-
PDF
- Split View
-
Views
-
Cite
Cite
Catherine M. Papadopoulos, Shih-Yen Tsai, Joseph L. Cheatwood, Melanie R. Bollnow, Bryan E. Kolb, Martin E. Schwab, Gwendolyn L. Kartje, Dendritic Plasticity in the Adult Rat Following Middle Cerebral Artery Occlusion and Nogo-A Neutralization, Cerebral Cortex, Volume 16, Issue 4, April 2006, Pages 529–536, https://doi.org/10.1093/cercor/bhi132
Close - Share Icon Share
Abstract
Our work has shown that following focal ischemic lesion in adult rats, neutralization of the axon growth inhibitor Nogo-A with the monoclonal antibody (mAb) IN-1 results in functional recovery. Furthermore, new axonal connections were formed from the contralesional cortex to subcortical areas corresponding to the observed functional recovery. The present study investigated whether dendritic changes, also known to subserve functional recovery, paralleled the axonal plasticity shown after ischemic lesion and treatment with mAb IN-1. Golgi–Cox-stained layer V pyramidal neurons in the contralesional sensorimotor cortex were examined for evidence of dendritic sprouting. Results demonstrated increased dendritic arborization and spine density in the mAb IN-1-treated animals with lesion. Interestingly, administration of mAb IN-1 without lesion resulted in transient dendritic outgrowth with no change in spine density. These results suggest a novel role for Nogo-A in limiting dendritic plasticity after stroke.
Introduction
Ischemic stroke, the lack of blood flow to the brain, is the third most common cause of death in developed countries. One-third of the 4.6 million stroke survivors in the USA are left permanently disabled, thus representing the leading cause of serious long-term neurological disability (American Heart Association, 2004). Neuronal loss due to stroke may result in chronic impairment of sensory and motor function that is resistant to therapeutic strategies.
Incomplete functional recovery after injury to the adult central nervous system (CNS) is a result of the inability of central neurons to form correct axonal and dendritic connections through regenerative or plastic responses. The failure of adult neurons to establish new connections after CNS lesions is most likely not an intrinsic deficit in the growth potential of the central neuron (Richardson et al., 1980; David and Aguayo, 1981), but rather an effect of the non-permissive adult environment. A major contributor to the presence of growth-inhibitory proteins that creates a non-permissive environment and limits the structural plasticity of injured mature neurons is CNS myelin (Schwab and Caroni, 1988; Qui et al., 2000). Several myelin-associated proteins and chondroitin-sulfate proteoglycans (CSPGs) have been characterized as inhibitors of axonal growth: oligodendrocyte myelin glycoprotein (OMgp) (Wang et al., 2002), myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994), Nogo-A (Spillman et al., 1997; Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000), and the CSPGs Versican V2 (Schweigreiter et al., 2004) and Brevican (Schmalfeldt et al., 2000). Nogo-A is expressed in oligodendrocytes and myelin, and in certain neuronal populations (Caroni and Schwab, 1988a; Chen et al., 2000; Huber et al., 2002; Buss et al., 2004; Dodd et al., 2005; this study). Based on several regeneration and plasticity studies, Nogo-A has proven to be a powerful inhibitor of axonal growth (for review, see Schwab, 2004). Regeneration studies have supported this inhibitory role by neutralization of Nogo-A with the specific antigen, monoclonal antibody (mAb) IN-1 (Fiedler et al., 2002) with in vitro growth assays (Caroni and Schwab, 1988b) and in vivo spinal cord injury studies (Schnell and Schwab, 1990; Bregman et al., 1995). Important to the present study, intraventricular administration of mAb IN-1 resulted in axonal plasticity of intact fiber tracts after cortical aspiration lesion (Kartje et al., 1999; Wenk et al., 1999) and improved functional recovery of skilled movements after unilateral pyramidotomy (Thallmair et al., 1998; Z'Graggen et al., 1998), cortical aspiration lesion (Emerick and Kartje, 2004) and permanent middle cerebral artery occlusion (MCAO) (Papadopoulos et al., 2002; Seymour et al., 2005) in adult rats.
Clinical studies have implicated the contralesional cortex as an important contributor to motor recovery after stroke (Cramer et al., 1997; Cuadrado et al., 1999). Functional imaging studies demonstrated increased cerebral blood flow (Weiller et al., 1993) as well as altered patterns of activation (Feydy et al., 2002; Johansen-Berg et al., 2002) in the intact cerebral hemisphere of human patients recovering from unilateral stroke that paralleled functional recovery. Our past work has demonstrated the critical role in functional recovery for neuronal (axonal) plasticity originating from the contralesional hemisphere to re-innervate deafferented areas after cortical lesions and treatment with mAb IN-1 (Wenk et al., 1999; Kartje et al., 1999; Papadopoulos et al., 2002). Also, using intracortical microstimulation mapping techniques, our laboratory has shown that reorganization in the contralesional forelimb motor cortex parallels functional recovery after aspiration lesion and treatment with mAb IN-1 (Emerick et al., 2003). Since dendritic plasticity is also associated with functional recovery after cortical lesions (Jones and Schallert, 1994; Kawamata et al., 1997b; Rowntree and Kolb, 1997; Biernaskie et al., 2004), we questioned whether neuronal (dendritic) changes paralleled the axonal plasticity shown after stroke and treatment with mAb IN-1. Therefore, we analyzed the dendritic arbors of layer V pyramidal neurons within the forelimb motor cortex of the contralesional hemisphere using a modified Golgi–Cox staining procedure. Our results demonstrate a dramatic increase in both the apical and basilar dendritic arborization and spine density of pyramidal neurons in the stroke/mAb IN-1-treated group compared with controls. These results point to a role for Nogo-A in restricting dendritic plasticity after stroke.
