-
PDF
- Split View
-
Views
-
Cite
Cite
DALE R. WALTERS, TRACY COWLEY, HANS WEBER, Rapid Accumulation of Trihydroxy Oxylipins and Resistance to the Bean Rust Pathogen Uromyces fabae Following Wounding in Vicia faba, Annals of Botany, Volume 97, Issue 5, May 2006, Pages 779–784, https://doi.org/10.1093/aob/mcl034
Close - Share Icon Share
Abstract
• Background and Aims Insect damage to plants leads to wound-activated responses directed to healing of damaged tissues, as well as activation of defences to prevent further insect damage. Negative cross-talk exists between the jasmonic acid-based signalling system that is activated upon insect attack and the salicylic acid-based system frequently activated following pathogen infection. Thus, insect attack may compromise the ability of the plant to defend itself against pathogens and vice versa. However, insect herbivory and mechanical wounding have been shown to reduce fungal infections on some plants, although the underlying mechanisms remain to be defined. This work examines the effects of mechanical wounding on rust infection both locally and systemically in the broad bean, Vicia faba and follows changes in oxylipins in wounded leaves and unwounded leaves on wounded plants.
• Methods The lamina of first leaves was wounded by crushing with forceps, and first and second leaves were then inoculated, separately, with the rust Uromyces fabae at various times over a 24 h period. Wounded first leaves and unwounded second leaves were harvested at intervals over a 24 h period and used for analysis of oxylipin profiles.
• Key Results Mechanical wounding of first leaves of broad bean led to significantly reduced rust infection in the wounded first leaf as well as the unwounded second leaf. Increased resistance to infection was induced in plants inoculated with rust just 1 h after wounding and was accompanied by rapid and significant accumulation of jasmonic acid and two trihydroxy oxylipins in both wounded first leaves and unwounded second leaves. The two trihydroxy oxylipins were found to possess antifungal properties, reducing germination of rust spores.
• Conclusions These results demonstrate the rapidity with which resistance to pathogen infection can be induced following wounding and provides a possible mechanism by which pathogen infection might be halted.
INTRODUCTION
Insect herbivory is a common source of injury to plants and leads to wound-activated responses directed to healing of damaged tissues and activation of defences to prevent further damage (Leon et al., 2001). Wounding leads to the rapid (minutes to several hours) induction of a series of events, including the generation and release of specific signals, the subsequent perception and transduction of those signals and, finally, activation of wound-related defences (Leon et al., 2001). Insect herbivory will activate defences both locally in the damaged tissue and systemically in distant tissues (Leon et al., 2001; Cowley and Walters, 2005a). Following on from the early events that occur within the first few minutes after wounding, secondary signals are generated, leading to the further propagation of defence responses locally and systemically. Prominent among these secondary signals are oxylipins (oxygenated fatty acids), including jasmonic acid (JA), which plays a dominant role in regulating some forms of induced plant resistance to insect attack (Wasternack and Parthier, 1997; Bostock, 2005). Many oxylipins and other lipid oxidation products are produced in plants under attack by herbivores, suggesting that they have several roles, including as anti-insect compounds (Feussner and Wasternack, 2002; Howe and Schilmiller, 2002). In contrast to host responses to insect attack, systemic acquired resistance (SAR), which develops systemically following prior challenge with a variety of agents including necrotizing pathogens, is dependent upon the action of salicylic acid (SA; Bostock, 2005). However, the situation is far more complex than this apparently simple split between JA regulation of defence against insects and SA regulation of defences against pathogens. Thus, there is much evidence for SA signalling in plant resistance to biotrophic pathogens and JA/ethylene signalling in resistance to necrotrophic pathogens (Glazebrook, 2005), while there are some reports suggesting a role for SA in signalling resistance to insect herbivores (Walling, 2000; Ollerstam and Larsson, 2003).
