-
PDF
- Split View
-
Views
-
Cite
Cite
Tricia S. Clement, Kerry E. Grens, Russell D. Fernald, Female affiliative preference depends on reproductive state in the African cichlid fish, Astatotilapia burtoni, Behavioral Ecology, Volume 16, Issue 1, Jan./Feb. 2005, Pages 83–88, https://doi.org/10.1093/beheco/arh134
Close - Share Icon Share
Abstract
Hormones play a pivotal role in reproductive behavior and have been implicated in mediating mate choice decisions. Here we asked whether the differences in female reproductive state dependent on changes in hormone levels correspond to changes in female affiliation with males. In the African cichlid fish, Astatotilapia burtoni, males shift between reproductive (territorial; T) and non-reproductive (non-territorial; NT) states depending on social context while females alternate between gravid (egg bearing; G) and non-gravid (NG) reproductive states independent of social conditions. Moreover, the brain-pituitary-gonadal axis controlling reproduction and reproductive hormones is substantially remodeled in both males and females depending on reproductive state. To measure affiliative preference, gravid and non-gravid females were given the choice of associating with T or NT males. Gravid females preferentially associated with T males, whereas non-gravid females showed no preference. To discover whether gravid females use male size independent of dominance status as a cue for their choice, gravid females were given a choice between territorial males of different sizes. Gravid females preferred the smaller of two T males, but the smaller T males were significantly more active. Our results show that associative change could be an important behavioral mediator between hormonal cues and reproductive success, and that females use a hierarchy of cues in decision-making, preferring to affiliate with T over NT males and, among T males, preferring more active animals.
It is often assumed that females choose males displaying the most exaggerated sexual traits—whether behavioral, morphological, or material, such as food and shelter. However, more factors may also contribute importantly to female mate choice decisions. A wide range of subtle and complex external factors have been shown to influence female choice, suggesting sophisticated integration of cues by females. Less well understood are the physiological substrates that are likely also crucial for successful female reproductive choice.
Genetic and epigenetic factors, circulating hormones, and learned behavior can contribute to a female's final mate choice. In particular, potential mediating effects of circulating peptide and steroid hormones on mating behavior have been described for some species (see Argiolas, 1999 for review). For example, infusion of one form of gonadotropin-releasing hormone (GnRH1) in the brain of the musk shrew, Suncus murinus, causes rapid and significant changes in rump presentation and tail wagging, while injection of a second form (GnRH2) has no effect (Schiml and Rissman, 2000). In contrast, in female sparrows, Zonotrichia leucophrys gambelii, copulation solicitation increases with GnRH2 intracerebral administration and is preserved longer with another type of GnRH (Maney et al., 1997).
In addition to their role in solicitation behavior, hormones are intimately involved in establishing a preference for conspecifics of the opposite sex during ontogeny (e.g., Adkins-Regan, 1998). Once the reproductive axes are established, circulating hormones can affect behavior. For example, both female Rhesus monkeys, Macaca mulatto, and female meadow voles, Microtus pennsylvanicus, prefer to associate with other females during non-breeding seasons and prefer males during breeding seasons (Ferkin and Zucker, 1991; Michael and Zumpe, 1993), suggesting a possible causal role for circulating hormones.
Although manipulating hormone levels experimentally can be informative, here we take advantage of the naturally fluctuating levels of hormones in the female reproductive cycle and examine whether and how decisions to affiliate with males of different reproductive quality change as a function of stage in the reproductive cycle. In females of the African cichlid fish, Astatotilapia (Haplochromis) burtoni, the brain-pituitary-gonadal axis controlling reproduction is substantially remodeled between gravid (egg-bearing) and non-gravid states (White and Fernald, 1993). Specifically, neurons containing gonadotropin releasing hormone (GnRH) enlarge significantly in gravid females, producing more GnRH that, in turn, causes production of eggs via gonadotropins from the pituitary. It is unknown, however, whether and how female choice changes in concert with modifications in the neuroendocrine system. Also unknown is the role of female behavior in successful reproduction in A. burtoni, although female choice in other cichlid species is an important selective force on male coloration and speciation through male selection (e.g., Dominey, 1984; Jordan et al., 2003; Lande et al., 2001; Seehausen et al., 1998).