Materials and Methods
Animals and In Vivo Manipulations
Adult male Long Evans, black-hooded rats (250–360 g) were divided into eight experimental groups: (i) MCAO only, six-week survival (n = 6); (ii) MCAO plus mAb IN-1, six-week survival (n = 6); (iii) MCAO plus control antibody treatment, six-week survival (n = 5); (iv) mAb IN-1 only, two-week survival (n = 5); (v) mAb IN-1 only, six-week survival (n = 3); (vi) control Ab only, two-week survival (n = 4); (vii) control Ab only, six-week survival (n = 3); and (viii) normal animals (n = 5). Animals were number-coded and investigators were blinded to the treatment groups. Experiments were approved by the Institutional Animal Care and Use Committees of Loyola University/Hines VA.
MCAO was performed as described in Chen et al. (1986). Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.P.). Bilateral common carotid arteries (CCA) were isolated, a vertical 2 cm long incision was made between the eye and ear, and the temporalis muscle was retracted. A burr hole was made to expose the left MCA and it was permanently occluded with a 10-0 suture. The CCA ipsilateral to the MCAO was permanently occluded with a 4-0 suture and the contralateral CCA was temporarily occluded for 45 min. Body temperature was maintained with a heating pad and monitored with a rectal probe. The wounds were then closed and animals warmed under a heating lamp until awake.
Antibody application was performed immediately after MCAO as in our previous study (Papadopoulos et al., 2002). A cell suspension (6 μl) containing a total of 1 × 105 of either mAb IN-1 (Caroni and Schwab, 1988b) or the control antibody (anti-HRP) (Schnell and Schwab, 1990) secreting mouse hybridoma cells was injected posterior to the lesion (4 mm caudal, 5 mm lateral and 5 mm ventral to bregma) on the side of the lesion. MCAO only animals received vehicle only injections in the same coordinates. All animals, including those in the MCAO only group, received cyclosporin A (10 mg/kg, i.P.) daily starting from the day before surgery to 7 days after surgery to prevent rejection of the hybridoma xenograft.
Histology
Either 2 or 6 weeks after MCAO and/or antibody treatments, animals were overdosed with sodium pentobarbital (100 mg/kg, i.P.) and transcardially perfused with 0.9% saline. Brains were removed and immersed whole in Golgi–Cox solution (Glaser and van der Loos, 1981) for 14 days and then placed in 30% sucrose solution for 2 days. Brains were cut into 200 μm coronal sections using a vibratome, slide mounted, and reacted according to the procedure described by Gibb and Kolb (1998).
Stroke Volume Analysis
The stroke volume was quantitatively analyzed on Golgi–Cox stained sections (+4.7 to −5.2 mm from bregma according to Paxinos and Watson, 1998) using a computer-interfaced imaging system (NIH Image 1.51) by the method as described in Kawamata et al. (1997a) (total area of the intact contralateral hemisphere – total area of the intact ipsilateral hemisphere multiplied by the total distance between the sections). Stroke size was expressed as a percentage of the intact contralateral hemispheric volume.
Nogo-A Immunostaining
A rat was euthanized and perfused intracardially with 0.9% saline and 4% paraformaldehyde. The brain was removed and flash frozen in isopentane, then stored in a −80°C freezer until cutting. Sections were cut at 50 μm on a freezing microtome and placed into tris-buffered saline (TBS). For immunohistochemistry, sections were first treated with a blocking solution of 10% normal goat serum (NGS)/TBS/0.5% Triton-X for 60 min. Sections were then incubated in TBS containing 0.5% Triton-X, 5% NGS, a rabbit polyclonal anti-Neurofilament 200 (1:250; Sigma, St Louis, MO) and the mouse IgG 11C7, a monoclonal antibody specific for Nogo-A (1:500; Novartis, Basel, Switzerland), overnight at 4°C. Sections were rinsed thoroughly and incubated in TBS containing 0.5% Triton-X, 5% NGS, a tetramethylrhodamine-conjugated goat anti-rabbit IgG (1:1000; Molecular Probes, Eugene, OR) and an AlexaFluor 488-conjugated goat anti-mouse IgG (1:1000; Molecular Probes) for 60 min at room temperature. After rinsing, sections were incubated in a 300 nM solution of 4′-6-diamidino-2-phenylindole (DAPI) in 0.1% Triton-X/TBS for 30 min. After a final rinse, sections were mounted onto slides from dilute TBS solution and coverslipped with the aqueous mounting medium Fluoromount-G (Electron Microscopy Sciences, Hatfield, PA).