There are many reports of both positive and negative cross-talk between the JA-based signalling that is activated upon insect attack and the SA-based signalling system activated following pathogen infection (Bostock, 2005). Negative cross-talk is manifested in the reports of attack by insects compromising the ability of the plant to defend itself against pathogens and vice versa (Bostock, 1999, 2005). However, there are examples of insect herbivory leading to reduced pathogen infection (Hatcher and Paul, 2000), although little is known of the mechanisms underlying these effects. Here, we examine the effects of wounding on rust infection in broad bean and, as a first step towards understanding the mechanisms underlying the responses observed, we examined oxylipin profiles in wounded and unwounded leaves.
MATERIALS AND METHODS
Growth of plants, wounding and inoculation with rust
Broad bean seeds (Vicia faba L. ‘Aquadulce’) were sown in 10-cm pots, one plant per pot, in Fisons Levington M3 compost. Plants were grown in a ventilated glasshouse at a temperature of 15 °C, venting at 22 °C, and daylight was supplemented with 400 W sodium lamps to produce a 16 h photoperiod. Plants with the first two leaves fully formed were used in experiments. First leaves of experimental plants were wounded by crushing the leaf three times across the lamina using forceps, a process which wounded approx. 40 % of the leaf area. In all experiments, plants were wounded at 08:00 h and then returned to the growth chamber for various periods (up to 24 h after wounding), after which wounded first leaves and unwounded second leaves of some plants were harvested and frozen immediately in liquid nitrogen for subsequent analysis. The remaining plants were used to assess the effect of wounding on rust infection. For this, first and second leaves were inoculated with rust (Uromyces fabae) by applying a spore suspension (0.3 g of spores in 100 mL of distilled water containing 0.01 % Tween-20) with a soft camel hair brush. Inoculated plants were then covered with polythene bags for 24 h to maintain the high humidity required for spore germination. Intensity of rust infection was assessed 2 weeks later by counting numbers of rust pustules on individual leaves. Infection was expressed as numbers of rust pustules cm−2 of leaf.
Oxylipin determination
Oxylipins were analysed using the oxylipin signature method of Weber et al. (1997) with modifications. Frozen leaves (1 g) were ground in a mortar to a fine powder and added to 3.5 mL of ice-cold methanol containing 100 ng of each internal standard. The solution was homogenized immediately for 1 min on ice, after which extraction of the fatty acids was continued by rotating the samples in tubes for 2 h at 4 °C. Ice-cold water (1.5 mL) was added and samples were kept for 5 min on ice. After centrifugation, the supernatant was adjusted to pH 8–9 with 1 m NH4OH and passed through a C18 cartridge (Bakerbond™ spe glass octadecyl, 8 mL, 500 mg; Mallinckrodt Baker, Griesheim, Germany), which had been pre-washed with methanol followed by methanol/water (70 : 30, v/v). The column was washed with an additional 7 mL of methanol/water (75 : 25 v/v) and both the eluates were combined. The pH of the eluates was adjusted to pH 3–4 with 10% formic acid and eluates were diluted to 50 mL with water. Samples were loaded on a C18 cartridge (Bakerbond™ spe glass octadecyl, 8 mL, 500 mg) which had been pre-conditioned with methanol, diethyl ether, methanol and water. The cartridge was washed with 7 mL of 15 % ethanol and 7 mL of water. Oxylipins were eluted with 10 mL of diethyl ether. The ether was dried over anhydrous magnesium sulphate and evaporated under a stream of nitrogen at 40 °C. Fatty acids were methylated with 450 μL of diazomethane in ether, dried under nitrogen and redissolved in 120 μL of acetonitrile for derivatization with N,O-bis[trimethylsilyl]trifluoroacetamide (BSTFA; Pierce Biotechnology, Rockford, IL, USA). Samples were dried under nitrogen and redissolved in 1 mL of hexane. The samples were loaded onto a silica cartridge (Bakerbond™ spe glass Florisil, 8 mL, 500 mg), which had been pre-washed first with diethyl ether and then hexane. The loaded column was washed with 5 mL of hexane, and the fatty acids were eluted with 7 mL of diethyl ether/ethyl acetate (1 : 1, v/v). After evaporation of the solvent under nitrogen, the samples were taken up in 10 μL of hexane and stored at –80 °C. Fatty acids were analysed by gas chromatography–mass spectrometry (Fisons MD-800; electron ionization mode, 70 eV electron potential, 11 p.s.i. column head pressure, HP-5MS column 30 m × 0.25 mm). The temperature gradient was 100 °C for 1 min, 100–160 °C at 20 °C min−1, 160–280 °C at 3 °C min−1 and 280–300 °C at 30 °C min−1. Internal standards used were methyl-3R,7R-dihydrojasmonate for the quantitation of JA, and 9,10,16-trihydroxyhexadecanoic acid for the quantitation of trihydroxy fatty acids. Quantitation was done using selective ion monitoring measuring ions m/z = 226 for methyl-3R,7R-dihydrojasmonate, m/z = 224 for JA, m/z = 259 for 9,10,16-trihydroxyhexadecenoic acid, m/z = 173 for 9,12,13-trihydroxy-10(E)-octadecenoic acid (TriOHE1) and m/z = 171 for 9,12,13-trihydroxy-10(E),15(Z)-octadecadienoic acid (TriOHE2). Recoveries of the internal standards were as follows: 54 % for dihydrojasmonic acid and 62 % for 9,10,16-trihydroxyhexadecanoic acid.
