatial resolution was 3.75 mm. To aid in localization of functional data, high resolution T1-weighted spoiled grass gradient recalled (SPGR) 3D MRI sequence with the following parameters was used: TR = 35 ms; TE = 6 ms; flip angle = 45°; 24 cm field of view; 124 slices in coronal plane; 256 × 192 matrix; acquired resolution = 1.5 × 0.9 × 1.2 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9 mm spatial resolution.
Functional Imaging Task Designs
The math task used in the present study has been shown to activate prefrontal and parietal regions (Menon et al., 2000b, 2002; Rivera et al., 2002). The task allows investigation of changes in brain activation associated with increasing arithmetic complexity. The task consisted of a 30 s rest epoch followed by six alternating 30 s epochs of easy (two-operand equation) experimental and control trials, another 30 s rest epoch, and then six alternating 30 s difficult (three-operand equation) experimental and control trials. The task ended with a third 30 s rest epoch. The easy experimental epochs included nine, two-operand randomly mixed addition or subtraction problems with either a correct or incorrect resultant (e.g. 1 + 2 = 3 or 5 – 2 = 4). Difficult experimental epochs consisted of nine, three-operand addition/subtraction problems (e.g. 6 – 3 + 5 = 8 or 6 + 2 – 3 = 4). All experimental and control stimuli were presented for 2625 ms with an inter-stimulus interval of 708 ms. Sixty-six percent of the results were correct and required a button press and the other 34% were incorrect. Equal numbers of button presses were required for experimental and control trials. During experimental epochs, subjects were instructed to respond with a button press only if the resultant of the math problem was correct. Control epochs comprised nine stimuli, each consisting of a string of five (easy) or seven (difficult) single digits. During control epochs, subjects were instructed to respond with a button press when a zero appeared in the string of digits.
Functional Data Analysis
Images were reconstructed by inverse Fourier transformation for each of the 120 time points into 64 × 64 × 18 image matrices (voxel size: 3.75 × 3.75 × 7 mm). Imaging data were pre-processed using Statistical Parametric Mapping (SPM99) (http://www.fil.ion.ucl.ac.uk/spm). Images were corrected for movement using least square minimization without higher-order corrections for spin history, and normalized to stereotaxic Talairach coordinates (Talairach and Tournoux, 1988). Images were then resampled every 2 mm using sinc interpolation and smoothed with a 4 mm Gaussian kernel to decrease spatial noise.
Statistical analysis was performed on individual and group data using the general linear model and the theory of Gaussian random fields as implemented in SPM99. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data (Friston, 1995). Activation foci were superposed on high-resolution T1-weighted images and their locations interpreted using known neuroanatomical landmarks.
A within-subjects procedure was used to model all the effects of interest for each subject. Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low-frequency noise was removed with a high-pass filter (0.5 cycles/min) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 s) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. For each subject, brain activation related to each fMRI task was determined by contrasting experimental and control conditions.
Group analysis was performed using a random-effects model that incorporated a two-stage hierarchical procedure. This model estimates the error variance for each condition of interest across subjects, rather than across scans (Holmes and Friston, 1998) and therefore provides a stronger generalization to the population from which data are acquired. In the first stage, contrast images for each subject and each effect of interest were generated as described above. In the second stage, these contrast images were analyzed using a general linear model to determine voxel-wise t-statistics. One contrast image was generated per subject, per effect of interest. A one-way t-test was then used to determine group activation for each effect. TS and control subjects were compared using an unpaired t-test. Finally, the t-statistics were normalized to Z-scores, and significant clusters of activation were determined using the joint expected probability distribution of height and extent of Z-scores (Poline et al., 1997), with height (Z > 1.67; P < 0.05) and extent (P < 0.05) thresholds. Within- and between-group contrast images were calculated to examine the effects of interest.
Results
Cognitive Assessment
Demographic information is listed in Table 1. Analysis of variance (ANOVA) indicated that participants with TS demonstrated significantly lower full-scale IQ (FSIQ) compared with controls (F = 97.6, df = 1,29, P = 0.01). However, this primarily involved performance IQ (F = 20.2, df = 1, 29, P <.0001) rather than verbal IQ (F = 2.7, df = 1,29, P = 0.11). This verbal > performance IQ discrepancy is typically observed among individuals with TS (Ross et al., 2002).
Demographic and behavioral performance data for the TS and control groups
. | TS . | Control . | F/U . | n . | P . |
|---|---|---|---|---|---|
| Age | 14.4 (4.8) | 14.6 (3.8) | 0.02 | 30 | 0.88 |
| Age range | 7.1–24.4 | 7.7–21 | |||
| FSIQ | 95 (21) | 114 (17) | 7.6 | 30 | 0.01 |
| PIQ | 89 (16) | 114 (14) | 20.2 | 30 | <0.0001 |
| VIQ | 102 (24) | 115 (18) | 2.9 | 30 | 0.10 |
| 2 operand (% correct) | 0.88 (0.25) | 0.97 (0.06) | 83 | 28 | 0.51 |
| RT | 1327 (257) | 1309 (346) | 0.02 | 28 | 0.88 |
| 3 operand (% correct) | 0.65 (0.21) | 0.71 (0.34) | 71 | 28 | 0.23 |
| RT | 2017 (519) | 2094 (483) | 0.17 | 28 | 0.69 |
. | TS . | Control . | F/U . | n . | P . |
|---|---|---|---|---|---|
| Age | 14.4 (4.8) | 14.6 (3.8) | 0.02 | 30 | 0.88 |
| Age range | 7.1–24.4 | 7.7–21 | |||
| FSIQ | 95 (21) | 114 (17) | 7.6 | 30 | 0.01 |
| PIQ | 89 (16) | 114 (14) | 20.2 | 30 | <0.0001 |
| VIQ | 102 (24) | 115 (18) | 2.9 | 30 | 0.10 |
| 2 operand (% correct) | 0.88 (0.25) | 0.97 (0.06) | 83 | 28 | 0.51 |
| RT | 1327 (257) | 1309 (346) | 0.02 | 28 | 0.88 |
| 3 operand (% correct) | 0.65 (0.21) | 0.71 (0.34) | 71 | 28 | 0.23 |
| RT | 2017 (519) | 2094 (483) | 0.17 | 28 | 0.69 |
Data are shown as mean (standard deviation). FSIQ, full-scale IQ score; VIQ, verbal IQ score; PIQ, performance IQ score; RT, reaction time (shown in ms).