Neuroanatomical Analysis
Seven layer V pyramidal neurons per animal located within the forelimb motor cortex and five layers II/III pyramidal neurons per animal located within the occipital cortex of the contralesional hemisphere were identified with an atlas (Zilles, 1985) and according to previous electrophysiological studies (Neafsey et al., 1986). Criteria for inclusion in the analyses were that the neuron had to be well impregnated, unobstructed by other dendrites, blood vessels or glial cells, and the dendritic arborizations intact and visible in the plane of section. For all analyses the slides were coded and investigators were blind to the treatment group.
Apical and basilar dendrites of layers II/III and V pyramidal neurons in the intact hemisphere opposite the lesion were traced at ×25 magnification with the aid of a camera lucida drawing tube. Both apical and basilar dendritic trees were analyzed for total dendritic length (Sholl, 1956) and total number of branch segments within a branch order (Coleman and Riesen, 1968). Branch order was determined by considering that branches emanating directly from the cell body (basilar) or the primary apical dendrite were first order; once bifurcation occurred, two secondary dendrites were formed and so on.
Dendritic spine densities were analyzed from layer V pyramidal neurons within the forelimb motor cortex of the intact hemisphere. Using a ×100 oil immersion lens, a 50 μm length of one terminal basilar branch segment of third or higher order and one terminal apical segment from the midpoint of the apical tree were drawn from seven neurons per animal. Spine density was expressed as the number of spines per μm. All the data presented in this study are expressed as means ± SEM. Statistical comparison for layer V and layers II/III neurons was assessed by one-way analysis of variance (ANOVA) followed by Newman–Keuls test for post hoc analyses.
Results
Lesions are Localized in the Sensorimotor Cortex
The topographical location of all lesions included the sensorimotor cortex ipsilateral to the occluded MCA (Fig. 2A, shaded area) with minimal subcortical damage. Measurement of the infarct volumes showed no difference between the stroke only (16.46 ± 5.01), stroke/control antibody (13.82 ± 3.98) and stroke/mAb IN-1 groups (11.70 ± 1.91) (P = 0.58, one-way ANOVA).
Forelimb Cortex Layer V Neurons are Strongly Nogo-A Positive
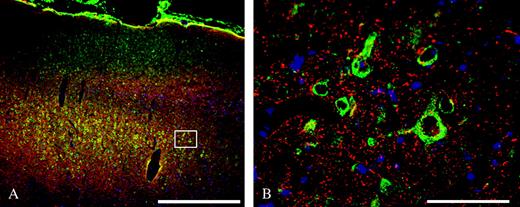
Immunohistochemical staining with the Nogo-A specific mouse monocloncal antibody 11C7 (labeled with a green fluorophore) and a rabbit polyclonal against the neuronal structural protein Neurofilament 200 (labeled with a red fluorophore) revealed that the inhibitory protein Nogo-A is present in many neurons across cortical layers II through VI of rat forelimb cortex (FL), as evidenced by the overlap of green and red fluorescence in the processed tissue (Fig. 1A). The cell body and dendritic processes of the large layer V pyramidal neurons stain very robustly for Nogo-A, but there is no visible staining in the nucleus (Fig. 1B).
Nogo-A is expressed on layer V pyramidal neurons in cortical area FL in the rat. (A) Low magnification (×32) view of Nogo-A (green), Neurofilament 200 (NF200; red) and DAPI (blue) in the cortical layers of area FL. Nogo-A staining is most robust on the large pyramidal neurons in layer V. Box represents the approximate field shown in (B). Scale bar = 500 μm. (B) Layer V pyramidal neurons staining positively for Nogo-A (green) and NF200 (red). Scale bar = 30 μm.
Dendritic Complexity is Enhanced after Stroke and mAb IN-1 Treatment
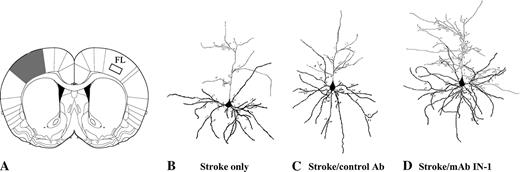
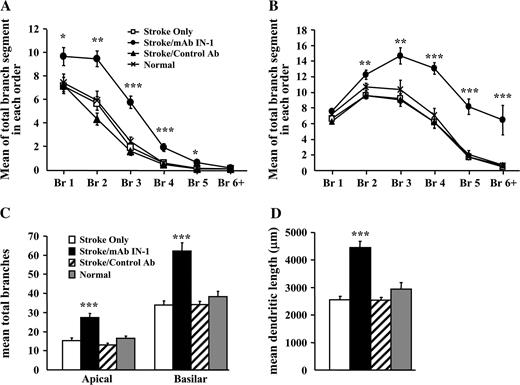
The significant functional recovery and increased axonal plasticity observed previously in rats after stroke and immediate or delayed mAb IN-1 treatment (Papadopoulos et al., 2002) led us to investigate whether dendritic changes paralleled these events. Both apical and basilar dendritic arborizaton and spine densities of Golgi–Cox stained layer V pyramidal neurons within the intact, contralesional forelimb motor cortex were examined (Fig. 2A, boxed area). Representative camera lucida drawings of pyramidal neurons from stroke only, stroke/control antibody and stroke/mAb IN-1 treatment groups are shown in Figure 2B–D. Apical dendrite patterns in animals with stroke lesion only, in stroke animals treated with control antibody and in normal animals showed no statistical difference between groups. However, stroke animals with mAb IN-1 treatment were significantly different from all control groups (Fig. 3A,C,D). mAb IN-1-treated animals had significantly greater apical dendrite arborization when measured for the number of branch segments within branch orders (Fig. 3A), total number of branches (P < 0.001, Fig. 3C) and dendritic length (P < 0.01, Fig. 3D).