Determining the effects of JA and trihydroxy oxylipins on germination of rust spores
Rust uredospores were collected from 10-d-old pustules and suspended in deionized water containing 0.05 mL mL−1 of Triton B (approx. 20 000 spores mL−1). Germination tests were carried out in Petri dishes containing 2 % water agar. Treated plates were amended with 1–60 μm of the trihydroxy oxylipins and JA (Larodan Fine Chemicals, Malmo, Sweden) (suspended in a mixture of acetone and water, 1+1 by volume), while control plates were amended with acetone and water only. Each Petri dish received 0.5 mL of the spore suspension evenly distributed on the medium, and plates were incubated at 20 °C for 8 h before a drop of lactophenol cotton blue was deposited in the centre of each dish to prevent further germination. Germinated and ungerminated spores (100 in total for each treatment, with each treatment replicated four times) were counted using a compound microscope (150×) in randomly selected ocular fields (1.91 mm2) in each Petri dish. Spores with a germ tube length greater than or equal to the spore diameter were considered germinated.
Statistical analysis
Data are presented as means ± s.e.m. of four or five replicates and were subjected to analysis of variance using the Genstat 5 statistical program (Lawes Agricultural Trust). Differences between means from controls and treatments were tested for significance using the Student's t-test. Experiments were repeated once with similar results.
RESULTS
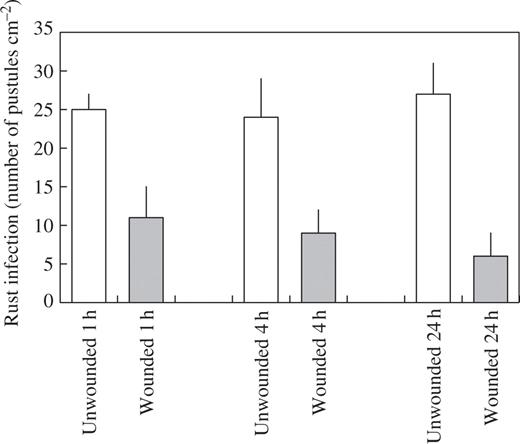
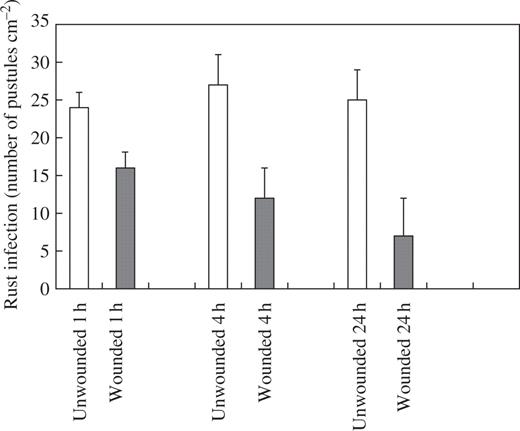
When plants were examined 2 weeks after inoculation, there was a 56 % reduction in rust infection on first leaves that had been inoculated 1 h after wounding (Fig. 1). First leaves that were inoculated 4 h following wounding showed a 63 % reduction in rust infection, while the largest reduction in infection (78 %) was found on first leaves that had been challenged with rust 24 h after wounding (Fig. 1). In addition to this local effect on rust infection, a systemic effect was also observed. Rust infection was reduced on second leaves of plants where the first leaf had been wounded, with the greatest reduction in infection obtained on second leaves 24 h after infliction of the wound to first leaves (Fig. 2).
