-
PDF
- Split View
-
Views
-
Cite
Cite
Colleen C. McLaughlin, Mark S. Baptiste, Maria J. Schymura, Philip C. Nasca, Michael S. Zdeb, Maternal and Infant Birth Characteristics and Hepatoblastoma, American Journal of Epidemiology, Volume 163, Issue 9, 1 May 2006, Pages 818–828, https://doi.org/10.1093/aje/kwj104
Close - Share Icon Share
Abstract
Hepatoblastoma is a rare embryonal tumor with unknown etiology. The authors conducted a case-cohort study using public health surveillance data sets to examine perinatal risk factors for hepatoblastoma. Hepatoblastoma cases (n = 58) diagnosed between 1985 and 2001 were identified from the New York State Cancer Registry and were matched to electronic birth records for 1985–2001 from New York State, excluding New York City. Controls (n = 6,056) were selected from the birth cohorts for the same years. Having a birth weight less than 1,000 g was associated with a strongly increased risk of hepatoblastoma (relative risk (RR) = 56.9, 95% confidence interval (CI): 24.0, 130.7). After adjustment for birth weight, a moderately increased risk of hepatoblastoma was found for younger maternal age (<20 years vs. 20–29 years: RR = 2.5, 95% CI: 1.0, 5.5), presumptive use of infertility treatment (RR = 9.2, 95% CI: 2.1, 31.5), maternal smoking (RR = 2.1, 95% CI: 1.0, 4.2), and higher maternal prepregnancy body mass index (body mass index of 25–29 vs. 20–24: RR = 2.9, 95% CI: 1.2, 7.6).
Relatively little is known about the etiology of hepatoblastoma, other than the fact that increased risk is associated with several congenital or genetic syndromes. Increased risk has been observed with familial adenomatous polyposis and Gardner's syndrome, both of which are related to the adenomatous polyposis coli (APC) gene (1, 2). The association between the APC tumor suppressor gene and hepatoblastoma maybe a consequence of the role APC plays in regulating β-catenin, since the β-catenin gene (cadherin-associated protein β1 (CTNNB1)) has been found to have a high frequency of mutations in persons with sporadic hepatoblastomas (3–6). Hepatoblastoma has also been observed among children with Beckwith-Wiedemann syndrome and hemihypertrophy (7, 8). Beckwith-Wiedemann syndrome, and perhaps some cases of hemihypertrophy, are caused by disregulation of chromosome 11p15, a region that includes several genes involved in growth regulation as well as the expression of insulin-like growth factor 2 (9, 10). Imprinting disorders related to chromosome 11p15 have also been observed in persons with sporadic hepatoblastomas (11–13).
A possibly increased risk of hepatoblastoma with prematurity was first reported in a case series from Michigan State University in 1996 (14). This was followed by several case series and reports of hepatoblastoma among premature (<38 weeks) or low birth weight (<2,500 g) infants from the United States and Japan (14–19). In an analysis of birth weights from the Japan Children's Cancer Registry, Ikeda et al. (17) reported that, over time, hepatoblastoma accounted for an increasing percentage of cancers among very low birth weight infants—increasing from 0.7 percent of tumors to 8.6 percent of tumors among these infants in 1985–1989 as compared with 1990–1993. In addition, a significant portion of the hepatoblastoma cases had extremely low birth weights (<1,000 g). Further analysis of the Japan Children's Cancer Registry data showed that the increase in the proportion of cancers among extremely low birth weight infants with hepatoblastoma coincided with improved survival of these infants starting in 1988, and there appeared to be a trend towards higher risk with lower birth weight (20). Similarly, increases in hepatoblastoma incidence in the United States appeared to coincide with increased survival of very low birth weight and extremely low birth weight infants (21). Increased risk of hepatoblastoma among very low birth weight infants, particularly extremely low birth weight infants, was subsequently confirmed in two case-control studies (22, 23).
We used existing data to perform a case-cohort study of hepatoblastoma and perinatal risk factors. We hypothesized that very low birth weight would be associated with hepatoblastoma risk. The other analyses described here were exploratory.
MATERIALS AND METHODS
Study subjects
Cases of hepatoblastoma were ascertained from the New York State Cancer Registry. All case infants aged 1 month to 5 years who had been born in New York State, excluding New York City, and were diagnosed with hepatoblastoma between 1985 and 2001 while living in New York State were eligible for inclusion in the study. Eligibility was determined by matching the hepatoblastoma cases in the cancer registry to electronic birth files maintained by the New York State Department of Health for 1985–2001. Because the New York State birth certificate differs from the certificates used in New York City and in other states, children who were found to have been born in New York City or out of state were excluded, as were children who were not matched to electronic birth certificate records.
Controls were selected from the electronic birth files of all children born in New York State, excluding New York City, between 1985 and 2001. This study was a subset of a larger study that included cases of childhood cancer at all cancer sites (24). Two controls were selected for each cancer case included in the larger study, giving us 6,056 controls. Because the data elements available from the birth records vary by year of birth, controls were frequency-matched to the cases in the larger study on the basis of birth year, to assure consistency of availability of information. For the purposes of individual cancer types, frequency of birth year was not necessarily proportional between cases and controls. No hepatoblastoma cases were also selected as controls, although seven control children were also in the cancer-birth match and therefore were known to have developed other forms of cancer. Children who died during the neonatal period (within 28 days of birth) were also excluded, because cases diagnosed at less than 1 month of age were excluded from the case series.
Perinatal data
The independent variables for this study came from the birth certificates of cases and controls. The birth certificates are completed by staff at the hospital where the child is born. Data from the birth certificates are routinely abstracted and entered into an electronic master livebirth file and were available for the birth cohorts included in this study (1985–2001). The New York State birth certificate underwent major revisions in 1988 and 1993. In addition to changes in the birth certificate itself, the data items available in the master livebirth file also changed, as did the coding of individual variables. Because of these changes, data on some independent variables were not available for analysis for all cases.
Analysis
This study had a case-cohort design; therefore, the odds ratio provides an estimate of the cumulative incidence (risk) ratio (25). Continuous variables, such as infant birth weight and maternal age, were grouped into ordinal categories based on a priori groupings determined by previous studies or on the distribution of the data within the study group. Separate analyses were conducted for hepatoblastoma overall and after stratification by age at diagnosis. Unconditional logistic regression was used for multivariate analysis. Because the prevalence of some exposures changed during the course of the study period, birth year was included in all regression models as a continuous variable. In addition, all models included birth weight.
In some cases, multiple variables from the birth files were combined to create a summary measure. For example, a child was considered to have been exposed to maternal hypertension during pregnancy if the mother was recorded as having had pregnancy-induced hypertension, preeclampsia, eclampsia, toxemia, or chronic hypertension. Size for gestational age was calculated on the basis of length of gestation and birth weight (26). Maternal use of government assistance included receipt of Aid to Families with Dependent Children or use of the Women, Infants, and Children Program, the Prenatal Care Assistance Program, the Supplemental Nutrition Assistance Program, or the Medicaid Obstetrical and Maternal Services Program.