A. burtoni live in the shallow shore pools and river estuaries of Lake Tanganyika, Tanzania, East Africa (Coulter, 1991). In this setting few males dominate resources (i.e., food and nesting sites), and adult males adopt one of two alternate phenotypes (Fernald and Hirata, 1977a,b). About 10–35% of the males are territorial (T), being brightly colored, having a blue or yellow body color, a dramatic black stripe through the eye, vertical black bars on the body, a black spot on the tip of the gill cover, and a large red patch just behind it. In contrast, non-territorial (NT) males are cryptically colored, making them difficult to distinguish from the substrate and from females that are similarly camouflaged. In sharp contrast to T males, NT males have a behavioral repertoire limited to fleeing and eating and they never attempt courtship. T males vigorously defend contiguous territories by exchanging threat displays at boundaries with their territorial neighbors. Their 17 distinct behavioral acts are important for territorial defense, for solicitation and courtship of females, and for constructing a spawning pit.
The phenotypic distinctions between territorial and non-territorial males extend to their reproductive physiology. T males are typically larger than NT males, have fully spermiated testes (Fraley and Fernald, 1982), and have higher androgen levels (Soma et al., 1996). Although the reproductive axis of NT males is completely inhibited (Fraley and Fernald, 1982), NT males can and do switch to T status opportunistically following periods of rapid growth, environmental disruption, or predation of neighboring T males (Fernald, 1977; Fernald and Hirata, 1977b; Hofmann et al., 1999: Hofmann and Fernald, 2000). Social status is not stable, however, and the duration of territorial tenure can be brief, depending on ecological and social conditions.
In social groups, A. burtoni females typically school with other females and NT males while T males court the females and vigorously chase intruders in defense of territories. However, to reproduce, gravid females must enter the male territory to spawn with a T male. Female behavior must change to allow mating, and we asked whether a shift in reproductive behavior reflected a change in female preference and if such change corresponded to the females' reproductive state. We tested female preference to associate with either T or NT males while either gravid or non-gravid, hypothesizing that affiliative behavior could depend on changes in the neuroendocrine system. Our results show that females change their affiliative preferences as a function of their reproductive state.
EXPERIMENT 1: DOES FEMALE REPRODUCTIVE STATE INFLUENCE HER CHOICE BEHAVIOR?
To discover whether affiliative behavior changes as a function of female reproductive state, gravid (egg-bearing) or non-gravid A. burtoni females were given the choice to spend time near either a territorial or a non-territorial A. burtoni male phenotype. A female's affiliation with a particular male is a reliable proxy of a male's ultimate reproductive success in guppies, Poecilia reticulata (Kodric-Brown, 1993). In addition, naturally observed female mating behavior of the African cichlid, Lethrinops c.f. parvidens, has shown that visits by females to male territories is correlated with circling, a courtship behavior. In turn, circling is correlated with actual spawning (Kellogg et al., 2000), so affiliation can be taken as a good indicator of ultimate spawning choice.
Males could not interact with each other (see below) and females could only view the males through a clear Plexiglas barrier. This procedure eliminated the possibility that female association would be based on male-male competition or non-visual cues and provides a direct test of female preference for territorial versus non-territorial A. burtoni male phenotypes. We controlled for the possibility that female choice was based on location by changing the position of the males in the choice paradigm and for possible diel variations by testing at different times of day.
METHODS
Subjects
We used Astotilapia burtoni originally derived from a wild-caught stock population. Prior to selection, the fish were maintained in aquaria under conditions similar to those of their natural environment (Fernald and Hirata, 1977b: 28°C water temperature, pH 8, and a 12:12 h light:dark cycle with full spectrum illumination). A layer of gravel covered the bottom of the aquaria, and terracotta pots were placed in each tank to allow establishment and maintenance of male territories. Fish were fed every morning ad libitum with cichlid pellets and flakes (AquaDine, Healdsburg, CA). All animals were treated in accordance with the Stanford University Institutional Animal Care and Use Committee (IACUC) guidelines (protocol #3110).