Effect of wounding first leaves of broad bean on rust infection of first leaves. Leaves were inoculated with rust 1, 4 and 24 h after wounding. Values represent means ± s.e.m. of five replicates. All treatments are significantly different from the unwounded controls at P ≤ 0.01.
Effect of wounding first leaves of broad bean on rust infection of second leaves. Second leaves were inoculated with rust 1, 4 and 24 h after wounding. Values represent means ± s.e.m. of five replicates. All treatments are significantly different from the unwounded controls: wounded 1 h P ≤ 0.05; wounded 4 and 24 h P ≤ 0.01.
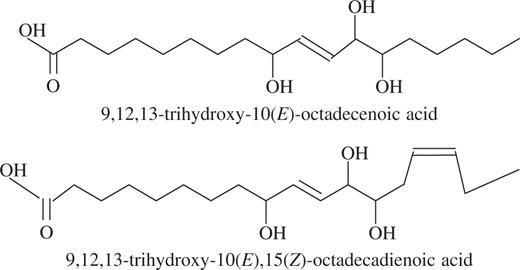
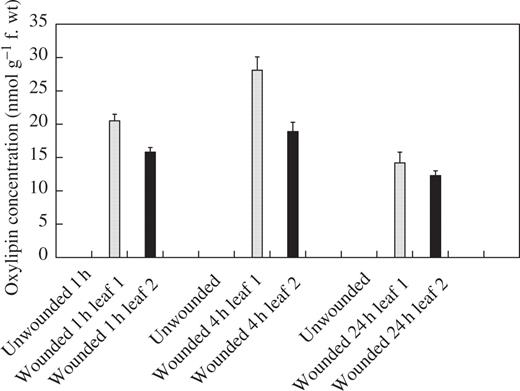
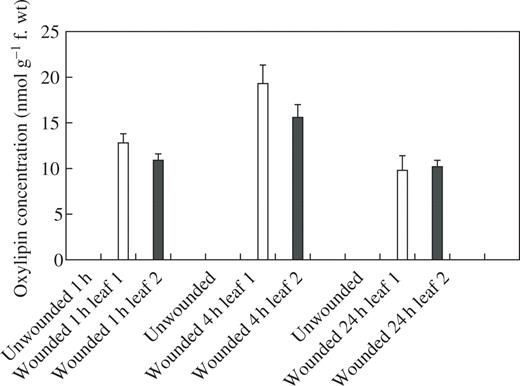
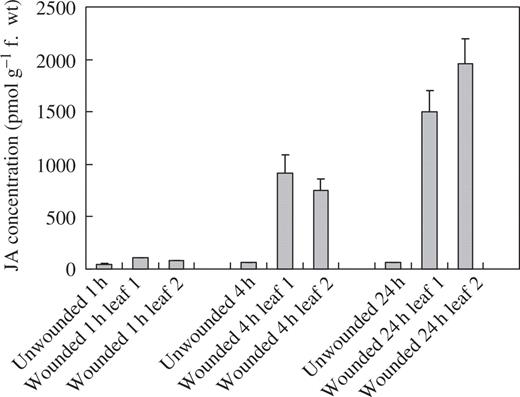
In an attempt to provide information on the mechanisms underlying the wound-induced reductions in rust infection described above, a study of the oxylipin profiles of unwounded and wounded leaves was undertaken, using a method for the global analysis and quantification of oxylipins (Weber et al., 1997). Using this method, two oxylipins appeared in the profiles of both wounded first leaves and second leaves on wounded plants, but were absent from profiles for unwounded plants. Based on their mass spectra and comparison with known standards, the two oxylipins were identified as TriHOE1 and TriHOE2 (Fig. 3). TriHOE1 was present in wounded first leaves and second leaves on wounded plants in appreciable concentrations (20.5 and 15.8 nmol g−1 f. wt, respectively, 1 h after wounding; Fig. 4). Concentrations of the TriHOE1 increased further 4 h after wounding (28.1 and 18.9 nmol g−1 f. wt in first and second leaves, respectively), but had declined to 14.2 and 12.3 nmol g−1 f. wt, 24 h after wounding (Fig. 4). Concentrations of TriHOE2 followed a similar trend, with highest concentrations recorded 4 h after wounding (19.3 and 15.6 nmol g−1 f. wt in wounded first leaves and unwounded second leaves, respectively; Fig. 5). JA was detected in unwounded leaves and its concentration increased considerably and significantly following wounding, in both the wounded first leaf and the unwounded second leaf (Fig. 6).