Starting in 1993, the New York State birth certificate included check-boxes for “in vitro fertilization” and “other fertilization.” “Other fertilization” potentially includes the use of fertility-enhancing drugs, artificial insemination, and other techniques which do not fall under the heading of in vitro fertilization. In addition to examining hepatoblastoma risk for these two exposures separately, we created two composite variables for examining exposure to infertility treatment. The first composite variable included exposure to either in vitro fertilization or other fertilization. The second composite variable included both of these exposures, as well as births involving triplets (no higher-order births were observed among either cases or controls). This variable was calculated only for births occurring in 1993 or later, since information on in vitro or other assisted fertilization was not available for earlier births. There was some overlap, such that one case and two controls who were part of triplet births were also coded as having been exposed to infertility treatment.
RESULTS
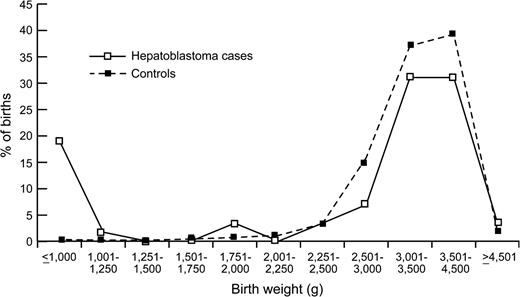
During the study period, 67 cases of hepatoblastoma were diagnosed in New York State, excluding New York City, among infants who were eligible for inclusion in the study. Birth certificate matches were made for 58 cases, resulting in a match proportion of 87 percent. Eleven (19 percent) of the 58 hepatoblastoma cases in the study had extremely low birth weight (<1,000 g; table 1). This compares with only 0.4 percent of controls in this birth weight range, resulting in a relative risk estimate of 56.9 for extremely low birth weight (95 percent confidence interval (CI): 24.0, 130.7) relative to normal birth weight (2,500–3,499 g). Although the test for trend produced a statistically significant p value, there was little evidence of a linear dose effect, and the birth weight distributions for cases and controls were very similar at birth weights above 1,000 g (figure 1). There was no evidence that the risk of hepatoblastoma with extremely low birth weight varied by age at diagnosis (data not shown). Cases and controls had different distributions of several other infant birth characteristics that are associated with low birth weight; cases had shorter gestation, lower 1-minute and 5-minute Apgar scores, and greater use of assisted ventilation. After adjustment for birth weight, however, male gender was the only infant characteristic that was associated with hepatoblastoma risk.
Distribution of the birth weights of hepatoblastoma cases and controls among children born in New York State, excluding New York City, 1985–2001.
Infant birth characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Birth weight (g) | |||||||||
| <1,000 | 11 | 19.0 | 24 | 0.4 | 56.9 | 24.0, 130.7 | |||
| 1,000–1,499 | 1 | 1.7 | 26 | 0.4 | 5.0 | 0.3, 25.5 | |||
| 1,500–2,499 | 4 | 6.9 | 339 | 5.6 | 1.6 | 0.5, 4.3 | |||
| 2,500–3,499 | 22 | 37.9 | 3,047 | 50.3 | 1 | Reference | |||
| 3,500–4,499 | 18 | 31.0 | 2,479 | 40.9 | 1.0 | 0.5, 1.9 | |||
| ≥4,500 | 2 | 3.4 | 130 | 2.1 | 2.1 | 0.3, 7.3 | <0.0001 | ||
| Missing data | 0 | 0.0 | 11 | 0.2 | |||||
| Gestational age (weeks) | |||||||||
| <35 | 12 | 20.7 | 198 | 3.3 | 1.0 | 0.2, 3.9 | |||
| 35–37 | 3 | 5.2 | 547 | 9.0 | 0.5 | 0.1, 1.6 | |||
| 38–41 | 35 | 60.3 | 4,099 | 67.7 | 1 | Reference | |||
| ≥42 | 5 | 8.6 | 1,041 | 17.2 | 0.6 | 0.2, 1.4 | 0.8159 | ||
| Missing data | 3 | 5.2 | 171 | 2.8 | |||||
| Size for gestational age | |||||||||
| Small (mean − 2 SD†) | 3 | 5.2 | 126 | 2.1 | 0.5 | 0.1, 2.1 | |||
| Normal (within 2 SD) | 49 | 84.5 | 5,312 | 87.7 | 1 | Reference | |||
| Large (mean + 2 SD) | 3 | 5.2 | 448 | 7.4 | 0.5 | 0.1, 2.6 | 0.9366 | ||
| Missing data | 3 | 5.2 | 170 | 2.8 | |||||
| 1-minute Apgar score | |||||||||
| 1–5 | 11 | 19.0 | 236 | 3.9 | 2.5 | 0.9, 6.8 | |||
| 6–8 | 23 | 39.7 | 2,335 | 38.6 | 1.5 | 0.8, 2.6 | |||
| 9–10 | 23 | 39.7 | 3,428 | 56.6 | 1 | Reference | 0.1988 | ||
| Missing data | 1 | 1.7 | 57 | 0.9 | |||||
| 5-minute Apgar score | |||||||||
| 1–8 | 12 | 20.7 | 473 | 7.8 | 1.4 | 0.5, 3.9 | |||
| 9 | 34 | 58.6 | 3,621 | 59.8 | 1.3 | 0.7, 2.7 | |||
| 10 | 11 | 19.0 | 1,910 | 31.5 | 1 | Reference | 0.4063 | ||
| Missing data | 1 | 1.7 | 52 | 0.9 | |||||
| Birth injury | |||||||||
| Yes | 2 | 3.4 | 178 | 2.9 | 0.7 | 0.1, 2.8 | |||
| No | 54 | 93.1 | 5,183 | 85.6 | 1 | Reference | |||
| Missing data | 2 | 3.4 | 695 | 11.5 | |||||
| Assisted ventilation (birth years 1988–2001) | |||||||||