Demographic and behavioral performance data for the TS and control groups
. | TS . | Control . | F/U . | n . | P . |
|---|---|---|---|---|---|
| Age | 14.4 (4.8) | 14.6 (3.8) | 0.02 | 30 | 0.88 |
| Age range | 7.1–24.4 | 7.7–21 | |||
| FSIQ | 95 (21) | 114 (17) | 7.6 | 30 | 0.01 |
| PIQ | 89 (16) | 114 (14) | 20.2 | 30 | <0.0001 |
| VIQ | 102 (24) | 115 (18) | 2.9 | 30 | 0.10 |
| 2 operand (% correct) | 0.88 (0.25) | 0.97 (0.06) | 83 | 28 | 0.51 |
| RT | 1327 (257) | 1309 (346) | 0.02 | 28 | 0.88 |
| 3 operand (% correct) | 0.65 (0.21) | 0.71 (0.34) | 71 | 28 | 0.23 |
| RT | 2017 (519) | 2094 (483) | 0.17 | 28 | 0.69 |
. | TS . | Control . | F/U . | n . | P . |
|---|---|---|---|---|---|
| Age | 14.4 (4.8) | 14.6 (3.8) | 0.02 | 30 | 0.88 |
| Age range | 7.1–24.4 | 7.7–21 | |||
| FSIQ | 95 (21) | 114 (17) | 7.6 | 30 | 0.01 |
| PIQ | 89 (16) | 114 (14) | 20.2 | 30 | <0.0001 |
| VIQ | 102 (24) | 115 (18) | 2.9 | 30 | 0.10 |
| 2 operand (% correct) | 0.88 (0.25) | 0.97 (0.06) | 83 | 28 | 0.51 |
| RT | 1327 (257) | 1309 (346) | 0.02 | 28 | 0.88 |
| 3 operand (% correct) | 0.65 (0.21) | 0.71 (0.34) | 71 | 28 | 0.23 |
| RT | 2017 (519) | 2094 (483) | 0.17 | 28 | 0.69 |
Data are shown as mean (standard deviation). FSIQ, full-scale IQ score; VIQ, verbal IQ score; PIQ, performance IQ score; RT, reaction time (shown in ms).
fMRI Behavioral Performance (Table 1)
Mann–Whitney nonparametric analyses indicated that there was no significant difference between the control and TS groups in terms of accuracy on either math task. One-way ANOVA indicated no significant group differences in reaction time for either math task. Behavioral data did not record correctly for one of the controls and one subject with TS.
Within Group Analyses (Table 2)
Two-Operand
Controls demonstrated significant activation in the right supramarginal gyrus (SMG) extending into the angular gyrus (ANG), intraparietal sulcus (IPS) and superior parietal lobule (SPL); left fusiform gyrus; right putamen extending into the caudate; left SPL extending into the SMG, ANG and IPS; and left middle frontal gyrus (MFG) extending into the inferior frontal (IFG) and precentral gyri (Supplementary Fig. 1a).
Within-group results indicating significant brain activation during the math tasks
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Control | <0.0001 | 2936 | 3.77 | −44, 34, 20 | Left middle frontal gyrus extending into inferior frontal and precentral gyri |
| <0.0001 | 2581 | 4.30 | −24, −70, 46 | Left superior parietal lobule extending into supramarginal gyrus, angular gyrus and intraparietal sulcus | |
| <0.0001 | 2483 | 4.54 | 42, −50. 45 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 1017 | 4.34 | 16, 2, 7 | Right putamen extending into caudate | |
| 0.0001 | 949 | 4.36 | −46, −55, −14 | Left fusiform | |
| TS | <0.0001 | 6424 | 4.08 | 8, 27, 32 | Right superior frontal gyrus extending into anterior cingulate |
| <0.0001 | 3821 | 4.39 | −42, −44, 43 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 2593 | 3.68 | 42, 36, 20 | Bilateral middle and inferior frontal gyri extending into left pre- and postcentral gyri | |
| <0.0001 | 1853 | 4.10 | 24, −56, 36 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| 0.003 | 853 | 3.31 | −44, −72, −10 | Left middle occipital gyrus extending into fusiform | |
| Three-operand | |||||
| Control | <0.0001 | 15625 | 5.35 | −24, −68, 48 | Left superior parietal lobule extending into supramarginal and angular gyri, intraparietal sulcus, fusiform, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate |
| <0.0001 | 2534 | 4.21 | 28, −68, 46 | Right superior parietal lobule extending into supramarginal gyrus, angular gyrus, intraparietal sulcus, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate | |
| TS | <0.0001 | 1814 | 3.71 | −46, 5, 29 | Left precentral gyrus extending into middle and inferior frontal gyri |
| <0.0001 | 1452 | 3.51 | −22, −79, 45 | Left superior occipital gyrus extending into supramarginal gyrus, angular gyrus, intraparietal sulcus | |
| <0.0001 | 1163 | 3.72 | 38, −49, 36 | Right supramarginal and angular gyri | |
| 0.008 | 760 | 3.72 | −30, −47, −13 | Left fusiform | |
| 0.017 | 684 | 3.63 | 2, 20, 47 | Right superior frontal gyrus extending into anterior cingulate |
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Control | <0.0001 | 2936 | 3.77 | −44, 34, 20 | Left middle frontal gyrus extending into inferior frontal and precentral gyri |
| <0.0001 | 2581 | 4.30 | −24, −70, 46 | Left superior parietal lobule extending into supramarginal gyrus, angular gyrus and intraparietal sulcus | |
| <0.0001 | 2483 | 4.54 | 42, −50. 45 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 1017 | 4.34 | 16, 2, 7 | Right putamen extending into caudate | |
| 0.0001 | 949 | 4.36 | −46, −55, −14 | Left fusiform | |
| TS | <0.0001 | 6424 | 4.08 | 8, 27, 32 | Right superior frontal gyrus extending into anterior cingulate |
| <0.0001 | 3821 | 4.39 | −42, −44, 43 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 2593 | 3.68 | 42, 36, 20 | Bilateral middle and inferior frontal gyri extending into left pre- and postcentral gyri | |
| <0.0001 | 1853 | 4.10 | 24, −56, 36 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| 0.003 | 853 | 3.31 | −44, −72, −10 | Left middle occipital gyrus extending into fusiform | |