Effect of wounding first leaves of broad bean on concentrations of TriHOE1 in first (open bar) and second leaves (black bar). TriHOE1 was not detected in unwounded plants. Values represent means ± s.e.m. of four replicates.
Effect of wounding first leaves of broad bean on concentrations of TriHOE2 in first (open bar) and second leaves (black bar). TriHOE2 was not detected in unwounded plants. Values represent means ± s.e.m. of four replicates.
Effect of wounding first leaves of broad bean on jasmonic acid (JA) concentrations in first and second leaves. Values represent means ± s.e.m. of four replicates. Treatments are significantly different from unwounded controls at 4 and 24 h (P ≤ 0.001).
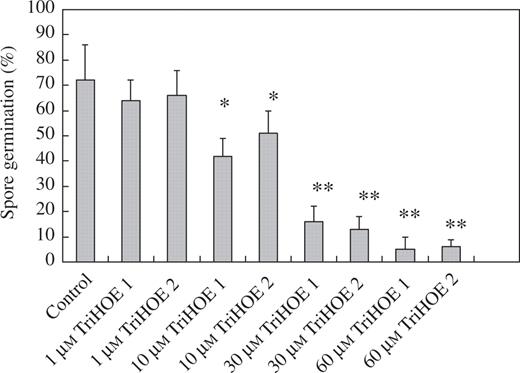
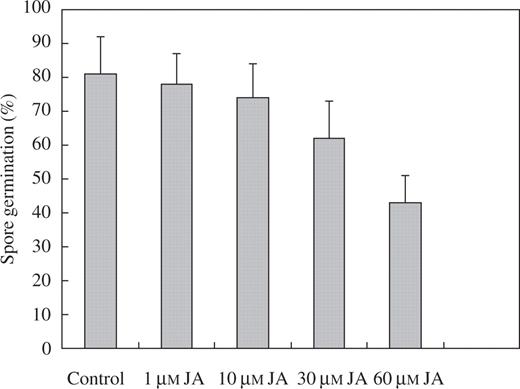
In an attempt to determine whether these two trihydroxy oxylipins could be responsible, at least in part, for the wound-induced reductions in rust infection, the effects of TriHOE1 and TriHOE2 on the germination of uredospores of the rust were examined. When rust uredospores were placed onto glass slides coated with 1–60 μm of the trihydroxy oxylipins, little inhibitory effect was observed with 1 μm of the oxylipins, while concentrations of ≥10 μm produced significant reductions in spore germination. When used at 60 μm, TriHOE1 reduced germination by 93 %, while TriHOE2 reduced germination by 89 % (Fig. 7). In contrast, JA exhibited considerably less inhibitory activity, with the only significant reduction in spore germination occurring at 60 μm (Fig. 8).
Effects of 1–60 μm TriHOE1 and TriHOE2 on germination of uredospores of U. fabae. Values represent means ± s.e.m. of four replicates (each replicate represents 100 counted spores). Significant differences from the control are shown at *P ≤ 0.01 and **P ≤ 0.001.