Qualitative assessment of increased apical and basilar dendritic arbor complexity in stroke/mAb IN-1-treated rats. (A) Schematic diagram illustrating a typical lesion (shaded area) after permanent MCAO. The location of the affected area includes the sensorimotor cortex. Boxed area indicates the contralesional forelimb motor cortex from which layer V pyramidal neurons were analyzed. (B–D) Representative camera lucida drawings of layer V pyramidal neurons respective of treatment condition, i.e. stroke only (B), stroke/control Ab (C) and stroke/mAb IN-1 (D). All data shown (A–D) are from 6 weeks after stroke. Panel A modified and reprinted from The Rat Brain in Stereotaxic Coordinates (CD-ROM), 5th Edition, George Paxinos and Charles Watson, p. 65, Copyright 2004, with permission from Elsevier.
Increased dendritic arbor complexity of layer V contralesional motor cortex neurons in stroke/mAb IN-1-treated rats. (A) Quantification of the total number of apical branch segments within a particular branch order showing the mean of seven neurons per animal for each treatment group: stroke only (n =6), stroke/control Ab (n = 5), stroke/mAb IN-1 (n = 6), and normal (n = 5). Br, branch order. (B) Quantification of the total number of basilar branch segments within a particular branch order showing the mean of seven neurons per animal for each treatment group: stroke only (n =6), stroke/control Ab (n = 5), stroke/mAb IN-1 (n = 6) and normal (n = 5). Br, branch order. (C) Quantification of the total number of branches within the apical and basilar arbors showing the mean of seven neurons per animal for each treatment group: stroke only (n =6), stroke/control Ab (n = 5), stroke/mAb IN-1 (n = 6) and normal (n = 5). (D) Quantification of total dendritic length showing the mean of seven neurons per animal for each treatment group: stroke only (n =6) (clear bars), stroke/control Ab (n = 5) (hatched bars), stroke/mAb IN-1 (n = 6) (black bars) and normal (n = 5) (gray bars). All data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA, Newman–Keuls test for post hoc comparison). All data shown (A–D) are from 6 weeks after stroke.
As with apical dendritic arborization, a similar pattern in basilar dendrites was observed. While the control groups showed no significant difference from each other, the stroke/mAb IN-1-treated animals showed an increased basilar arbor complexity when compared with controls. Analyses demonstrated that the stroke/mAb IN-1-treated group had significantly greater number of branch segments per branch order (Fig. 3B), total number of branches (P < 0.001, Fig. 3C) and dendritic length (P < 0.001, Fig. 3D).
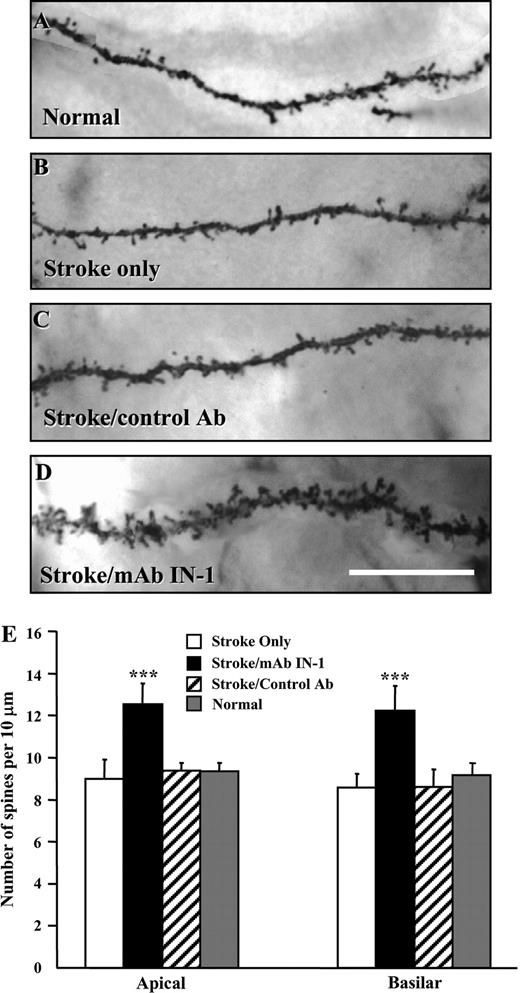
Quantitative analysis of spine densities in layer V pyramidal neurons of the contralesional forelimb motor cortex showed a significant increase in the number of spines of both apical and basilar terminal branch segments in stroke animals treated with mAb IN-1 when compared with stroke only, stroke/control Ab and normal groups (P < 0.001, Fig. 4).