Selection of females
Eight gravid females were selected based on observation. Because females can appear gravid from enlarged stomachs immediately after feeding, gravid females were identified by observation in the late afternoon. Females identified as gravid by observation for this experiment that were subsequently killed for the analysis of their tissue for another study all had ripe egg masses confirming their gravid status.
Eight non-gravid females were selected using the criteria that they had just released a brood following two weeks of mouthbrooding. Females previously selected in this manner and then sacrificed at days one, three, and six following brood release had no egg masses. A non-gravid female was then housed with a single gravid female for at least two days prior to the first day of testing. Once selected, all females were kept as pairs of gravid and non-gravid in tanks (76.3 × 30.5 × 30.5 cm) throughout the experiment for identification purposes.
Selection of males
To select males for the experiment, we used behavioral observations to identify T and NT individuals. Males had been previously tagged to distinguish individuals and were observed for three min, three times per week for one week at the same time each day. Behaviors were identified using a standard reference (Fernald, 1977) and included chasing or biting females or non-territorial males, chasing or biting toward territorial males as well as threat and border displays, and fighting; reproductive acts including digging a spawning pit, courting, and spawning; and submissive acts such as fleeing. The number of occurrences of each behavior was recorded, as well as the overall coloration of the fish and the presence or absence of an eyebar. When a male occupied a territory the location was noted. Because males engage in aggressive acts in defense of an existing territory as well as during the acquisition of a new territory, only males actually defending a discernable territory were classified as “territorial.” Males were then classified as either territorial or non-territorial on the basis of their behavior and coloration.
Observation test aquarium
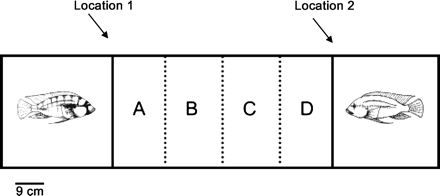
The experiment was conducted in a rectangular tank (147[l] × 37[d] × 28[h] cm). Each end could be partitioned by clear or opaque Plexiglas dividers into 37 × 37 cm compartments for the target males, leaving a center compartment for the female being tested (74 × 37 cm). The center section was externally marked into equally spaced quadrants so the experimenter could quantify the time a female spent in each quadrant (Figure 1).
Test tank: One male was placed in the compartments at each end of the tank, as shown, and separated from the central compartment by a clear Plexiglas barrier. During baseline trials an opaque barrier at locations 1 and 2 isolated the three compartments. Dashed lines show the boundaries of the four quadrants (A, B, C, D) used to measure female position within her compartment.
Procedures
Preliminary experiments were carried out to identify conditions that would minimize the stress on the animals, because previous work has shown they are sensitive to stressful situations (Fox et al., 1997). No animals used in these preliminary experiments were tested in any of the reported experiments. We found that exchanging male locations within one day resulted in males failing to interact with the female, and they exhibited dark coloration and an overall stressed appearance. Instead of repeatedly moving T and NT males between the left and right sides of the tank each day, we devised a method that allowed the males to remain in each side of the test chamber the greatest amount of time, which reduced stress considerably, as evidenced by increased activity levels and normal coloration. In the experiments reported here all side-bias controls for males were done across days with females only receiving one test trial a day for four days.
Since males are confined at each end of the test aquarium 147 cm apart, they cannot interact physically with each other. However, when males are separated by a Plexiglas divider with no space between their chambers, they can and do interact. To test whether males interacted across the 147 cm between them, we examined 16 pairs of territorial males (e.g., males most likely to display aggressive, interactive behaviors) by placing them in the end compartments with no female present (Figure 1). Behavior for each male was recorded in two conditions: with and without the paired male visible. In neither case did we observe any directed interactions between the two males. Instead, males typically either remained stationary in the tank or under the shelter provided. These results were obtained even when the fish had a significantly longer period of accommodation (48 h) than typical for the experiments reported with females. Correspondingly, we saw no evidence that males interacted during the test trials with the female present, since all interactions seemed directed either toward their own reflection or to the female. Thus, preferences exhibited by females in this test situation are solely a result of male-female interactions and are not influenced by male-male interactions.