Effects of 1–60 μm JA on germination of uredospores of U. fabae. Values represent means ± s.e.m. of four replicates (each replicate represents 100 counted spores). Only 60 μm JA was significantly different from control (*P ≤ 0.01).
DISCUSSION
These data show that wounding the first leaves of broad bean plants induces protection against rust infection both in the wounded leaf and in the unwounded leaf on the same plant. These data agree with previous reports of insect herbivory on pathogen infection. For example, laboratory experiments by Hatcher et al. (1994, 1995) demonstrated the inhibitory effect of feeding by the beetle Gastrophysa viridula on rust infection on Rumex spp., while in field experiments, Hatcher and Paul (2000) showed that herbivory by G. viridula significantly reduced infection not only by rust (U. rumicis), but also by fungal pathogens from different functional groups: Venturia rumicis and Ramularia rubella. There are a number of other reports of reduced pathogen infection following insect feeding. Thus, Padgett et al. (1994) found that defoliation of soybean by larvae of the moth Pseudoplusia includens led to reduced infection by the pathogen Diaporthe phaseolorum, while Colletotrichum orbiculare lesion density decreased on watermelon previously infested with thrips or aphids (Russo et al., 1997). However, there are also reports of insect feeding compromising the ability of plants to ward off pathogen attack (e.g. Klepzig et al., 1997) as well as reports of no effect of insect feeding on pathogen susceptibility (e.g. Moran and Schultz, 1998). Perhaps such inconsistency should not be surprising in view of the complexity of signalling for pest and disease resistance (Bostock, 2005). Although SA is necessary for the full expression of both local resistance and SAR to pathogen infection (Gaffney et al., 1993; Delaney et al., 1994) and JA is involved in systemic responses to wounding and insect herbivory (Constabel et al., 1995; McConn et al., 1997), JA is an important regulator of defences against necrotrophic pathogens (Glazebrook, 2005), while SA has been shown to be involved in signalling for resistance to insect attack (Walling, 2000; Ollerstam and Larsson, 2003; Bostock, 2005). As befits the complexity of signalling for resistance to pathogens and pests, there are reports of both positive and negative cross-talk between the different signalling pathways (Bostock, 2005). Pharmacological experiments have demonstrated negative cross-talk between plant responses to pathogen infection and responses to wounding (Doherty et al., 1988; Doares et al., 1995), and Felton et al. (1999) showed that in tobacco expressing reduced levels of phenylalanine ammonia lyase (PAL), SAR to tobacco mosaic virus infection was reduced, while systemic resistance to grazing by larvae of Heliothis virescens was increased.
Nevertheless, for the various reports of herbivore-induced reductions in plant susceptibility to fungal pathogens, the underlying mechanisms remain largely undefined. Mechanical wounding of leaves is known to induce local and systemic accumulation of proteinase inhibitor, polyphenol oxidase, PAL, chalcone synthase and hydroxyproline-rich glycoprotein RNAs (Green and Ryan, 1972; Lawton and Lamb, 1987; Constabel et al., 1995). In fact, mechanical wounding of one leaf of young rice plants led to reduced infection of unwounded leaves by the rice blast pathogen Magnaporthe grisea and transient accumulation of JA in both the wounded and unwounded leaves (Schweizer et al., 1998). The wound-induced reductions of rust infection and the increase in JA in broad bean confirm this work, although the appearance of systemic protection in the present work is considerably more rapid and the pathogen protected against is a biotroph and not a necrotroph. Although JA-dependent responses do not seem to play a major role in resistance against biotrophs, they can be effective in Arabidopsis against Peronospora parasitica and Erysiphe spp. if induced prior to pathogen challenge (Glazebrook, 2005). Whether the increased levels of JA were involved in signalling for defence responses to rust infection or were exerting a direct, inhibitory effect on the fungus in planta is not clear from this work. Thus, although reductions in rust infection were detected both locally and systemically just 1 h after wounding, rust infection was reduced even further 24 h after wounding (Figs 1 and 2). In contrast, JA concentrations were relatively unchanged 1 h after wounding, with very large increases occurring 4 and 24 h after damage (Fig. 6). If JA was involved in signalling for defence responses to rust infection, then increases in JA prior to any reductions in rust infection might have been expected. It is possible that wounding increased endogenous levels of SA in the bean plants, since it is known that methyl salicylate is generated following aphid feeding in maize (Bernasconi et al., 1998), and feeding by larvae of the noctuid Helicoverpa zea induces systemic increases in SA in cotton (Bi et al., 1997). However, since we did not determine SA levels in this work, we cannot rule out the possibility that the effects on rust infection were mediated via SA. Moreover, although maximum JA levels coincided with the greatest reductions in rust infection, a direct inhibitory effect on the rust in planta seems unlikely given the lack of any significant antifungal effect of exogenous JA on germination of rust spores (Fig. 8). Clearly, clarifying the role of JA in resistance to rust infection in wounded broad bean will require further work.