Increased spine density of layer V contralesional motor cortex neurons in stroke/mAb IN-1-treated rats. (A–D) Representative photomicrographs of dendritic spines from layer V pyramidal neurons in the forelimb motor cortex respective of treatment condition, i.e. normal (A), stroke only (B), stroke/control Ab (C), and stroke/mAb IN-1 (D). Note the increased spine density in stroke/mAb IN-1 animals and their apparently different morphology, i.e. many multi-headed spines when compared with controls. Scale bar equals 10 μm. (E) Quantification of basilar and apical spine density from a terminal branch segment showing the mean of seven branch segments per animal for each treatment group: stroke only (n =6), stroke/control Ab (n = 5), stroke/mAb IN-1 (n = 6) and normal (n = 4). All data are represented as mean ± SEM. ***P < 0.001 (one-way ANOVA, Newman–Keuls test for post hoc comparison). All data shown (A–E) are from 6 weeks after stroke.
Lasting Enhanced Dendritic Complexity is Specific to the Motor Cortex after Stroke and mAb IN-1 Treatment
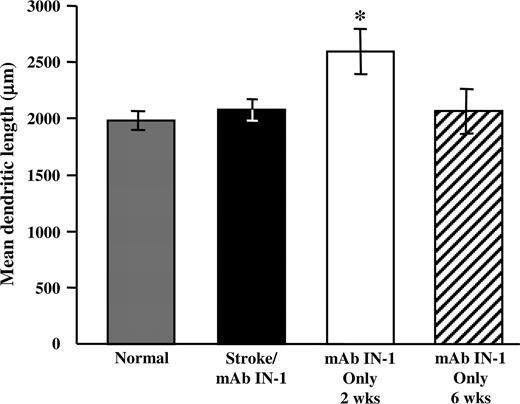
The significant dendritic plasticity observed in the contralesional forelimb motor cortex of stroke/mAb IN-1 animals raises the question whether the effect on plasticity is specific to an area or is found globally throughout the cortex. To determine this, we assessed the dendritic structures in the lateral area of the secondary visual cortex (Oc2L), a distant region from the motor cortex. Apical and basilar dendritic arbors of layers II/III neurons within the contralesional occipital cortex were examined. Quantitative analysis demonstrated no difference in occipital neuron total dendritic length between the stroke/mAb IN-1 group, normal animals, and normal animals treated with mAb IN-1 at 6 weeks (P > 0.05, Fig. 5). However, total dendritic length was enhanced after 2 weeks of mAb IN-1 treatment in normal animals, but this difference resolved at 6 weeks (P < 0.05).
Lasting increased dendritic plasticity is specific to the contralesional motor cortex after stroke/mAb IN-1. Quantification of total dendritic length from layers II/III neurons in the occipital cortex showing the mean of five neurons per animal for each treatment group: stroke/mAb IN-1 (six-week survival; n = 6), normal (n = 5), mAb IN-1 only (two-week survival; n = 5) and mAb IN-1 only (six-week survival; n = 3). No significant difference is shown in occipital neuron total dendritic length between the stroke/mAb IN-1 group, normal animals and animals treated with mAb IN-1only at 6 weeks survival (P > 0.05, Fig. 5). However, total dendritic length was significantly enhanced in animals treated with mAb IN-1 only at two-week survival, indicating a transient response of occipital neurons to treatment with mAb IN-1(P < 0.05). All data are represented as mean ± SEM. *P < 0.05 (one-way ANOVA, Newman–Keuls test for post hoc comparison).
Dendritic Complexity is Transient after mAb IN-1 Treatment in Normal Animals
Treatment with mAb IN-1 in the absence of CNS injury induces transient axonal outgrowth in adult cerebellar Purkinje neurons (Buffo et al., 2000) and corticospinal neurons (Bareyre et al., 2002). We therefore investigated whether mAb IN-1 administration without stroke had the same effect on dendritic outgrowth. Apical and basilar dendritic arbors and spine densities of layer V pyramidal neurons within the forelimb motor cortex were examined.