| Three-operand | |||||
| Control | <0.0001 | 15625 | 5.35 | −24, −68, 48 | Left superior parietal lobule extending into supramarginal and angular gyri, intraparietal sulcus, fusiform, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate |
| <0.0001 | 2534 | 4.21 | 28, −68, 46 | Right superior parietal lobule extending into supramarginal gyrus, angular gyrus, intraparietal sulcus, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate | |
| TS | <0.0001 | 1814 | 3.71 | −46, 5, 29 | Left precentral gyrus extending into middle and inferior frontal gyri |
| <0.0001 | 1452 | 3.51 | −22, −79, 45 | Left superior occipital gyrus extending into supramarginal gyrus, angular gyrus, intraparietal sulcus | |
| <0.0001 | 1163 | 3.72 | 38, −49, 36 | Right supramarginal and angular gyri | |
| 0.008 | 760 | 3.72 | −30, −47, −13 | Left fusiform | |
| 0.017 | 684 | 3.63 | 2, 20, 47 | Right superior frontal gyrus extending into anterior cingulate |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05).
Within-group results indicating significant brain activation during the math tasks
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Control | <0.0001 | 2936 | 3.77 | −44, 34, 20 | Left middle frontal gyrus extending into inferior frontal and precentral gyri |
| <0.0001 | 2581 | 4.30 | −24, −70, 46 | Left superior parietal lobule extending into supramarginal gyrus, angular gyrus and intraparietal sulcus | |
| <0.0001 | 2483 | 4.54 | 42, −50. 45 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 1017 | 4.34 | 16, 2, 7 | Right putamen extending into caudate | |
| 0.0001 | 949 | 4.36 | −46, −55, −14 | Left fusiform | |
| TS | <0.0001 | 6424 | 4.08 | 8, 27, 32 | Right superior frontal gyrus extending into anterior cingulate |
| <0.0001 | 3821 | 4.39 | −42, −44, 43 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 2593 | 3.68 | 42, 36, 20 | Bilateral middle and inferior frontal gyri extending into left pre- and postcentral gyri | |
| <0.0001 | 1853 | 4.10 | 24, −56, 36 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| 0.003 | 853 | 3.31 | −44, −72, −10 | Left middle occipital gyrus extending into fusiform | |
| Three-operand | |||||
| Control | <0.0001 | 15625 | 5.35 | −24, −68, 48 | Left superior parietal lobule extending into supramarginal and angular gyri, intraparietal sulcus, fusiform, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate |
| <0.0001 | 2534 | 4.21 | 28, −68, 46 | Right superior parietal lobule extending into supramarginal gyrus, angular gyrus, intraparietal sulcus, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate | |
| TS | <0.0001 | 1814 | 3.71 | −46, 5, 29 | Left precentral gyrus extending into middle and inferior frontal gyri |
| <0.0001 | 1452 | 3.51 | −22, −79, 45 | Left superior occipital gyrus extending into supramarginal gyrus, angular gyrus, intraparietal sulcus | |
| <0.0001 | 1163 | 3.72 | 38, −49, 36 | Right supramarginal and angular gyri | |
| 0.008 | 760 | 3.72 | −30, −47, −13 | Left fusiform | |
| 0.017 | 684 | 3.63 | 2, 20, 47 | Right superior frontal gyrus extending into anterior cingulate |
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Control | <0.0001 | 2936 | 3.77 | −44, 34, 20 | Left middle frontal gyrus extending into inferior frontal and precentral gyri |
| <0.0001 | 2581 | 4.30 | −24, −70, 46 | Left superior parietal lobule extending into supramarginal gyrus, angular gyrus and intraparietal sulcus | |
| <0.0001 | 2483 | 4.54 | 42, −50. 45 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 1017 | 4.34 | 16, 2, 7 | Right putamen extending into caudate | |
| 0.0001 | 949 | 4.36 | −46, −55, −14 | Left fusiform | |
| TS | <0.0001 | 6424 | 4.08 | 8, 27, 32 | Right superior frontal gyrus extending into anterior cingulate |
| <0.0001 | 3821 | 4.39 | −42, −44, 43 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| <0.0001 | 2593 | 3.68 | 42, 36, 20 | Bilateral middle and inferior frontal gyri extending into left pre- and postcentral gyri | |
| <0.0001 | 1853 | 4.10 | 24, −56, 36 | Right supramarginal gyrus extending into angular gyrus, intraparietal sulcus and superior parietal lobule | |
| 0.003 | 853 | 3.31 | −44, −72, −10 | Left middle occipital gyrus extending into fusiform | |
| Three-operand | |||||
| Control | <0.0001 | 15625 | 5.35 | −24, −68, 48 | Left superior parietal lobule extending into supramarginal and angular gyri, intraparietal sulcus, fusiform, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate |
| <0.0001 | 2534 | 4.21 | 28, −68, 46 | Right superior parietal lobule extending into supramarginal gyrus, angular gyrus, intraparietal sulcus, cuneus, middle temporal gyrus inferior, middle and superior frontal gyri, precentral gyrus, putamen and caudate | |
| TS | <0.0001 | 1814 | 3.71 | −46, 5, 29 | Left precentral gyrus extending into middle and inferior frontal gyri |
| <0.0001 | 1452 | 3.51 | −22, −79, 45 | Left superior occipital gyrus extending into supramarginal gyrus, angular gyrus, intraparietal sulcus | |
| <0.0001 | 1163 | 3.72 | 38, −49, 36 | Right supramarginal and angular gyri | |
| 0.008 | 760 | 3.72 | −30, −47, −13 | Left fusiform | |
| 0.017 | 684 | 3.63 | 2, 20, 47 | Right superior frontal gyrus extending into anterior cingulate |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05).