| Yes | 7 | 12.1 | 251 | 4.1 | 0.5 | 0.1, 1.4 | |||
| No | 41 | 70.7 | 3,891 | 64.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,914 | 31.6 | |||||
| Meconium (birth years 1988–2001) | |||||||||
| Yes | 5 | 8.6 | 399 | 6.6 | 1.4 | 0.5, 3.4 | |||
| No | 43 | 74.1 | 3,835 | 63.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,822 | 30.1 | |||||
| Gender | |||||||||
| Male | 40 | 69.0 | 3,133 | 51.7 | 2.1 | 1.2, 3.9 | |||
| Female | 18 | 31.0 | 2,916 | 48.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 7 | 0.1 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Birth weight (g) | |||||||||
| <1,000 | 11 | 19.0 | 24 | 0.4 | 56.9 | 24.0, 130.7 | |||
| 1,000–1,499 | 1 | 1.7 | 26 | 0.4 | 5.0 | 0.3, 25.5 | |||
| 1,500–2,499 | 4 | 6.9 | 339 | 5.6 | 1.6 | 0.5, 4.3 | |||
| 2,500–3,499 | 22 | 37.9 | 3,047 | 50.3 | 1 | Reference | |||
| 3,500–4,499 | 18 | 31.0 | 2,479 | 40.9 | 1.0 | 0.5, 1.9 | |||
| ≥4,500 | 2 | 3.4 | 130 | 2.1 | 2.1 | 0.3, 7.3 | <0.0001 | ||
| Missing data | 0 | 0.0 | 11 | 0.2 | |||||
| Gestational age (weeks) | |||||||||
| <35 | 12 | 20.7 | 198 | 3.3 | 1.0 | 0.2, 3.9 | |||
| 35–37 | 3 | 5.2 | 547 | 9.0 | 0.5 | 0.1, 1.6 | |||
| 38–41 | 35 | 60.3 | 4,099 | 67.7 | 1 | Reference | |||
| ≥42 | 5 | 8.6 | 1,041 | 17.2 | 0.6 | 0.2, 1.4 | 0.8159 | ||
| Missing data | 3 | 5.2 | 171 | 2.8 | |||||
| Size for gestational age | |||||||||
| Small (mean − 2 SD†) | 3 | 5.2 | 126 | 2.1 | 0.5 | 0.1, 2.1 | |||
| Normal (within 2 SD) | 49 | 84.5 | 5,312 | 87.7 | 1 | Reference | |||
| Large (mean + 2 SD) | 3 | 5.2 | 448 | 7.4 | 0.5 | 0.1, 2.6 | 0.9366 | ||
| Missing data | 3 | 5.2 | 170 | 2.8 | |||||
| 1-minute Apgar score | |||||||||
| 1–5 | 11 | 19.0 | 236 | 3.9 | 2.5 | 0.9, 6.8 | |||
| 6–8 | 23 | 39.7 | 2,335 | 38.6 | 1.5 | 0.8, 2.6 | |||
| 9–10 | 23 | 39.7 | 3,428 | 56.6 | 1 | Reference | 0.1988 | ||
| Missing data | 1 | 1.7 | 57 | 0.9 | |||||
| 5-minute Apgar score | |||||||||
| 1–8 | 12 | 20.7 | 473 | 7.8 | 1.4 | 0.5, 3.9 | |||
| 9 | 34 | 58.6 | 3,621 | 59.8 | 1.3 | 0.7, 2.7 | |||
| 10 | 11 | 19.0 | 1,910 | 31.5 | 1 | Reference | 0.4063 | ||
| Missing data | 1 | 1.7 | 52 | 0.9 | |||||
| Birth injury | |||||||||
| Yes | 2 | 3.4 | 178 | 2.9 | 0.7 | 0.1, 2.8 | |||
| No | 54 | 93.1 | 5,183 | 85.6 | 1 | Reference | |||
| Missing data | 2 | 3.4 | 695 | 11.5 | |||||
| Assisted ventilation (birth years 1988–2001) | |||||||||
| Yes | 7 | 12.1 | 251 | 4.1 | 0.5 | 0.1, 1.4 | |||
| No | 41 | 70.7 | 3,891 | 64.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,914 | 31.6 | |||||
| Meconium (birth years 1988–2001) | |||||||||
| Yes | 5 | 8.6 | 399 | 6.6 | 1.4 | 0.5, 3.4 | |||
| No | 43 | 74.1 | 3,835 | 63.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,822 | 30.1 | |||||
| Gender | |||||||||
| Male | 40 | 69.0 | 3,133 | 51.7 | 2.1 | 1.2, 3.9 | |||
| Female | 18 | 31.0 | 2,916 | 48.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 7 | 0.1 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
SD, standard deviations.
Infant birth characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Birth weight (g) | |||||||||
| <1,000 | 11 | 19.0 | 24 | 0.4 | 56.9 | 24.0, 130.7 | |||
| 1,000–1,499 | 1 | 1.7 | 26 | 0.4 | 5.0 | 0.3, 25.5 | |||
| 1,500–2,499 | 4 | 6.9 | 339 | 5.6 | 1.6 | 0.5, 4.3 | |||
| 2,500–3,499 | 22 | 37.9 | 3,047 | 50.3 | 1 | Reference | |||
| 3,500–4,499 | 18 | 31.0 | 2,479 | 40.9 | 1.0 | 0.5, 1.9 | |||
| ≥4,500 | 2 | 3.4 | 130 | 2.1 | 2.1 | 0.3, 7.3 | <0.0001 | ||
| Missing data | 0 | 0.0 | 11 | 0.2 | |||||
| Gestational age (weeks) | |||||||||
| <35 | 12 | 20.7 | 198 | 3.3 | 1.0 | 0.2, 3.9 | |||
| 35–37 | 3 | 5.2 | 547 | 9.0 | 0.5 | 0.1, 1.6 | |||
| 38–41 | 35 | 60.3 | 4,099 | 67.7 | 1 | Reference | |||
| ≥42 | 5 | 8.6 | 1,041 | 17.2 | 0.6 | 0.2, 1.4 | 0.8159 | ||
| Missing data | 3 | 5.2 | 171 | 2.8 | |||||
| Size for gestational age | |||||||||
| Small (mean − 2 SD†) | 3 | 5.2 | 126 | 2.1 | 0.5 | 0.1, 2.1 | |||
| Normal (within 2 SD) | 49 | 84.5 | 5,312 | 87.7 | 1 | Reference | |||
| Large (mean + 2 SD) | 3 | 5.2 | 448 | 7.4 | 0.5 | 0.1, 2.6 | 0.9366 | ||
| Missing data | 3 | 5.2 | 170 | 2.8 | |||||
| 1-minute Apgar score | |||||||||
| 1–5 | 11 | 19.0 | 236 | 3.9 | 2.5 | 0.9, 6.8 | |||
| 6–8 | 23 | 39.7 | 2,335 | 38.6 | 1.5 | 0.8, 2.6 | |||
| 9–10 | 23 | 39.7 | 3,428 | 56.6 | 1 | Reference | 0.1988 | ||
| Missing data | 1 | 1.7 | 57 | 0.9 | |||||
| 5-minute Apgar score | |||||||||
| 1–8 | 12 | 20.7 | 473 | 7.8 | 1.4 | 0.5, 3.9 | |||
| 9 | 34 | 58.6 | 3,621 | 59.8 | 1.3 | 0.7, 2.7 | |||
| 10 | 11 | 19.0 | 1,910 | 31.5 | 1 | Reference | 0.4063 | ||