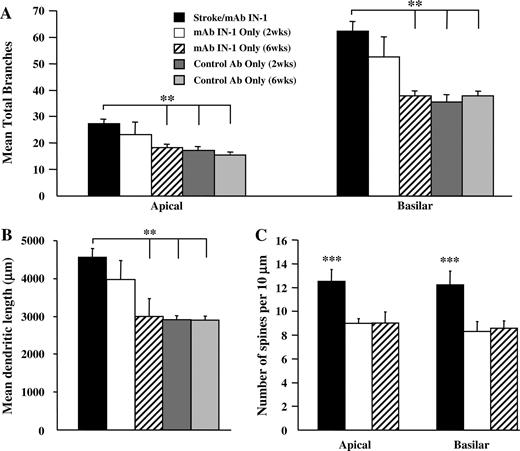
At 2 weeks after antibody administration, control antibody-treated animals showed no increase in dendritic complexity when compared with stroke/mAb IN-1 animals in all parameters measured (P < 0.01) (Fig. 6A,B). Conversely, the dendritic arbors of mAb IN-1-treated animals were similar to those of stroke/mAb IN-1 animals in both total number of branches (Fig. 6A) and length (Fig. 6B), showing an overall increase in dendritic outgrowth. Despite this effect on dendrite growth, spine density in mAb IN-1-treated animals without stroke remained unaffected by antibody treatment. This was significantly different to stroke/mAb IN-1-treated animals that showed more spines per dendritic length (P < 0.001) (Fig. 6C).
Transient increase in dendritic arbor complexity of layer V contralesional motor cortex neurons after mAb IN-1 treatment in normal rats. (A) Quantification of the total number of branches within the apical and basilar arbors showing the mean of seven neurons per animal for each treatment group: stroke/mAb IN-1 (n = 6), mAb IN-1 only (two-week survival; n = 4), mAb IN-1 only (six-week survival; n = 3), control Ab only (two-week survival; n = 4) and control Ab only (six-week survival; n = 3). (B) Quantification of total dendritic length showing the mean of seven neurons per animal for each treatment group: stroke/mAb IN-1 (n = 6), mAb IN-1 only (two-week survival; n = 4), mAb IN-1 only (six-week survival; n = 3), control Ab only (two-week survival; n = 4) and control Ab only (six-week survival; n = 3). (C) Quantification of basilar and apical spine density from a terminal branch segment showing the mean of seven branch segments per animal for each treatment group: stroke/mAb IN-1 (n = 6), mAb IN-1 only (two-week survival; n = 4), mAb IN-1 only (six-week survival; n = 3). All data are represented as mean ± SEM. **P < 0.01; ***P < 0.001 (one-way ANOVA, Newman–Keuls test for post hoc comparison).
At 6 weeks after antibody administration, animals treated with mAb IN-1 were similar to control antibody-treated animals in dendritic length and total branch number when compared with the increased dendritic complexity of stroke/mAb IN-1-treated animals (P < 0.01) (Fig. 6A,B). This indicated that the dendritic growth triggered by mAb IN-1 alone without stroke at 2 weeks was transient over time. In addition, spine density remained unaffected in mAb IN-1-treated animals when compared with the increased density in the stroke/mAb IN-1 animals (P < 0.001) (Fig. 6C).
Discussion
The present study demonstrates that treatment with the mAb IN-1 to neutralize Nogo-A after stroke in adult rats results in a significant increase in the dendritic arbors and spine density of layer V pyramidal neurons within the forelimb motor cortex of the opposite, intact hemisphere. Additionally, analysis of mAb IN-1-treated animals without lesion revealed a transient increase in dendritic arborization of layer V pyramidal neurons with no increase in spine density. An increase in neuronal complexity as seen here has been correlated with improved motor function in animals after cortical lesions (Jones and Schallert, 1994; Kawamata et al., 1997b; Rowntree and Kolb, 1997; Biernaskie et al., 2004). Importantly, strategies such as enriched environment and drug intervention have been shown to enhance dendritic plasticity and lead to better functional outcome (for review, see Kolb and Metz, 2003).
In recent years, Nogo-A has been identified as a primary inhibitor of axonal growth after injury in the adult CNS. Indeed, our earlier work has shown that neutralizion of Nogo-A with the mAb IN-1 resulted in axonal plasticity from cortical neurons in the opposite cerebral hemisphere after unilateral stroke (Papadopoulos et al., 2002; Seymour et al., 2005) or aspiration lesion (Emerick and Kartje, 2004). This axonal plasticity paralleled the improved behavioral recovery on skilled motor tests as observed in these animals. Using intracortical microstimulation, we have recently shown that, following cortical lesion and treatment with anti-Nogo-A antibody, cortical reorganization occurred in the opposite, intact hemisphere (Emerick et al., 2003). We now show an important role for Nogo-A in regulating dendritic growth after ischemic stroke in this contralesional hemisphere.
The dendritic plasticity observed in the present study may be the result of a complexity of factors. Early events such as physiological and molecular alterations may facilitate a growth permissive environment after stroke. Alterations in physiological properties have been shown to promote excitability by disinhibition of the contralesional hemisphere after focal brain injury in human subjects (Liepert et al., 2000; Manganotti et al., 2002) and animal studies (Rouiller et al., 1994; Fabri and Manzoni, 1996; Buchkremer-Ratzmann and Witte, 1997), and facilitate the induction of synaptic plasticity (Carmichael and Chesselet, 2002). In addition, the cortex opposite the lesion has an increased expression of factors implicated in plasticity such as the immediate early genes c-fos and nerve-growth factor inducible-B (Johansson et al., 2000; Keyvani et al., 2002), phosphorylated cAMP-response element (Tanaka et al., 1999, 2001; Keyvani et al., 2002), and activity-regulated cytoskeleton-associated protein, a protein enriched in dendrites (Keyvani et al., 2002). Several neurotrophins are also up-regulated in the hemisphere contralateral to the stroke lesion, including nerve growth factor (Lee et al., 1996), insulin-like growth factor (Garcia-Estrada et al., 1992) and basic fibroblast growth factor (Schallert et al., 2000). Moreover, the growth-associated protein GAP-43 is up-regulated in the contralesional hemisphere with a subsequent increase in synaptophysin levels, suggesting the development of mature synapses (Stroemer et al., 1995).