The TS group demonstrated areas of significant activation similar to controls in bilateral SMG, ANG, IPS and SPL, as well as right superior frontal gyrus (SFG) extending into the anterior cingulate; bilateral MFG extending into the inferior frontal gyrus (IFG), pre- and post-central gyri; and left middle occipital gyrus extending into the fusiform (Supplementary Fig. 2a).
Three-Operand
Significant activation in the control group was found in the bilateral (although greater on the left) SPL, SMG, ANG and IPS, cuneus, left fusiform, bilateral MFG, SFG, IFG, precentral gyrus, posterior middle temporal gyrus (MTG), putamen and caudate (Supplementary Fig. 1b).
Although to a lesser extent, the TS group showed similar areas significant activation to controls in the right SMG, ANG, IPS, left fusiform; left superior occipital gyrus extending into left SMG, ANG and IPS; and right SFG extending into the anterior cingulate; and left precentral gyrus extending into the MFG and IFG (Supplementary Fig. 2b).
Between Group Analyses (Table 3)
Two-Operand
Control Group minus TS Group.
There were no areas of significantly greater activation for controls compared with the TS group.
Between-group results indicating significant brain activation during the math tasks
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Con–TS | NS | ||||
| TS–Con | <0.0001 | 1534 | 3.77 | −6, 0, 44 | Anterior cingulate |
| <0.0001 | 1225 | 3.86 | −42, −10, 35 | Left precentral gyrus extending into middle frontal, postcentral and supramarginal gyri | |
| 0.014 | 678 | 3.22 | −46, −4, −7 | Left superior temporal gyrus extending into middle temporal and inferior frontal gyri | |
| Three-operand | |||||
| Con–TS | <0.0001 | 4759 | 3.90 | −44, −39, 26 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus, superior parietal lobule, fusiform and posterior middle temporal gyrus |
| <0.0001 | 2905 | 4.25 | −6, 35, 6 | Anterior cingulate extending into medial bilateral superior frontal gyrus | |
| 0.001 | 914 | 3.44 | 44, 35, 33 | Right middle frontal gyrus extending into inferior frontal gyrus | |
| 0.026 | 575 | 3.31 | −42, 10, 36 | Left inferior frontal gyrus Extending into middle frontal gyrus | |
| 0.032 | 553 | 3.98 | 6, −73, 18 | Cuneus | |
| TS–Con | NS |
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Con–TS | NS | ||||
| TS–Con | <0.0001 | 1534 | 3.77 | −6, 0, 44 | Anterior cingulate |
| <0.0001 | 1225 | 3.86 | −42, −10, 35 | Left precentral gyrus extending into middle frontal, postcentral and supramarginal gyri | |
| 0.014 | 678 | 3.22 | −46, −4, −7 | Left superior temporal gyrus extending into middle temporal and inferior frontal gyri | |
| Three-operand | |||||
| Con–TS | <0.0001 | 4759 | 3.90 | −44, −39, 26 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus, superior parietal lobule, fusiform and posterior middle temporal gyrus |
| <0.0001 | 2905 | 4.25 | −6, 35, 6 | Anterior cingulate extending into medial bilateral superior frontal gyrus | |
| 0.001 | 914 | 3.44 | 44, 35, 33 | Right middle frontal gyrus extending into inferior frontal gyrus | |
| 0.026 | 575 | 3.31 | −42, 10, 36 | Left inferior frontal gyrus Extending into middle frontal gyrus | |
| 0.032 | 553 | 3.98 | 6, −73, 18 | Cuneus | |
| TS–Con | NS |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05). NS, not significant.
Between-group results indicating significant brain activation during the math tasks
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Con–TS | NS | ||||
| TS–Con | <0.0001 | 1534 | 3.77 | −6, 0, 44 | Anterior cingulate |
| <0.0001 | 1225 | 3.86 | −42, −10, 35 | Left precentral gyrus extending into middle frontal, postcentral and supramarginal gyri | |
| 0.014 | 678 | 3.22 | −46, −4, −7 | Left superior temporal gyrus extending into middle temporal and inferior frontal gyri | |
| Three-operand | |||||
| Con–TS | <0.0001 | 4759 | 3.90 | −44, −39, 26 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus, superior parietal lobule, fusiform and posterior middle temporal gyrus |
| <0.0001 | 2905 | 4.25 | −6, 35, 6 | Anterior cingulate extending into medial bilateral superior frontal gyrus | |
| 0.001 | 914 | 3.44 | 44, 35, 33 | Right middle frontal gyrus extending into inferior frontal gyrus | |
| 0.026 | 575 | 3.31 | −42, 10, 36 | Left inferior frontal gyrus Extending into middle frontal gyrus | |
| 0.032 | 553 | 3.98 | 6, −73, 18 | Cuneus | |
| TS–Con | NS |
| Task . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| Two-operand | |||||
| Con–TS | NS | ||||
| TS–Con | <0.0001 | 1534 | 3.77 | −6, 0, 44 | Anterior cingulate |
| <0.0001 | 1225 | 3.86 | −42, −10, 35 | Left precentral gyrus extending into middle frontal, postcentral and supramarginal gyri | |
| 0.014 | 678 | 3.22 | −46, −4, −7 | Left superior temporal gyrus extending into middle temporal and inferior frontal gyri | |
| Three-operand | |||||
| Con–TS | <0.0001 | 4759 | 3.90 | −44, −39, 26 | Left supramarginal gyrus extending into angular gyrus, intraparietal sulcus, superior parietal lobule, fusiform and posterior middle temporal gyrus |
| <0.0001 | 2905 | 4.25 | −6, 35, 6 | Anterior cingulate extending into medial bilateral superior frontal gyrus | |
| 0.001 | 914 | 3.44 | 44, 35, 33 | Right middle frontal gyrus extending into inferior frontal gyrus | |
| 0.026 | 575 | 3.31 | −42, 10, 36 | Left inferior frontal gyrus Extending into middle frontal gyrus | |
| 0.032 | 553 | 3.98 | 6, −73, 18 | Cuneus | |
| TS–Con | NS |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05). NS, not significant.