A territorial and a non-territorial male were selected from the general population and each placed in one end of the test aquarium at least three days prior to the first test session. A mixed-sex community of fish inhabited the center compartment of the test chamber, separated by clear Plexiglas, to reduce stress and provide stimulation for the males. The community was comprised of conspecifics, mostly females and non-territorial males. All fish maintained in the test aquarium remained on identical feeding and lighting schedule as those in the general colony. Choice sessions were performed four days a week during the morning and afternoon. Each female received only one choice session per day (either morning or afternoon). Order of choice trials was counterbalanced between gravid and non-gravid females so that an equal number of gravid females were tested beginning with the territorial male in the right compartment of the tank and with the territorial male in the left compartment. Similarly, both gravid and non-gravid females equally experienced their first test trial in the morning and in the afternoon.
Baseline control trial
To control for possible female preferences towards a particular part of the test tank, independent of male stimuli, females were tested with opaque barriers placed between each end of the test tank and the center compartment. The female was allowed to acclimate to the chamber for one min while no data was collected. Following acclimation, the female's movements around the chamber were recorded as time spent in each quadrant for 10 min.
Female in “fixed” location
Following baseline data collection, the female subject was acclimated in a clear glass cylinder in the center of the test chamber for two reasons: (1) to ensure equivalent exposure to each male prior to the test trials, and (2) to ensure that every female started each trial from the same location. Once the female was in place, the ‘choice’ or focal males were made visible by removing the opaque barrier and leaving the transparent barrier in place, and the female remained in the glass container in the center of the tank for 10 min.
Test trials
After the “female fixed” trial, the glass container was removed from the tank and the female was allowed to swim freely around the center compartment. After acclimating for 1 min, the time spent in each quadrant for the subsequent 10 min was recorded as the female swam around the chamber. All time measurements obtained during test trials were corrected for the corresponding baseline trial by subtracting time spent in each quadrant during baseline observations from the time spent in that same quadrant during the test trial. Although males were matched for activity level during the selection process, relative activity level of both focal males was recorded while the female viewed them. Activity level was judged on a scale from 0 to 3: (0) stationary, (1) occasional movements, but without distinctive behavior, (2) active, but only interacting with his reflection, and (3) interacting with the female by tracking her movements. If both males were highly active they would both be scored 3, and correspondingly, if both males were stationary, they both received a score of 0.
RESULTS AND DISCUSSION
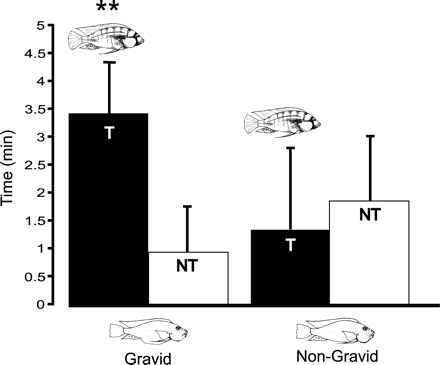
Our central finding is that gravid females spent more time near T males than NT males, whereas non-gravid females spent their time equally near T and NT males. The center two quadrants of the female's compartment (B and C of Figure 1) were considered choice neutral and were not used for any measure of preference. Time spent in the two quadrants closest to the males (A and D of Figure 1) were analyzed as follows. The average time gravid females spent with T males was 3.4 min as compared to only 0.9 min spent with NT males (see left side of Figure 2). In contrast, non-gravid females spent an average of 1.2 min with the T male and an average of 2.0 min with the NT male (see right side of Figure 2). A three-way mixed factor repeated measures ANOVA was used to assess the interaction among three factors: (1) female status (gravid or non-gravid); (2) male status (territorial or non-territorial); and (3) trial order (a.m. territorial male on left, a.m. non-territorial on left or p.m. territorial male on left, p.m. non-territorial on left). The only significant interaction was female status versus male status (F1,11 = 5.04; p = .046). In contrast, there was no significant main effect of female status (F1,11 = 1.55; p = .24), male status (F1,11 = 3.22; p = .10), or of trial order (F1,11 = 0.601; p = .62) on female preference. When analyzed, the activity levels of the focal males during the testing period did not significantly differ (F1,48 = 2.630; p = .111]. Comparing gravid and non-gravid females, the gravid females showed a significant main effect of male status (F1,29 = 5.30; p = .03), whereas for non-gravid females there was no significant main effect (F = 0.221; p = .64].