Dendritic structural events have also been reported in the hemisphere opposite the lesion. After unilateral cortical electrolytic lesions, dendritic sprouting in the contralesional cortex was shown by an increase in the apical and basilar dendritic complexity of layer V pyramidal neurons up to 18 days following the lesion with a subsequent decrease by day 30 (Schallert and Jones, 1993; Jones et al., 1996). However, others using electrolytic or aspiration lesions found no evidence of transient dendritic growth in the contralesional hemisphere (Forgie et al., 1996; Prusky and Whishaw, 1996). In our study we examined dendritic changes at 6 weeks post-stroke in order to investigate a time point when any possible transient growth would have returned to normal. Interestingly, in our study normal animals treated with mAb IN-1 without stroke showed a transient increase of dendritic arbors at 2 weeks that returned to normal by 6 weeks post-treatment, indicating that Nogo-A neutralization has an effect on dendritic plasticity that is not dependent upon a coexisting cortical lesion.
We previously reported that treatment with mAb IN-1 or other antibodies against Nogo-A increases cortico-efferent plasticity from the opposite, forelimb motor cortex into deafferented motor areas with concomitant improved functional outcome after pyramidotomy (Thallmair et al., 1998; Z'Graggen et al., 1998), cortical aspiration lesions (Emerick and Kartje, 2004) and ischemic stroke (Papadopoulos et al., 2002; Wiessner et al., 2003). Here we report that after unilateral stroke, animals treated with mAb IN-1 showed a significant increase in apical and basilar dendritic growth and spine density in the same population of layer V pyramidal neurons of the opposite, forelimb motor cortex. These results implicate Nogo-A as a ‘stop’ signal for both dendritic and axonal growth, since neutralization with an anti-Nogo-A antibody leads to increased dendritic arborization and spine formation. The physiological mechanism responsible for the dendritic sprouting of these same cortical neurons shown here is speculative. However, an important hypothesis to note is that dendritic growth is regulated by afferent innervation and synaptic contact in an activity-dependent manner (Kossel et al., 1997; Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999; McAllister, 2000; Whitford et al., 2002). Consequently, the robust axonal sprouting observed after treatment with mAb IN-1 in previous studies may provide the afferent innervation for the present dendritic growth. Whether the activity originates from local axonal collaterals in the homotopic cortex, from distant transcallosal connections or from the combination of both has yet to be determined.
Previous studies have shown that treatment with mAb IN-1 to block Nogo-A in normal non-injured adult animals promoted transient axonal sprouting of the CST (Bareyre et al., 2002) and cerebellar Purkinje cells (Buffo et al., 2000). However, after corticospinal tract injury and treatment with mAb IN-1, axonal sprouting was target specific and stable, and was paralleled by an increased expression of guidance molecules and growth-related proteins (Bareyre et al., 2002). In the present study we show that after stroke/mAb IN-1 treatment the increase in dendritic complexity is stable and possibly responding to the same mechanistic cues as axons. Similar to axonal studies in non-injured animals, administration of mAb IN-1 in normal animals without stroke resulted in transient growth of dendritic length and branching which returned to normal by 6 weeks, and showed no increase in the number of spines at any time. Furthermore, our results show that the effect on dendrites is not specific to motor neurons, i.e. the occipital neurons also showed a transient effect from anti-Nogo treatment and returned to normal at 6 weeks. Therefore, our effect of sustained dendritic plasticity in the contralesional motor cortex is not due to the intrinsic properties of motor neurons, but most likely has some relationship to the location in the contralesional motor cortex. The increased plasticity is specific to the contralesional forelimb motor cortex, a region directly affected by injury-related factors needed for guidance, attraction and stabilization. These results support the notion of lesion-induced events playing an important role in the stable remodeling of dendrites after mAb IN-1 treatment.
The role of myelin-associated Nogo-A in axonal growth inhibition is well established. We now show a role for Nogo-A, which is present in CNS myelin and certain neuronal subtypes (Dodd et al., 2005), including layer V and layers II/III pyramidal neurons (Huber et al., 2002; this study), in dendritic growth restriction as well. Treatment with mAb IN-1 to neutralize Nogo-A may contribute to the plasticity of the system through the orchestration of the same growth promoting mechanisms in axons and dendrites and their subsequent stabilization. This combination of axonal sprouting to deafferented targets and increased dendritic arborization in the contralesional cortex following ischemic stroke and mAb IN-1 treatment most likely contributes to the dramatic functional recovery as reported after anti-Nogo-A therapy.