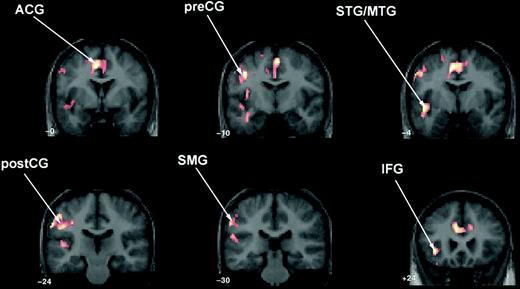
TS Group minus Control Group (Fig. 1).
The TS groups showed greater activation than controls in the anterior cingulate; left precentral gyrus extending into the MFG, postcentral gyrus and SMG; and left superior temporal gyrus (STG) extending into MTG and IFG.
Two-operand math task: between-group contrast demonstrating significantly greater activation in the TS group compared with controls in anterior cingulate (ACG); left precentral gyrus (preCG) extending into postcentral (postCG) and supramarginal gyri (SMG); left superior temporal gyrus (STG) extending into middle temporal (MTG) and inferior frontal gyri (IFG). The control minus TS contrast for two-operand math was not significant.
Three-Operand
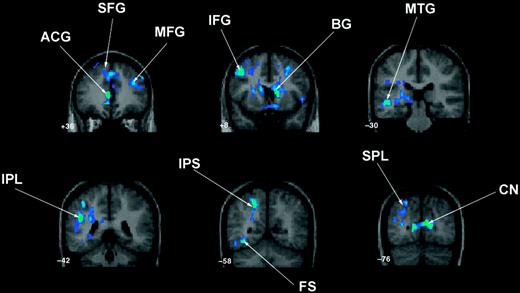
Control Group minus TS Group (Fig. 2).
Controls showed significantly greater activation compared with the TS group in the left SMG, ANG, IPS, SPL, fusiform and posterior MTG, anterior cingulate extending into medial bilateral SFG, bilateral MFG and IFG, caudate and cuneus.
Three-operand math: between-group contrast demonstrating significantly greater activation in controls compared with the TS group in left inferior parietal lobe (IPL) extending into intraparietal sulcus (IPS), superior parietal lobule (SPL), fusiform (FS) and posterior middle temporal gyrus (MTG); anterior cingulate (ACG) extending into bilateral medial superior frontal gyrus (SFG); bilateral middle (MFG) and inferior frontal (IFG) gyri; cuneus (CN) and basal ganglia (BG). (b) Surface renderings of these results. The TS minus control contrast for three-operand math was not significant.
TS Group Minus Control Group.
The TS group demonstrated no areas of significantly greater activation compared with controls.
All of the above between-group results remained unchanged, even after IQ was used as a covariate in the analyses.
Discussion
In the present study we report functional brain activation differences between girls with TS) and age-matched typically developing controls while performing an arithmetic task. Both groups demonstrated significant activation in expected parietal and frontal regions associated with arithmetic processing. Several studies illustrate the importance of a fronto-parietal network in arithmetic processing. Superior and middle frontal gyri are involved in the executive processes underlying mental calculation, particularly working memory (Zago et al., 2001). The inferior frontal gyrus is known to be involved in working memory as well as linguistic processing aspects of arithmetic function (Gruber et al., 2001; Zago et al., 2001; Delazer et al., 2003). The prefrontal cortex is additionally necessary for speed of processing in arithmetic tasks (Menon et al., 2000b). Activation in premotor cortex has been previously observed during arithmetic tasks and is likely associated with motor response preparation (Menon et al., 2000b).
Both groups additionally demonstrated significant activation in the inferior parietal lobe, including the supramarginal and angular gyri as well as the intraparietal sulcus, regions known to be essential for arithmetic processing (Zago and Tzourio-Mazoyer, 2002; Dehaene et al., 2003). Dissociating the specific mathematical roles of these regions is difficult. Whereas the intraparietal sulcus may be more involved in approximate calculation and subtraction, the angular gyrus is potentially more active in exact calculation and multiplication (Dehaene et al., 1999; Stanescu-Cosson et al., 2000). Additionally, Dehaene et al. (2003) have suggested that the angular gyrus is involved in verbal requirements of arithmetic tasks. Furthermore, activation in the angular gyrus has been shown to be related to arithmetic proficiency (Delazer et al., 2003; Menon et al., 2000a). The supramarginal gyrus appears to serve working memory aspects of mathematical reasoning, possibly by storing intermediate results of complex calculations (Zago and Tzourio-Mazoyer, 2002).
Although both groups activated a similar pattern of brain regions associated with arithmetic function, they differed in terms of their neural and behavioral responses to increased task demands. Between-group contrasts indicated that the TS group demonstrated similar areas of frontal and parietal activation to controls during the two-operand math task but to a significantly greater degree. These regions included left dorsolateral and ventrolateral prefrontal cortex, supramarginal gyrus and premotor cortex.