Gravid and non-gravid female affiliation with territorial (T) and non-territorial (NT) male phenotypes. The graphs show average time females affiliated with T and NT males, who were confined to either end of a tank. Affiliation time is corrected by baseline measurements of time spent at either end while males were obscured.
Clearly, in the absence of male-male competition, females selectively affiliate with a particular male phenotype when reproductively active. Gravid females affiliate preferentially with territorial males whereas non-gravid females do not show a preference.
These results show that females appear flexible in their behavior during their reproductive cycle, though it is not clear what the basis is for their choice. T males have several salient visual features compared with NT males that might be used, including larger physical size, brighter body coloration, and prominent chromatic body patterns. The most likely of these characteristics, based on field (Fernald and Hirata, 1977a) and laboratory studies (Fernald, 1977), is relative body size. For this reason, in a second experiment we examined the role of T male body size in female affiliation preference when status is held constant.
EXPERIMENT 2: DOES FEMALE CHOICE DEPEND ON MALE SIZE?
Because NT males are generally smaller than T males, the gravid females tested in Experiment 1 may have used male size as a basis for their affiliative choice. To discover whether male size was a critical cue, gravid females were given a choice between T males of different sizes. In all other respects, T males were the same; they each had a predominantly yellow body coloring with a red patch behind the eye, the ability to turn on and off a black eyebar, and possessed bright white eggspots. We predicted that if gravid female preference were based on male size, females would choose the larger of two T males. Alternatively, if size were not a key factor, females would exhibit no significant preference for either male.
METHOD
Subjects, selection, and apparatus
Subjects, their housing, and the experimental apparatus were identical to those used in Experiment 1. Gravid females were chosen on the same basis as in Experiment 1. The larger T male was chosen to be at least 1.3 times longer than the smaller T male for each test. This specific criterion was chosen because previous observations with males suggested a size discrepancy of this magnitude provides a salient social cue (White SA, Fernald RD, unpublished observations).
Procedure
All procedural aspects of Experiment 2 were identical to those described for Experiment 1 with the exception that only gravid females were tested and the choice was between a large T male and small T male.
RESULTS AND DISCUSSION
Given the choice, gravid females spent more time with a small T male (4.8 m) than with a large T male (−1.1 m). As in Experiment 1, these are corrected scores (e.g., baseline time subtracted from observed time) and only time spent in the two quadrants closest to the males was used for analysis. Thus, the negative average value of time females spent with the large T shows that females spent more time in the quadrant nearest the large T during baseline measurement when he was not visible than during the test trial when he was visible. A three-way mixed factor repeated measures ANOVA was used to assess the interaction among three factors: (1) male size (large, small); (2) trial order (a.m. larger territorial male on left, a.m. smaller territorial on left, p.m. larger territorial male on left, p.m. smaller territorial on left); and (3) time of day. There was a main effect of male size (F1,11 = 4.74; p = .04) but not of trial order (F1,11 = 0.378; p = .77) or time of day (F1,11 = 0.367; p = .55).
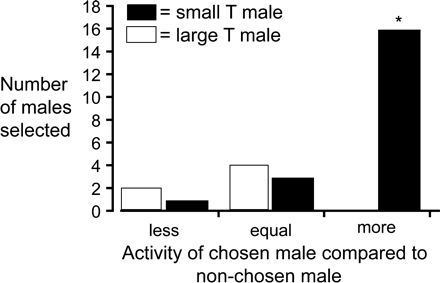
During observations, we noted differences in the activity levels among T males during the testing period. Analyzing this difference revealed that small T males were significantly more active than large T males (F1,11 = 11.22; p = .004), suggesting that females affiliate preferentially with the more active male. Indeed, females spent an average of 3.21 min with the more active male and an average of −1.37 min with the less active male when scores were corrected. Thus, if on a particular test trial the smaller T male was more active, the gravid female preferred to affiliate with that male, and if the larger T male was more active, females spent more time near that male (see Figure 3). However, because overall small T males were more active than the large T males, females spent significantly more time near the small T males.