| Missing data | 1 | 1.7 | 52 | 0.9 | |||||
| Birth injury | |||||||||
| Yes | 2 | 3.4 | 178 | 2.9 | 0.7 | 0.1, 2.8 | |||
| No | 54 | 93.1 | 5,183 | 85.6 | 1 | Reference | |||
| Missing data | 2 | 3.4 | 695 | 11.5 | |||||
| Assisted ventilation (birth years 1988–2001) | |||||||||
| Yes | 7 | 12.1 | 251 | 4.1 | 0.5 | 0.1, 1.4 | |||
| No | 41 | 70.7 | 3,891 | 64.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,914 | 31.6 | |||||
| Meconium (birth years 1988–2001) | |||||||||
| Yes | 5 | 8.6 | 399 | 6.6 | 1.4 | 0.5, 3.4 | |||
| No | 43 | 74.1 | 3,835 | 63.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,822 | 30.1 | |||||
| Gender | |||||||||
| Male | 40 | 69.0 | 3,133 | 51.7 | 2.1 | 1.2, 3.9 | |||
| Female | 18 | 31.0 | 2,916 | 48.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 7 | 0.1 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Birth weight (g) | |||||||||
| <1,000 | 11 | 19.0 | 24 | 0.4 | 56.9 | 24.0, 130.7 | |||
| 1,000–1,499 | 1 | 1.7 | 26 | 0.4 | 5.0 | 0.3, 25.5 | |||
| 1,500–2,499 | 4 | 6.9 | 339 | 5.6 | 1.6 | 0.5, 4.3 | |||
| 2,500–3,499 | 22 | 37.9 | 3,047 | 50.3 | 1 | Reference | |||
| 3,500–4,499 | 18 | 31.0 | 2,479 | 40.9 | 1.0 | 0.5, 1.9 | |||
| ≥4,500 | 2 | 3.4 | 130 | 2.1 | 2.1 | 0.3, 7.3 | <0.0001 | ||
| Missing data | 0 | 0.0 | 11 | 0.2 | |||||
| Gestational age (weeks) | |||||||||
| <35 | 12 | 20.7 | 198 | 3.3 | 1.0 | 0.2, 3.9 | |||
| 35–37 | 3 | 5.2 | 547 | 9.0 | 0.5 | 0.1, 1.6 | |||
| 38–41 | 35 | 60.3 | 4,099 | 67.7 | 1 | Reference | |||
| ≥42 | 5 | 8.6 | 1,041 | 17.2 | 0.6 | 0.2, 1.4 | 0.8159 | ||
| Missing data | 3 | 5.2 | 171 | 2.8 | |||||
| Size for gestational age | |||||||||
| Small (mean − 2 SD†) | 3 | 5.2 | 126 | 2.1 | 0.5 | 0.1, 2.1 | |||
| Normal (within 2 SD) | 49 | 84.5 | 5,312 | 87.7 | 1 | Reference | |||
| Large (mean + 2 SD) | 3 | 5.2 | 448 | 7.4 | 0.5 | 0.1, 2.6 | 0.9366 | ||
| Missing data | 3 | 5.2 | 170 | 2.8 | |||||
| 1-minute Apgar score | |||||||||
| 1–5 | 11 | 19.0 | 236 | 3.9 | 2.5 | 0.9, 6.8 | |||
| 6–8 | 23 | 39.7 | 2,335 | 38.6 | 1.5 | 0.8, 2.6 | |||
| 9–10 | 23 | 39.7 | 3,428 | 56.6 | 1 | Reference | 0.1988 | ||
| Missing data | 1 | 1.7 | 57 | 0.9 | |||||
| 5-minute Apgar score | |||||||||
| 1–8 | 12 | 20.7 | 473 | 7.8 | 1.4 | 0.5, 3.9 | |||
| 9 | 34 | 58.6 | 3,621 | 59.8 | 1.3 | 0.7, 2.7 | |||
| 10 | 11 | 19.0 | 1,910 | 31.5 | 1 | Reference | 0.4063 | ||
| Missing data | 1 | 1.7 | 52 | 0.9 | |||||
| Birth injury | |||||||||
| Yes | 2 | 3.4 | 178 | 2.9 | 0.7 | 0.1, 2.8 | |||
| No | 54 | 93.1 | 5,183 | 85.6 | 1 | Reference | |||
| Missing data | 2 | 3.4 | 695 | 11.5 | |||||
| Assisted ventilation (birth years 1988–2001) | |||||||||
| Yes | 7 | 12.1 | 251 | 4.1 | 0.5 | 0.1, 1.4 | |||
| No | 41 | 70.7 | 3,891 | 64.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,914 | 31.6 | |||||
| Meconium (birth years 1988–2001) | |||||||||
| Yes | 5 | 8.6 | 399 | 6.6 | 1.4 | 0.5, 3.4 | |||
| No | 43 | 74.1 | 3,835 | 63.3 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,822 | 30.1 | |||||
| Gender | |||||||||
| Male | 40 | 69.0 | 3,133 | 51.7 | 2.1 | 1.2, 3.9 | |||
| Female | 18 | 31.0 | 2,916 | 48.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 7 | 0.1 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
SD, standard deviations.
Both younger (<20 years) and older (≥40 years) maternal ages were associated with increased risk relative to a maternal age of 20–29 years, although the relative risk for maternal age ≥40 years did not reach statistical significance and was based on only three cases (table 2). Paternal age and other demographic or socioeconomic characteristics were not associated with hepatoblastoma after adjustment for birth year and birth weight. Greater maternal weight was also associated with increased risk (table 3). Information on maternal height was not collected on the New York State birth certificate until 1993, so data on body mass index (weight (kg)/height (m)2) were not available for over half of the study subjects. With regard to body mass index, children born to women classified as overweight (body mass index 25–29) were at three times' higher risk of hepatoblastoma than were children born to normal-weight women (body mass index 20–24) (relative risk (RR) = 2.9, 95 percent CI: 1.2, 7.6). The relative risk was also elevated for children born to obese mothers (body mass index >29), although the confidence intervals were wide and included the null value (RR = 2.1, 95 percent CI: 0.7, 6.1). Weight gain during pregnancy was not associated with risk after adjustment for birth year and infant birth weight.