The present work also demonstrates the rapid accumulation of two trihydroxy oxylipins in both wounded and unwounded leaves on the same plant. It has been shown previously that trihydroxy oxylipins are capable of inducing plant defence responses and protection against powdery mildew in barley (Cowley and Walters, 2005b), and other workers have demonstrated antifungal activity for several trihydroxy oxylipins (Kato et al., 1986; Masui et al., 1989). Of course, for the trihydroxy oxylipins to exert an effect on rust infection and subsequent growth in planta, they would need to be present in the right place and at concentrations sufficient to inhibit the fungus. Although this work does not provide this information, it does present the first report of rapid wound-induced trihydroxy oxylipin accumulation both locally and systemically, associated with significant reductions in rust infection. These data, together with evidence that the jasmonic acid pathway might be involved in systemic induced resistance to pathogen infection (Vijayan et al., 1998; Staswick and Lehman, 1999; Walters et al., 2002) and the effects of beetle grazing on fungal infection (Hatcher and Paul, 2000), suggest that cross-phyla-induced resistance might be more common than previously thought.
The Scottish Agricultural College receives financial support from the Scottish Executive Environment and Rural Affairs Department.
LITERATURE CITED
Bostock RM.
Bostock RM.
Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S.
Bi JL, Murphy JB, Felton GW.
Constabel CP, Bergey DR, Ryan CA.
Cowley T, Walters DR.
Cowley T, Walters D.
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weyman K, Negrotto D, et al.
Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA.
Doherty HM, Selvendran RR, Bowles DJ.
Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA.
Feussner I, Wasternack C.
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J.
Glazebrook J.
Green TR, Ryan CA.
Hatcher PE, Paul ND.
Hatcher PE, Paul ND, Ayres PG, Whittaker JB.
Hatcher PE, Ayres PG, Paul ND.
Howe GA, Schilmiller AL.
Kato T, Yamaguchi Y, Ohnuma S, Uyehara T, Namai T, Kodama M, Shiobara Y.
Klepzig KD, Robison DJ, Smalley EB, Raffa KF.
Lawton MA, Lamb CJ.
Leon J, Rojo E, Sanchez-Serrano JJ.
Masui H, Kondo T, Kojima M.
McConn M, Creelman RA, Bell E, Mullet JE, Browse J.
Moran P, Schultz JC.
Ollerstam O, Larsson S.
Padgett GB, Russin JS, Snow JP, Boethel DJ, Berggren GT.
Russo VM, Russo BM, Peters M, Perkins-Veazie P, Cartwright B.
Schweizer P, Buchala A, Dudler R, Metraux J-P.
Staswick PE, Lehman CC.
Vijayan, P, Shockey J, Levesque CA, Cook RJ, Browse J.
Walling LL.
Walters DR, Cowley T, Mitchell AF.
Wasternack C, Parthier B.
Author notes
1Crop and Soil Research Group, Scottish Agricultural College, King's Buildings, West Mains Road, Edinburgh EH9 3JG, UK and 2DNA Array Facility, Center for Integrated Genomics, BEP, University of Lausanne, CH-1015 Lausanne, Switzerland