Individuals with TS also showed significantly greater activation in the anterior cingulate compared with controls during the two-operand math task. This is in contrast to Molko and colleagues's previous study of arithmetic processing in TS that demonstrated decreased anterior cingulate activation (Molko et al., 2003). This difference in findings likely reflects differences in sample characteristics (age, presence of chromosomal mosaicism) and math task paradigm. The role of the anterior cingulate in arithmetic processing is not well understood. The anterior cingulate may be involved in working memory (Kondo et al., 2004) and/or other more specific aspects of arithmetic processing (Chochon et al., 1999; Menon et al., 2002). Additionally, the anterior cingulate is believed to serve several other cognitive functions including response inhibition, task management, attention, performance monitoring and mental effort, among others (Peterson et al., 1999; MacDonald et al., 2000; Allman et al., 2001; Barch et al., 2001). However, processes such as performance monitoring and response inhibition occurring in the experimental condition would be balanced by the control conditions. Activation of the anterior cingulate due to increased mental effort has been shown to occur primarily in less efficient performers while more skilled performers tend to show reduced anterior cingulate activation (Raichle et al., 1994). This is consistent with the present findings of increased activation in this area in the TS group compared with controls.
The TS group additionally showed significantly greater activation than controls in the temporal lobe during the two-operand math task. However, these areas were not activated in the TS within-group two-operand contrast and therefore, possible deactivation differences were examined post hoc by subtracting the two-operand control task from the experimental task in the control group. This contrast demonstrated activation in the same temporal lobe areas, suggesting that these differences may actually represent deactivation deficits in the TS group. Deactivation in superior and middle temporal gyri tends to occur in association with visually presented stimuli (Laurienti et al., 2002) and therefore these findings may suggest that temporal lobe activation was not appropriately inhibited in the TS group and/or that they attempted to rely more heavily on verbal strategies to solve the arithmetic problems. Moreover, we previously demonstrated morphological abnormalities in the superior temporal gyrus associated with TS (Kesler et al., 2003). Further investigation is necessary to elucidate the relationship between inhibitory processes and arithmetic function in TS.
The TS group engaged brain regions similar to controls during the three-operand task, but to a significantly lesser extent, even after accounting for IQ differences. Controls showed significantly greater activation than the TS group in dorsolateral and ventrolateral prefrontal and inferior parietal regions during the three-operand task. Compared with TS, controls also showed greater activation of the left fusiform during the three-operand math task. The fusiform's role in arithmetic processing is believed to include numbers perceptual processing and storage of configural visual representations, and has previously been shown to have increased activation with increased number of operands (Rickard et al., 2000; Zago et al., 2001). Additionally, fusiform activation is mediated by increased level of expertise in a variety of tasks, including mental calculation (Tarr and Gauthier, 2000; Hanakawa et al., 2003; McCandliss et al., 2003).
Both groups recruited posterior visuo-spatial networks during both math tasks in the superior parietal lobule; however, controls activated this area to a significantly greater extent than individuals with TS. The superior parietal lobe appears to be involved in approximate versus exact calculation (Dehaene et al., 1999) and some researchers have postulated that it is responsible for attention orientation on a mental number line where numbers are spatially organized based on their proximity (Dehaene et al., 2003). Alternatively, the visual association cortex, activated by the TS group, has no known direct role in arithmetic processing role.
Controls also showed increased activation in the basal ganglia compared with the TS group during the three-operand task. The controls demonstrated significant putamen and caudate activation during both arithmetic tasks, based on within-group analyses, whereas the TS group did not. A previous study of arithmetic processing in typically developing individuals by our laboratory indicated recruitment of caudate activation in response to increasing task difficulty (Menon et al., 2000b). Many studies of arithmetic processing have not shown significant caudate activation, and individuals with disease processes affecting caudate integrity, such as Parkinson's and Huntington's diseases, do not tend to demonstrate significant arithmetic deficits (Rickard et al., 2000). However, a recent case study of a patient with Fahr's disease, which results in calcification of the basal ganglia, suggested a relationship between basal ganglia and number processing, particularly involving cortico-subcortical connections responsible for executive functions such as problem solving, mental flexibility and organization. Additionally, the caudate is known to be important in spatial working memory (Postle and D'Esposito, 1999). Therefore, the role of the caudate in arithmetic processing may involve indirect functions of executive control and spatial working memory.
The reasons for the decreased profile of brain activation in the TS group during the three-operand task despite relatively intact task performance are unclear. Post hoc analyses comparing differences in brain activation between the two- and three-operand tasks both within and between groups (see Tables 4 and 5 and Supplementary Figs 3 and 4) indicate that the TS group did show significantly more fronto-parietal activation in the two-operand versus the three-operand task and that this difference was significantly greater than that of controls. Additionally, the TS group showed no significant activation in the three-operand minus two-operand contrast at the 0.05 level. They did demonstrate significant frontal-striatal and temporal lobe activation in this contrast at the 0.1 level. These findings might argue for decreased effort during the three-operand task.