We thank Robin Gibb and Grazna Gorny for expert technical assistance in Golgi–Cox staining techniques. We gratefully acknowledge Novartis for providing us with the 11C7 antibody. This work was supported by the Department of Veterans Affairs, NIH Grant NS 40960, a Schmidt Fellowship to C.M.P. and the Swiss NSF.
References
American Heart Association (
Bareyre FM, Haudenschild B, Schwab ME (
Biernaskie J, Chernenko G, Corbett D (
Bregman BS, Kunkel-Bagden E, Schnell L Dai HN, Gao D, Schwab ME (
Buchkremer-Ratzmann I, Witte OW (
Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F (
Buss A, Sellhaus B, Wolmsley A, Noth J, Schwab ME, Brook GA (
Carmichael ST, Chesselet M-F (
Caroni P, Schwab ME (
Caroni P, Schwab ME (
Chen MS, Huber AB, Van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (
Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD (
Coleman PD, Riesen AH (
Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR (
Cuadrado ML, Egido JA, Gonzalez-Gutierrez JL, Varela-de-Seijas E (
David S, Aguayo AJ (
Dodd DA, Niederoest B, Bloechlinger S, Dupuis L, Loeffler JP, Schwab ME (
Emerick A, Kartje GL (
Emerick AJ, Neafsey EJ, Schwab ME, Kartje GL (
Engert F, Bonhoeffer T (
Fabri M, Manzoni T (
Feydy A, Carlier R, Roby-Brami A, Busssel B, Cazalis F, Pierot L, Burnod Y, Maier MA (
Fiedler M, Horn C, Bandtlow C, Schwab ME, Skerra A (
Forgie ML, Gibb R, Kolb B (
Garcia-Estrada J, Garcia-Segura LM, Torres-Aleman I (
Gibb R, Kolb B (
Glaser EM, van der Loos H (
GrandPre T, Nakamura F, Vartanian T, Strittmatter SM (
Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME (
Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM (
Johansson IM, Wester P, Hakova M, Gu W, Seckl JR, Olsson T (
Jones TA, Schallert T (
Jones TA, Kleim JA, Greenough WT (
Kartje GL, Schulz, MK, Lopez A, Schnell L, Schwab ME (
Kawamata T, Speliotes EK, Finklestein SP (
Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP (
Keyvani K, Witte OW, Paulus W (
Kossel AH, Williams CV, Schweizer M, Kater SB (
Lee TH, Kato H, Kogure K, Itoyama Y (
Liepert J, Hamzei F, Weiller C (
Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A (
Maletic-Savatic M, Malinow R, Svoboda K (
McAllister AK (
McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ Braun PE (
Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (
Neafsey EJ, Bold EL, Haas G, Hurley-Gius KB, Quirk G, Sievert CF, Terreberry RR (
Papadopoulos CM, Tsai S-Y, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL (
Paxinos G, Watson C (
Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS (
Prusky G, Whishaw IQ (
Qui JQ, Cai D, Filbin MT (
Richardson PM, McGuinness UM, Aguayo AJ (
Rouiller EM, Babablian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M (
Rowntree R, Kolb B (
Schallert T, Jones TA (
Schallert T, Leasure JL, Kolb B (
Schnell L, Schwab ME (
Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR (
Schwab ME, Caroni P (
Schweigreiter R, Walmsley AR, Niederost B, Zimmermann DR, Oertle T, Casademunt E, Frentzel S, Dechant G, Mir A, Bandtlow CE (
Seymour AB, Andrews EM, Tsai S-Y, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL (May 11, 2005) Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab doi: 10.1038/sj.jcbfm.9600134.
Spillmann AA, Amberger VR, Schwab ME (
Stroemer RP, Kent TA, Hulsebosch CE (
Tanaka K, Nagata E, Suzuki S et al. (
Tanaka K, Nogawa S, Ito D, et al. (
Thallmair M, Metz GAS, Z'Graggen WJ, Raineteau O, Kartje GL, Schwab ME (
Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z (
Weiller C, Ramsey SC, Wise RJS, Friston KJ Frackowiak RSJ (
Wenk CA, Thallmair M, Kartje GL, Schwab ME (
Whitford KL, Dijkhuizen P, Polleux F, Ghosh A (
Wiessner C, Bareyre FM, Ballegrini PR, Mir AK, Fretzel S, Zurini M, Schnell L, Oertle T, Schwab ME (
Z'Graggen WJ, Metz GAS, Kartje GL, Thallmair M, Schwab ME (
Author notes
1Research, Hines VA Hospital, Hines, IL 60141, USA, 2Neurology Service, Hines VA Hospital, Hines, IL 60141, USA, 3Department of Neurology, Loyola University, Maywood, IL 60153, USA, 4Department of Psychology, University of Lethbridge, Lethbridge, AB, Canada, 5Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology, Zurich, Switzerland and 6Department of Cell Biology, Neurobiology and Anatomy, Loyola University, Maywood, IL 60153, USA