Parental demographic characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal age (years) | |||||||||
| <20 | 9 | 15.5 | 532 | 8.8 | 2.5 | 1.0, 5.5 | |||
| 20–29 | 20 | 34.5 | 3,248 | 53.6 | 1 | Reference | |||
| 30–39 | 26 | 44.8 | 2,189 | 36.1 | 1.6 | 0.9, 3.0 | |||
| ≥40 | 3 | 5.2 | 86 | 1.4 | 2.2 | 0.4, 7.9 | 0.8506 | ||
| Missing data | 0 | 0.0 | 1 | 0.0 | |||||
| Paternal age (years) | |||||||||
| <20 | 2 | 3.4 | 116 | 1.9 | 1.7 | 0.2, 7.3 | |||
| 20–29 | 14 | 24.1 | 2,181 | 36.0 | 1 | Reference | |||
| 30–39 | 24 | 41.4 | 2,573 | 42.5 | 1.3 | 0.6, 2.6 | |||
| ≥40 | 6 | 10.3 | 399 | 6.6 | 1.7 | 0.6, 4.5 | 0.6246 | ||
| Missing data | 12 | 20.7 | 787 | 13.0 | |||||
| Education | |||||||||
| No college | 19 | 32.8 | 2,361 | 39.0 | 1.2 | 0.7, 2.2 | |||
| Some college | 34 | 58.6 | 3,308 | 54.6 | 1 | Reference | |||
| Missing data for both parents | 5 | 8.6 | 387 | 6.4 | |||||
| Race/ethnicity | |||||||||
| White non-Hispanic | 41 | 70.7 | 4,456 | 73.6 | 1 | Reference | |||
| Either parent Hispanic | 9 | 15.5 | 799 | 13.2 | 1.1 | 0.5, 2.4 | |||
| Either parent Black | 5 | 8.6 | 448 | 7.4 | 0.7 | 0.2, 1.8 | |||
| Other/unknown | 3 | 5.2 | 353 | 5.8 | |||||
| Government assistance (birth years 1988–2001) | |||||||||
| Yes | 15 | 25.9 | 918 | 15.2 | 1.4 | 0.7, 2.7 | |||
| No | 29 | 50.0 | 2,096 | 34.6 | 1 | Reference | |||
| Missing data | 14 | 24.1 | 3,042 | 50.2 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal age (years) | |||||||||
| <20 | 9 | 15.5 | 532 | 8.8 | 2.5 | 1.0, 5.5 | |||
| 20–29 | 20 | 34.5 | 3,248 | 53.6 | 1 | Reference | |||
| 30–39 | 26 | 44.8 | 2,189 | 36.1 | 1.6 | 0.9, 3.0 | |||
| ≥40 | 3 | 5.2 | 86 | 1.4 | 2.2 | 0.4, 7.9 | 0.8506 | ||
| Missing data | 0 | 0.0 | 1 | 0.0 | |||||
| Paternal age (years) | |||||||||
| <20 | 2 | 3.4 | 116 | 1.9 | 1.7 | 0.2, 7.3 | |||
| 20–29 | 14 | 24.1 | 2,181 | 36.0 | 1 | Reference | |||
| 30–39 | 24 | 41.4 | 2,573 | 42.5 | 1.3 | 0.6, 2.6 | |||
| ≥40 | 6 | 10.3 | 399 | 6.6 | 1.7 | 0.6, 4.5 | 0.6246 | ||
| Missing data | 12 | 20.7 | 787 | 13.0 | |||||
| Education | |||||||||
| No college | 19 | 32.8 | 2,361 | 39.0 | 1.2 | 0.7, 2.2 | |||
| Some college | 34 | 58.6 | 3,308 | 54.6 | 1 | Reference | |||
| Missing data for both parents | 5 | 8.6 | 387 | 6.4 | |||||
| Race/ethnicity | |||||||||
| White non-Hispanic | 41 | 70.7 | 4,456 | 73.6 | 1 | Reference | |||
| Either parent Hispanic | 9 | 15.5 | 799 | 13.2 | 1.1 | 0.5, 2.4 | |||
| Either parent Black | 5 | 8.6 | 448 | 7.4 | 0.7 | 0.2, 1.8 | |||
| Other/unknown | 3 | 5.2 | 353 | 5.8 | |||||
| Government assistance (birth years 1988–2001) | |||||||||
| Yes | 15 | 25.9 | 918 | 15.2 | 1.4 | 0.7, 2.7 | |||
| No | 29 | 50.0 | 2,096 | 34.6 | 1 | Reference | |||
| Missing data | 14 | 24.1 | 3,042 | 50.2 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
Parental demographic characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal age (years) | |||||||||
| <20 | 9 | 15.5 | 532 | 8.8 | 2.5 | 1.0, 5.5 | |||
| 20–29 | 20 | 34.5 | 3,248 | 53.6 | 1 | Reference | |||
| 30–39 | 26 | 44.8 | 2,189 | 36.1 | 1.6 | 0.9, 3.0 | |||
| ≥40 | 3 | 5.2 | 86 | 1.4 | 2.2 | 0.4, 7.9 | 0.8506 | ||
| Missing data | 0 | 0.0 | 1 | 0.0 | |||||
| Paternal age (years) | |||||||||
| <20 | 2 | 3.4 | 116 | 1.9 | 1.7 | 0.2, 7.3 | |||
| 20–29 | 14 | 24.1 | 2,181 | 36.0 | 1 | Reference | |||
| 30–39 | 24 | 41.4 | 2,573 | 42.5 | 1.3 | 0.6, 2.6 | |||
| ≥40 | 6 | 10.3 | 399 | 6.6 | 1.7 | 0.6, 4.5 | 0.6246 | ||
| Missing data | 12 | 20.7 | 787 | 13.0 | |||||
| Education | |||||||||
| No college | 19 | 32.8 | 2,361 | 39.0 | 1.2 | 0.7, 2.2 | |||
| Some college | 34 | 58.6 | 3,308 | 54.6 | 1 | Reference | |||
| Missing data for both parents | 5 | 8.6 | 387 | 6.4 | |||||
| Race/ethnicity | |||||||||
| White non-Hispanic | 41 | 70.7 | 4,456 | 73.6 | 1 | Reference | |||
| Either parent Hispanic | 9 | 15.5 | 799 | 13.2 | 1.1 | 0.5, 2.4 | |||
| Either parent Black | 5 | 8.6 | 448 | 7.4 | 0.7 | 0.2, 1.8 | |||
| Other/unknown | 3 | 5.2 | 353 | 5.8 | |||||
| Government assistance (birth years 1988–2001) | |||||||||
| Yes | 15 | 25.9 | 918 | 15.2 | 1.4 | 0.7, 2.7 | |||
| No | 29 | 50.0 | 2,096 | 34.6 | 1 | Reference | |||
| Missing data | 14 | 24.1 | 3,042 | 50.2 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal age (years) | |||||||||
| <20 | 9 | 15.5 | 532 | 8.8 | 2.5 | 1.0, 5.5 | |||
| 20–29 | 20 | 34.5 | 3,248 | 53.6 | 1 | Reference | |||
| 30–39 | 26 | 44.8 | 2,189 | 36.1 | 1.6 | 0.9, 3.0 | |||
| ≥40 | 3 | 5.2 | 86 | 1.4 | 2.2 | 0.4, 7.9 | 0.8506 | ||
| Missing data | 0 | 0.0 | 1 | 0.0 | |||||
| Paternal age (years) | |||||||||
| <20 | 2 | 3.4 | 116 | 1.9 | 1.7 | 0.2, 7.3 | |||
| 20–29 | 14 | 24.1 | 2,181 | 36.0 | 1 | Reference | |||
| 30–39 | 24 | 41.4 | 2,573 | 42.5 | 1.3 | 0.6, 2.6 | |||
| ≥40 | 6 | 10.3 | 399 | 6.6 | 1.7 | 0.6, 4.5 | 0.6246 | ||
| Missing data | 12 | 20.7 | 787 | 13.0 | |||||
| Education | |||||||||
| No college | 19 | 32.8 | 2,361 | 39.0 | 1.2 | 0.7, 2.2 | |||
| Some college | 34 | 58.6 | 3,308 | 54.6 | 1 | Reference | |||
| Missing data for both parents | 5 | 8.6 | 387 | 6.4 | |||||
| Race/ethnicity | |||||||||
| White non-Hispanic | 41 | 70.7 | 4,456 | 73.6 | 1 | Reference | |||
| Either parent Hispanic | 9 | 15.5 | 799 | 13.2 | 1.1 | 0.5, 2.4 | |||
| Either parent Black | 5 | 8.6 | 448 | 7.4 | 0.7 | 0.2, 1.8 | |||