Within-group results for post hoc task comparisons
| Contrast . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Two-operand–three-operand | |||||||||
| Control | 00.013 | 596 | 3.31 | −34, −4, 0 | Left putamen | ||||
| 00.006 | 665 | 3.52 | 24, 23, −8 | Right inferior frontal gyrus | |||||
| 00.000 | 1266 | 3.94 | 6, −10, 32 | Cingulate | |||||
| TS | 0.012 | 591 | 3.48 | −40, −1, 20 | Left precentral gyrus | ||||
| <0.0001 | 1398 | 3.72 | 4, −26, 33 | Parietal cingulate, left pre- and post-central gyri, bilateral superior frontal gyrus, left inferior parietal lobe | |||||
| Three-operand–two-operand | |||||||||
| Control | <0.0001 | 2191 | 3.96 | 6, 57, 12 | Bilateral superior and middle frontal gyri, medial frontal gyrus | ||||
| TS | NS | ||||||||
| Contrast . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Two-operand–three-operand | |||||||||
| Control | 00.013 | 596 | 3.31 | −34, −4, 0 | Left putamen | ||||
| 00.006 | 665 | 3.52 | 24, 23, −8 | Right inferior frontal gyrus | |||||
| 00.000 | 1266 | 3.94 | 6, −10, 32 | Cingulate | |||||
| TS | 0.012 | 591 | 3.48 | −40, −1, 20 | Left precentral gyrus | ||||
| <0.0001 | 1398 | 3.72 | 4, −26, 33 | Parietal cingulate, left pre- and post-central gyri, bilateral superior frontal gyrus, left inferior parietal lobe | |||||
| Three-operand–two-operand | |||||||||
| Control | <0.0001 | 2191 | 3.96 | 6, 57, 12 | Bilateral superior and middle frontal gyri, medial frontal gyrus | ||||
| TS | NS | ||||||||
Thresholds: height (Z > 1.67; P <.05) and extent (P <.05). NS, not significant.
Within-group results for post hoc task comparisons
| Contrast . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Two-operand–three-operand | |||||||||
| Control | 00.013 | 596 | 3.31 | −34, −4, 0 | Left putamen | ||||
| 00.006 | 665 | 3.52 | 24, 23, −8 | Right inferior frontal gyrus | |||||
| 00.000 | 1266 | 3.94 | 6, −10, 32 | Cingulate | |||||
| TS | 0.012 | 591 | 3.48 | −40, −1, 20 | Left precentral gyrus | ||||
| <0.0001 | 1398 | 3.72 | 4, −26, 33 | Parietal cingulate, left pre- and post-central gyri, bilateral superior frontal gyrus, left inferior parietal lobe | |||||
| Three-operand–two-operand | |||||||||
| Control | <0.0001 | 2191 | 3.96 | 6, 57, 12 | Bilateral superior and middle frontal gyri, medial frontal gyrus | ||||
| TS | NS | ||||||||
| Contrast . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Two-operand–three-operand | |||||||||
| Control | 00.013 | 596 | 3.31 | −34, −4, 0 | Left putamen | ||||
| 00.006 | 665 | 3.52 | 24, 23, −8 | Right inferior frontal gyrus | |||||
| 00.000 | 1266 | 3.94 | 6, −10, 32 | Cingulate | |||||
| TS | 0.012 | 591 | 3.48 | −40, −1, 20 | Left precentral gyrus | ||||
| <0.0001 | 1398 | 3.72 | 4, −26, 33 | Parietal cingulate, left pre- and post-central gyri, bilateral superior frontal gyrus, left inferior parietal lobe | |||||
| Three-operand–two-operand | |||||||||
| Control | <0.0001 | 2191 | 3.96 | 6, 57, 12 | Bilateral superior and middle frontal gyri, medial frontal gyrus | ||||
| TS | NS | ||||||||
Thresholds: height (Z > 1.67; P <.05) and extent (P <.05). NS, not significant.
Between-group results for post hoc task comparisons
| Contrasta . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| TS–Con (2op–3op) | 0.004 | 750 | 3.32 | 28, −9, 52 | Right precentral gyrus |
| Con–TS (3op–2op) | <0.0001 | 2292 | 3.90 | −12, 27, 30 | Cingulate, right medial superior frontal gyrus, bilateral inferior and middle frontal gyri |
| TS–Con (3op–2op) | <0.0001 | 1211 | 3.83 | 26, 26, −8 | Right inferior frontal gyrus, putamen, caudate, right superior temporal gyrus |
| Con–TS (2op–3op) |
| Contrasta . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| TS–Con (2op–3op) | 0.004 | 750 | 3.32 | 28, −9, 52 | Right precentral gyrus |
| Con–TS (3op–2op) | <0.0001 | 2292 | 3.90 | −12, 27, 30 | Cingulate, right medial superior frontal gyrus, bilateral inferior and middle frontal gyri |
| TS–Con (3op–2op) | <0.0001 | 1211 | 3.83 | 26, 26, −8 | Right inferior frontal gyrus, putamen, caudate, right superior temporal gyrus |
| Con–TS (2op–3op) |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05). 2op, two-operand; 3op, three-operand.
Due to double subtraction in the between-group contrasts, these pairs of contrasts are identical.
Between-group results for post hoc task comparisons
| Contrasta . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| TS–Con (2op–3op) | 0.004 | 750 | 3.32 | 28, −9, 52 | Right precentral gyrus |
| Con–TS (3op–2op) | <0.0001 | 2292 | 3.90 | −12, 27, 30 | Cingulate, right medial superior frontal gyrus, bilateral inferior and middle frontal gyri |
| TS–Con (3op–2op) | <0.0001 | 1211 | 3.83 | 26, 26, −8 | Right inferior frontal gyrus, putamen, caudate, right superior temporal gyrus |
| Con–TS (2op–3op) |
| Contrasta . | P-value (corrected) . | No. of voxels . | Z-score . | Peak location (Talairach coordinates) . | Activation description . |
|---|---|---|---|---|---|
| TS–Con (2op–3op) | 0.004 | 750 | 3.32 | 28, −9, 52 | Right precentral gyrus |
| Con–TS (3op–2op) | <0.0001 | 2292 | 3.90 | −12, 27, 30 | Cingulate, right medial superior frontal gyrus, bilateral inferior and middle frontal gyri |
| TS–Con (3op–2op) | <0.0001 | 1211 | 3.83 | 26, 26, −8 | Right inferior frontal gyrus, putamen, caudate, right superior temporal gyrus |
| Con–TS (2op–3op) |
Thresholds: height (Z > 1.67; P < 0.05) and extent (P < 0.05). 2op, two-operand; 3op, three-operand.
Due to double subtraction in the between-group contrasts, these pairs of contrasts are identical.