Affiliative preferences of gravid females as a function of the activity level of the chosen male. Gravid females were given a choice to affiliate with either a small or a large territorial male. The number of times a male from either size group was chosen is plotted as a function of the three categories of male activity. The relative activity levels of males selected by the female were calculated as the difference in activity between opponents (e.g., selected minus non-selected) and the male. Male activity level was ranked from 0 (none) to 3 (most active). The data show that females typically chose small males in preference to large males and that small males were usually more active. The asterisk indicates that females chose the more active male significantly more times than the equal or less active male.
Taken together, these experiments show that when activity level was held constant and status and size varied, gravid females chose to associate with the dominant territorial male. When male status was held constant and size and activity level varied, gravid females preferred to associate with the smaller, more active territorial male. Thus, gravid females tested in Experiment 1 appear not to be choosing the T male solely on the basis of larger size.
GENERAL DISCUSSION
The basis for female choice is often shown to be differential ornamentation or color among competing males, but little is known about possible internal factors influencing female choice. In other species, there are well known differences in female reproductive behavior dependent on circulating hormones. In fish, there is some evidence that the physical condition of female sticklebacks, Gasterosteus aculeatus, (Bakker et al., 1999)—including the amount of time she has been gravid and her level of energy stores (Luttbeg et al., 2001)—influences mate choice. In A. burtoni, overall female condition is affected by reproductive state since females are likely to be nutrient deficient after mouthbrooding for two weeks. However, how the nutritional state interacts with reproductive readiness is unknown. In our experiments non-gravid females were tested over the course of a week after the release of fry. It would be expected that nutritional state would significantly change over the course of this time, but affiliative preference in these females did not appear to be affected by such shifts. It is possible that our females do change behavior upon condition shifts, but our gross estimates of time spent affiliating with males were unable to parse out such subtle effects.
Our experiments show that both the male and female reproductive states and male activity level influence female preference for a particular male. Gravid females choose to spend time near dominant, territorial males rather than camouflaged, non-territorial males, whereas non-gravid females do not show a preference. In colonies, both females and NT males are chased vigorously by T males; females may conserve energy by exhibiting no preference during times of reproductive inactivity. However, as females become gravid they must associate with T males for spawning to occur. Thus, the preference shown by gravid females for T males may be a behavioral priming mechanism that facilitates spawning.
In the studies presented here, preference for more active males when size and status were controlled raise the question of whether males are more active because females are in their vicinity. In other fish species strength of female preference was correlated positively with the proportion of time a male spent in courtship while a female was associating with him (Amundsen and Forsgren, 2001; Wong and Jennions, 2003). In our experiments males did not engage directly in courtship behavior; however, increased activity level may be a predictable precursor of courtship. Although the level of choosiness of males and females is predicted to depend on their relative parental investment, Werner and Lotem (2003) show that in a haplochromine cichlid, Astatotilapia flaviijosephi, males with no parental investment preferred to court larger females, suggesting that males choose females likely to lay more eggs. Interestingly, this does not appear to be the case in our system. Male activity level did not appear to be directly correlated with female reproductive status. Furthermore, preliminary data from experiments in which males were given a choice between gravid and non-gravid females suggests that males do not differentially associate with these females (Clement TS, unpublished data).
In our studies, male and female subjects were separated by a Plexiglas barrier and hence female behavior towards males was not influenced by physical contact. We know that sound does not play a role in communication in A. burtoni (Hirata and Fernald, 1975) and that olfactory cues were not available. Thus, it is likely that females relied exclusively on visual information, which appears to be vital for mate preference in many fish. For example, in several cichlid species, genus Metriaclima, females choose to associate with conspecifics over heterospecifics, even under monochromatic lighting conditions and in the absence of olfactory cues (Jordan et al., 2003). In guppies, Poecilia reticulata, females associate with males that reflect ultraviolet light as opposed to males whose ultraviolet light reflectance is blocked (Kodric-Brown and Johnson, 2002). And in the Lake Malawi cichlid, Pseudotropheus lombardoi, females demonstrate a precise preference for the size and number of male egg spots (Couldridge, 2002). Evidently, the many types of visual cues—light reflectance, color patterns, shape, and behavior—must convey to the female key characteristics of her potential mate. Based on our data it is likely that A. burtoni females use a hierarchy of visual cues to choose a mate.