| Other/unknown | 3 | 5.2 | 353 | 5.8 | |||||
| Government assistance (birth years 1988–2001) | |||||||||
| Yes | 15 | 25.9 | 918 | 15.2 | 1.4 | 0.7, 2.7 | |||
| No | 29 | 50.0 | 2,096 | 34.6 | 1 | Reference | |||
| Missing data | 14 | 24.1 | 3,042 | 50.2 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
Maternal medical, pregnancy, and reproductive history and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal prepregnancy weight (pounds†) (birth years 1988–2001) | |||||||||
| <125 | 11 | 19.0 | 1,199 | 19.8 | 1.3 | 0.5, 3.0 | |||
| 125–149 | 12 | 20.7 | 1,466 | 24.2 | 1 | Reference | |||
| 150–174 | 12 | 20.7 | 735 | 12.1 | 2.2 | 0.9, 5.1 | |||
| ≥175 | 13 | 22.4 | 558 | 9.2 | 2.5 | 1.1, 5.9 | 0.1473 | ||
| Missing data | 10 | 17.2 | 2,098 | 34.6 | |||||
| Maternal prepregnancy body mass index‡ (birth years 1993–2001) | |||||||||
| <20 | 3 | 5.2 | 207 | 3.4 | 1.4 | 0.3, 4.9 | |||
| 20–24 | 10 | 17.2 | 895 | 14.8 | 1 | Reference | |||
| 25–29 | 12 | 20.7 | 447 | 7.4 | 2.9 | 1.2, 7.6 | |||
| ≥30 | 8 | 13.8 | 301 | 5.0 | 2.1 | 0.7, 6.1 | 0.1582 | ||
| Missing data | 25 | 43.1 | 4,206 | 69.5 | |||||
| Maternal pregnancy weight gain (pounds†) (birth years 1988–2001) | |||||||||
| <10 | 4 | 6.9 | 120 | 2.0 | 1.5 | 0.4, 4.6 | |||
| 10–19 | 9 | 15.5 | 394 | 6.5 | 1.0 | 0.4, 2.5 | |||
| 20–29 | 16 | 27.6 | 1,143 | 18.9 | 1 | Reference | |||
| 30–39 | 10 | 17.2 | 1,236 | 20.4 | 0.7 | 0.3, 1.5 | |||
| ≥40 | 7 | 12.1 | 986 | 16.3 | 0.5 | 0.2, 1.3 | 0.2753 | ||
| Missing data | 12 | 20.7 | 2,177 | 35.9 | |||||
| Maternal hypertension | |||||||||
| Yes | 2 | 3.4 | 254 | 4.2 | 0.2 | 0.0, 0.9 | |||
| No | 53 | 91.4 | 5,486 | 90.6 | 1 | Reference | |||
| Missing data | 3 | 5.2 | 316 | 5.2 | |||||
| Maternal lung disease (birth years 1988–2001) | |||||||||
| Yes | 3 | 5.2 | 88 | 1.5 | 1.6 | 0.3, 5.4 | |||
| No | 45 | 77.6 | 3,997 | 66.0 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | |||||
| Maternal prior fetal loss | |||||||||
| Yes | 15 | 25.9 | 1,171 | 19.3 | 1.3 | 0.7, 2.4 | |||
| No | 43 | 74.1 | 4,738 | 78.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 147 | 2.4 | |||||
| Maternal prior livebirth | |||||||||
| Yes | 31 | 53.4 | 3,558 | 58.8 | 1.0 | 0.6, 1.8 | |||
| No | 27 | 46.6 | 2,458 | 40.6 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 40 | 0.7 | |||||
| Maternal smoking (birth years 1988–2001) | |||||||||
| Yes | 12 | 20.7 | 742 | 12.3 | 2.1 | 1.0, 4.2 | |||
| No | 36 | 62.1 | 3,439 | 56.8 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,875 | 31.0 | |||||
| Presumptive fertility treatment§ (birth years 1993–2001) | |||||||||
| No treatment | 27 | 46.6 | 1,894 | 31.3 | 1 | Reference | |||
| Presumptive treatment | 5 | 8.6 | 23 | 0.4 | 9.2 | 2.1, 31.5 | |||
| Missing data | 26 | 44.8 | 4,139 | 68.3 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal prepregnancy weight (pounds†) (birth years 1988–2001) | |||||||||
| <125 | 11 | 19.0 | 1,199 | 19.8 | 1.3 | 0.5, 3.0 | |||
| 125–149 | 12 | 20.7 | 1,466 | 24.2 | 1 | Reference | |||
| 150–174 | 12 | 20.7 | 735 | 12.1 | 2.2 | 0.9, 5.1 | |||
| ≥175 | 13 | 22.4 | 558 | 9.2 | 2.5 | 1.1, 5.9 | 0.1473 | ||
| Missing data | 10 | 17.2 | 2,098 | 34.6 | |||||
| Maternal prepregnancy body mass index‡ (birth years 1993–2001) | |||||||||
| <20 | 3 | 5.2 | 207 | 3.4 | 1.4 | 0.3, 4.9 | |||
| 20–24 | 10 | 17.2 | 895 | 14.8 | 1 | Reference | |||
| 25–29 | 12 | 20.7 | 447 | 7.4 | 2.9 | 1.2, 7.6 | |||
| ≥30 | 8 | 13.8 | 301 | 5.0 | 2.1 | 0.7, 6.1 | 0.1582 | ||
| Missing data | 25 | 43.1 | 4,206 | 69.5 | |||||
| Maternal pregnancy weight gain (pounds†) (birth years 1988–2001) | |||||||||
| <10 | 4 | 6.9 | 120 | 2.0 | 1.5 | 0.4, 4.6 | |||
| 10–19 | 9 | 15.5 | 394 | 6.5 | 1.0 | 0.4, 2.5 | |||
| 20–29 | 16 | 27.6 | 1,143 | 18.9 | 1 | Reference | |||
| 30–39 | 10 | 17.2 | 1,236 | 20.4 | 0.7 | 0.3, 1.5 | |||
| ≥40 | 7 | 12.1 | 986 | 16.3 | 0.5 | 0.2, 1.3 | 0.2753 | ||
| Missing data | 12 | 20.7 | 2,177 | 35.9 | |||||
| Maternal hypertension | |||||||||
| Yes | 2 | 3.4 | 254 | 4.2 | 0.2 | 0.0, 0.9 | |||
| No | 53 | 91.4 | 5,486 | 90.6 | 1 | Reference | |||
| Missing data | 3 | 5.2 | 316 | 5.2 | |||||
| Maternal lung disease (birth years 1988–2001) | |||||||||
| Yes | 3 | 5.2 | 88 | 1.5 | 1.6 | 0.3, 5.4 | |||
| No | 45 | 77.6 | 3,997 | 66.0 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | |||||
| Maternal prior fetal loss | |||||||||
| Yes | 15 | 25.9 | 1,171 | 19.3 | 1.3 | 0.7, 2.4 | |||
| No | 43 | 74.1 | 4,738 | 78.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 147 | 2.4 | |||||
| Maternal prior livebirth | |||||||||
| Yes | 31 | 53.4 | 3,558 | 58.8 | 1.0 | 0.6, 1.8 | |||
| No | 27 | 46.6 | 2,458 | 40.6 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 40 | 0.7 | |||||
| Maternal smoking (birth years 1988–2001) | |||||||||
| Yes | 12 | 20.7 | 742 | 12.3 | 2.1 | 1.0, 4.2 | |||
| No | 36 | 62.1 | 3,439 | 56.8 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,875 | 31.0 | |||||
| Presumptive fertility treatment§ (birth years 1993–2001) | |||||||||
| No treatment | 27 | 46.6 | 1,894 | 31.3 | 1 | Reference | |||
| Presumptive treatment | 5 | 8.6 | 23 | 0.4 | 9.2 | 2.1, 31.5 | |||
| Missing data | 26 | 44.8 | 4,139 | 68.3 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
1 pound = 0.45 kg.