However, previous studies of neurofunction in TS demonstrate that decreased activation may not necessarily mean that individuals with TS did not engage readily in the task. Specifically, during a spatial orientation task, individuals with TS demonstrated instances of lower activation but comparable performance as well as greater activation and lower performance compared with controls (Kesler et al., 2004). During a visual working memory task, subjects with TS showed greater activation associated with lower performance as well as lower activation associated with lower performance (Haberecht et al., 2001). Furthermore, previous studies of typically developing individuals indicate that decreased activation actually corresponds to more efficient performance in certain situations (Tomasi et al., 2004). Therefore, increased or decreased activation does not always have a straightforward interpretation.
Based on the post hoc analyses, controls relied more heavily on working memory and attention regions (IFG, basal ganglia) in the two-operand task compared with the three-operand task, rather than on parietal regions. This is interesting given that previous research has suggested that increased proficiency in math tasks causes increased reliance on parietal areas because the response becomes more automatic (Delazer et al., 2003). Thus, while easier than the three-operand task, the two-operand task was not necessarily automatic for the control subjects. Controls then recruited additional dorsolateral (MFG)/dorsomedial (SFG) frontal regions in the three-operand versus two-operand task. The three-operand task did not increase working memory or attention load for controls, but increased reliance on regions responsible for strategies and algorithms.
The profile of differences in brain activation between the two-operand and three-operand tasks for the TS group suggests a less efficient approach to the task compared with controls. Compared with the three-operand task, the TS group engaged a host of systems during the two-operand task, including those underlying executive strategies and algorithms (SFG), approximate calculation (angular gyrus) and the more primitive techniques of subvocalization and finger counting (pre/postcentral gyri). The pre- and postcentral activations demonstrated by the TS group correspond to somatotopic regions responsible for lips, mouth, fingers and hand and thus have been suggested to reflect the use of arithmetic strategies such as subvocalization and finger counting (Delazer et al., 2003).
The controls responded to the increased demand of the three-operand task by increasing activation in regions supporting higher order (executive) cognitive processes. This likely reflects an increase in effort compared with the two-operand task. However, the TS group appeared to change strategies in response to the more difficult task, engaging working memory (IFG) and attention (caudate) systems with the addition of temporal regions responsible for language processing (MTG, STG). Individuals with TS may demonstrate superior language abilities (Temple, 2002) and therefore may approach difficult arithmetic tasks by relying on these strengths.
Arithmetic processing results from a distributed network of functions and thus may involve a profile of impaired and preserved arithmetic abilities in certain populations. This study suggests more diffuse neurofunctional deficits associated with mathematical impairment in TS than previous studies. It also suggests that, compared with controls, individuals with TS demonstrate different activation profiles involving both anterior and posterior systems known to subserve a variety of abilities involved in arithmetic function including working memory, linguistic skills, processing speed, quantity manipulation, expertise and visuo-spatial ability. The math paradigm used here isolated calculation, executive function and working memory. Continued studies with more sophisticated arithmetic task design are required to determine if there are disproportional deficits in any of these arithmetic-related abilities.
This work was supported by NIH grants MH01142, HD31715, HD40761, HD47520 and MH50047.
References
Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (
Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (
Brown WE, Kesler SK, Eliez S, Warsofsky IS, Haberecht MF, Patwardhan A, Ross JL, Neely EK, Zing S, Yankowitz J, Reiss AL (
Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht MF, Reiss AL (
Bruandet M, Molko N, Cohen L, Dehaene S (
Buchanan L, Pavlovic J, Rovet J (
Chochon F, Cohen L, van de Moortele PF, Dehaene S (
Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S (
Dehaene S, Piazza M, Pinel P, Cohen L (
Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T (
Friston KJ (
Glover GH, Lai S (
Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A (
Haberecht MF, Menon V, Warsofsky IS, White CD, Dyer-Friedman J, Glover GH, Neely EK, Reiss AL (
Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H (
Holmes AP, Friston KJ (
Jacobs PA, Betts PR, Cockwell AE, Crolla JA, Mackenzie MJ, Robinson DO, Youings SA (
Jacobs P, Dalton P, James R, Mosse K, Power M, Robinson D, Skuse D (
Jones KL (
Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL (
Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, Reiss AL (
Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H (
Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE (
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (
Mazzocco MM (
McCandliss BD, Cohen L, Dehaene S (
Menon V, Rivera SM, White CD, Eliez S, Glover GH, Reiss AL (
Menon V, Rivera SM, White CD, Glover GH, Reiss AL (
Menon V, Mackenzie K, Rivera SM, Reiss AL (
Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S (
Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI, Schapiro MB (
Murphy DG, Mentis MJ, Pietrini P, Grady C, Daly E, Haxby JV, De La Granja M, Allen G, Largay K, White BJ, Powell CM, Horwitz B, Rapoport SI, Schapiro MB (
Nijhuis-van der Sanden MW, Eling PA, Otten BJ (
Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (
Poline JB, Worsley KJ, Evans AC, Friston KJ (
Postle BR, D'Esposito M (
Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE (
Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL (
Rickard TC, Romero SG, Basso G, Wharton C, Flitman S, Grafman J (
Rivera SM, Menon V, White CD, Glaser B, Reiss AL (
Romans SM, Roeltgen DP, Kushner H, Ross JL (
Ross JL, Stefanatos GA, Kushner H, Zinn A, Bondy C, Roeltgen D (
Rovet J, Szekely C, Hockenberry MN (
Stanescu-Cosson R, Pinel P, van De Moortele PF, Le Bihan D, Cohen L, Dehaene S (
Talairach J, Tournoux P (
Tamm L, Menon V, Reiss AL (
Tarr MJ, Gauthier I (
Temple CM (
Temple CM, Marriott AJ (
Temple CM, Sherwood S (
Tomasi D, Ernst T, Caparelli EC, Chang L (
Wechsler D (
Wechsler D (
Wiktor A, Van Dyke DL (
Zago L, Tzourio-Mazoyer N (
Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio-Mazoyer N (
Zinn A, Page DC, Fisher EMC (