The demonstration that female preference depends on reproductive state raises several interesting evolutionary questions. For example, how might reproductively important hormones influence affiliation preference? There is considerable evidence that circulating hormones play a role in female behavior in many species (e.g., Becker et al., 1993). In A. burtoni females, gonadotropin releasing hormone levels are increased dramatically in the gravid state, leading to increased production of hormones by the ovary. Among the many changes in females during this time, elevated estrogen or progesterone levels during time of reproductive activity may influence the shift to preference for T males, and there are numerous sites where the influence could be exerted.
Hormones have been shown to cause fluctuations in sexual motivation, as seen in primates (Wallen, 2001). It is also possible that hormones influence sensory systems directly. In human females perception through sensory modalities, such as pain perception (Gaumond et al., 2002; Hellstrom and Lundberg, 2000; Kerem, et al., 2002), olfaction (Kawai, et al., 1999; Purdon, et al., 2001; Watanabe, et al., 2002), audition (Davis and Ahroon, 1982), and vision (Burke and Eisner, 2002), is influenced by hormones and changes throughout the menstrual cycle (for review see Parlee, 1983). Interestingly, evidence for visual mate preference as a function of reproductive state has been reported for female humans where menstrual cycle stage correlates with preference for type of male face. Ovulating females preferred more masculine faces, but when the same females were not ovulating they showed a bias toward more feminine male faces (Penton-Voak et al., 1999).
Interestingly, in fish steroid hormone receptors are present in the eye (Kobayashi et al., 1998; Wickham et al., 2000), and it is conceivable that cyclic changes in hormone levels could alter visual sensitivity and/or pattern procession to influence preference and behavior. Manipulations of key hormones and/or their receptors may reveal potential mechanisms for the differential selectivity of females demonstrated here.
We thank Anna K. Greenwood, Michael D. Scanlon, Jo Martin, Megan Corty, and two anonymous reviewers for helpful comments on a previous version and Amanda L. Knier for technical help. This research was supported by NIH NS 34950 Javits Award to R.D.F. and the Albion Walter Hewlett Stanford Graduate Fellowship to K.E.G.
References
Amundsen T, and Forsgren E,
Bakker TCM, Künzler R, and Mazzi D,
Becker JB, Breedlove SM, Crews D (eds),
Couldridge VCK,
Davis MJ, and Ahroon WA,
Dominey WJ,
Ferkin MH, and Zucker I,
Fernald RD,
Fernald RD, and Hirata NR,
Fernald RD, and Hirata NR,
Fox HE, White SA, Kao MHF, and Fernald RD,
Fraley NB, and Fernald RD,
Gaumond I, Arsenault P, and Marchand S,
Hellstrom B, and Lundberg U,
Hirata NR, and Fernald RD,
Hofmann HA, Benson ME, and Fernald RD,
Hofmann HA, and Fernald RD,
Jordan R, Kellogg K, Juanes F, and Stauffer J Jr,
Kawai F, Kurahashi T, and Kaneko A,
Kellogg KA, Stauffer JR Jr., and McKaye KR,
Kerem M, Akbayrak T, Bumin G, Yigiter K, Armutlu K, and Kerimoglu D,
Kobayashi K, Kobayashi H, Ueda M, and Honda Y,
Kodric-Brown A,
Kodric-Brown A, and Johnson SC,
Lande R, Seehausen O, and van Alphen JJM,
Luttbeg B, Towner MC, Wandesforde-Smith A, Mangel M, and Foster SA,
Maney DL, Richardson RD, and Windfield JC,
Michael RP, and Zumpe D,
Parlee MB,
Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray IK, and Minamisawa R,
Purdon SE, Klein S, and Flor-Henry P,
Schiml PA, and Rissman EF,
Seehausen O, Witte F, van Alphen JJM, and Bouton N,
Soma KK, Francis RC, Wingfield JC, and Fernald RD,
Wallen K,
Watanabe K, Umezu K, and Kurahashi T,
Werner NY, and Lotem A,
White SA, and Fernald RD,
Wickham LA, Gao J, Toda I, Rocha EM, Ono M, and Sullivan DA,