Weight (kg)/height (m)2.
Includes in vitro fertilization, other fertilization, or triplet plurality. Births of triplets occurring prior to 1993 were not included in presumptive treatment.
Maternal medical, pregnancy, and reproductive history and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal prepregnancy weight (pounds†) (birth years 1988–2001) | |||||||||
| <125 | 11 | 19.0 | 1,199 | 19.8 | 1.3 | 0.5, 3.0 | |||
| 125–149 | 12 | 20.7 | 1,466 | 24.2 | 1 | Reference | |||
| 150–174 | 12 | 20.7 | 735 | 12.1 | 2.2 | 0.9, 5.1 | |||
| ≥175 | 13 | 22.4 | 558 | 9.2 | 2.5 | 1.1, 5.9 | 0.1473 | ||
| Missing data | 10 | 17.2 | 2,098 | 34.6 | |||||
| Maternal prepregnancy body mass index‡ (birth years 1993–2001) | |||||||||
| <20 | 3 | 5.2 | 207 | 3.4 | 1.4 | 0.3, 4.9 | |||
| 20–24 | 10 | 17.2 | 895 | 14.8 | 1 | Reference | |||
| 25–29 | 12 | 20.7 | 447 | 7.4 | 2.9 | 1.2, 7.6 | |||
| ≥30 | 8 | 13.8 | 301 | 5.0 | 2.1 | 0.7, 6.1 | 0.1582 | ||
| Missing data | 25 | 43.1 | 4,206 | 69.5 | |||||
| Maternal pregnancy weight gain (pounds†) (birth years 1988–2001) | |||||||||
| <10 | 4 | 6.9 | 120 | 2.0 | 1.5 | 0.4, 4.6 | |||
| 10–19 | 9 | 15.5 | 394 | 6.5 | 1.0 | 0.4, 2.5 | |||
| 20–29 | 16 | 27.6 | 1,143 | 18.9 | 1 | Reference | |||
| 30–39 | 10 | 17.2 | 1,236 | 20.4 | 0.7 | 0.3, 1.5 | |||
| ≥40 | 7 | 12.1 | 986 | 16.3 | 0.5 | 0.2, 1.3 | 0.2753 | ||
| Missing data | 12 | 20.7 | 2,177 | 35.9 | |||||
| Maternal hypertension | |||||||||
| Yes | 2 | 3.4 | 254 | 4.2 | 0.2 | 0.0, 0.9 | |||
| No | 53 | 91.4 | 5,486 | 90.6 | 1 | Reference | |||
| Missing data | 3 | 5.2 | 316 | 5.2 | |||||
| Maternal lung disease (birth years 1988–2001) | |||||||||
| Yes | 3 | 5.2 | 88 | 1.5 | 1.6 | 0.3, 5.4 | |||
| No | 45 | 77.6 | 3,997 | 66.0 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | |||||
| Maternal prior fetal loss | |||||||||
| Yes | 15 | 25.9 | 1,171 | 19.3 | 1.3 | 0.7, 2.4 | |||
| No | 43 | 74.1 | 4,738 | 78.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 147 | 2.4 | |||||
| Maternal prior livebirth | |||||||||
| Yes | 31 | 53.4 | 3,558 | 58.8 | 1.0 | 0.6, 1.8 | |||
| No | 27 | 46.6 | 2,458 | 40.6 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 40 | 0.7 | |||||
| Maternal smoking (birth years 1988–2001) | |||||||||
| Yes | 12 | 20.7 | 742 | 12.3 | 2.1 | 1.0, 4.2 | |||
| No | 36 | 62.1 | 3,439 | 56.8 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,875 | 31.0 | |||||
| Presumptive fertility treatment§ (birth years 1993–2001) | |||||||||
| No treatment | 27 | 46.6 | 1,894 | 31.3 | 1 | Reference | |||
| Presumptive treatment | 5 | 8.6 | 23 | 0.4 | 9.2 | 2.1, 31.5 | |||
| Missing data | 26 | 44.8 | 4,139 | 68.3 | |||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | p for trend* . | ||
|---|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | . | ||
| Maternal prepregnancy weight (pounds†) (birth years 1988–2001) | |||||||||
| <125 | 11 | 19.0 | 1,199 | 19.8 | 1.3 | 0.5, 3.0 | |||
| 125–149 | 12 | 20.7 | 1,466 | 24.2 | 1 | Reference | |||
| 150–174 | 12 | 20.7 | 735 | 12.1 | 2.2 | 0.9, 5.1 | |||
| ≥175 | 13 | 22.4 | 558 | 9.2 | 2.5 | 1.1, 5.9 | 0.1473 | ||
| Missing data | 10 | 17.2 | 2,098 | 34.6 | |||||
| Maternal prepregnancy body mass index‡ (birth years 1993–2001) | |||||||||
| <20 | 3 | 5.2 | 207 | 3.4 | 1.4 | 0.3, 4.9 | |||
| 20–24 | 10 | 17.2 | 895 | 14.8 | 1 | Reference | |||
| 25–29 | 12 | 20.7 | 447 | 7.4 | 2.9 | 1.2, 7.6 | |||
| ≥30 | 8 | 13.8 | 301 | 5.0 | 2.1 | 0.7, 6.1 | 0.1582 | ||
| Missing data | 25 | 43.1 | 4,206 | 69.5 | |||||
| Maternal pregnancy weight gain (pounds†) (birth years 1988–2001) | |||||||||
| <10 | 4 | 6.9 | 120 | 2.0 | 1.5 | 0.4, 4.6 | |||
| 10–19 | 9 | 15.5 | 394 | 6.5 | 1.0 | 0.4, 2.5 | |||
| 20–29 | 16 | 27.6 | 1,143 | 18.9 | 1 | Reference | |||
| 30–39 | 10 | 17.2 | 1,236 | 20.4 | 0.7 | 0.3, 1.5 | |||
| ≥40 | 7 | 12.1 | 986 | 16.3 | 0.5 | 0.2, 1.3 | 0.2753 | ||
| Missing data | 12 | 20.7 | 2,177 | 35.9 | |||||
| Maternal hypertension | |||||||||
| Yes | 2 | 3.4 | 254 | 4.2 | 0.2 | 0.0, 0.9 | |||
| No | 53 | 91.4 | 5,486 | 90.6 | 1 | Reference | |||
| Missing data | 3 | 5.2 | 316 | 5.2 | |||||
| Maternal lung disease (birth years 1988–2001) | |||||||||
| Yes | 3 | 5.2 | 88 | 1.5 | 1.6 | 0.3, 5.4 | |||
| No | 45 | 77.6 | 3,997 | 66.0 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | |||||
| Maternal prior fetal loss | |||||||||
| Yes | 15 | 25.9 | 1,171 | 19.3 | 1.3 | 0.7, 2.4 | |||
| No | 43 | 74.1 | 4,738 | 78.2 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 147 | 2.4 | |||||
| Maternal prior livebirth | |||||||||
| Yes | 31 | 53.4 | 3,558 | 58.8 | 1.0 | 0.6, 1.8 | |||
| No | 27 | 46.6 | 2,458 | 40.6 | 1 | Reference | |||
| Missing data | 0 | 0.0 | 40 | 0.7 | |||||
| Maternal smoking (birth years 1988–2001) | |||||||||
| Yes | 12 | 20.7 | 742 | 12.3 | 2.1 | 1.0, 4.2 | |||
| No | 36 | 62.1 | 3,439 | 56.8 | 1 | Reference | |||
| Missing data | 10 | 17.2 | 1,875 | 31.0 | |||||
| Presumptive fertility treatment§ (birth years 1993–2001) | |||||||||
| No treatment | 27 | 46.6 | 1,894 | 31.3 | 1 | Reference | |||
| Presumptive treatment | 5 | 8.6 | 23 | 0.4 | 9.2 | 2.1, 31.5 | |||
| Missing data | 26 | 44.8 | 4,139 | 68.3 | |||||
Relative risk and trend p value were adjusted for birth year and birth weight.
1 pound = 0.45 kg.
Weight (kg)/height (m)2.
Includes in vitro fertilization, other fertilization, or triplet plurality. Births of triplets occurring prior to 1993 were not included in presumptive treatment.
Maternal smoking was also associated with a statistically significant elevated relative risk (RR = 2.1, 95 percent CI: 1.0, 4.2) (table 3). Further adjustment for maternal age, maternal weight, and infant gender did not change the point estimate of the relative risk for maternal smoking. The increased risk of hepatoblastoma with maternal smoking was stronger for children diagnosed with hepatoblastoma at the age of 2 years or older, although there were only 11 cases in this age group with data on maternal smoking (births occurring in 1988 or later). After adjustment for birth year, birth weight, maternal age, and maternal prepregnancy weight, the relative risk for maternal smoking among children diagnosed at age 2 years or older was 6.0 (95 percent CI: 1.6, 22.4), while that for children diagnosed prior to age 2 years was 1.4 (95 percent CI: 0.5, 3.3).
There was also some evidence of heterogeneity by birth weight, with the relative risk for smoking and hepatoblastoma being stronger for children with normal birth weight than for low birth weight children. Among children with birth weights of 2,500 g or more, the relative risk for maternal smoking was 2.7 (95 percent CI: 1.2, 5.5), while that for low birth weight children was 0.2 (95 percent CI: 0.0, 1.3). The interaction term for low birth weight and smoking was statistically significant in the logistic regression model (p = 0.039). For hepatoblastoma diagnosed after the age of 2 years, the relative risk for maternal smoking among children without low birth weight was 5.8 (95 percent CI: 1.4, 25.1), while that for low birth weight children was 2.1 (95 percent CI: 0.9, 24.8), although the number of children included in these stratified models was very low (three cases with low birth weight and eight cases without low birth weight). No case children diagnosed at a younger age had both exposure to smoking and low birth weight, so the interaction between birth weight and smoking could not be examined in this age group.
One case (1.7 percent) and seven controls (0.1 percent) were listed as having been conceived through in vitro fertilization (RR = 3.0, 95 percent CI: 0.1, 50.9; adjusted for birth weight). Three cases (5.2 percent) and 16 controls (0.3 percent) were conceived through other fertilization methods (adjusted RR = 9.4, 95 percent CI: 2.0, 43.2). In addition, two cases (3.4 percent) and seven controls (0.1 percent) were part of a triplet birth (table 4; for triplet births relative to singleton births, adjusted RR = 4.7, 95 percent CI: 0.5, 44.9), which may be a marker for infertility treatment. Both of the cases and two of the seven controls who were triplets were born in 1993 or later. Combining these three exposures, five out of 32 (15.6 percent) cases born in 1993 or later were potentially exposed to fertility treatment, whereas only 23 out of 1,917 controls (1.2 percent) were potentially exposed (table 3).
Delivery characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | ||
|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | ||
| Plurality of birth | ||||||||
| Singleton | 53 | 91.4 | 5,894 | 97.3 | 1 | Reference | ||
| Twin | 3 | 5.2 | 155 | 2.6 | 0.8 | 0.2, 3.3 | ||
| Triplet | 2 | 3.4 | 7 | 0.1 | 4.7 | 0.5, 44.9 | ||
| Birth position | ||||||||
| Vertex | 5 | 8.6 | 119 | 2.0 | 1 | Reference | ||
| Nonvertex | 51 | 87.9 | 5,665 | 93.5 | 1.0 | 0.3, 2.7 | ||
| Missing data | 2 | 3.4 | 272 | 4.5 | ||||
| Analgesia during labor (birth years 1988–2001) | ||||||||
| Yes | 16 | 27.6 | 1,670 | 27.6 | 1.0 | 0.5, 1.8 | ||
| No | 32 | 55.2 | 2,490 | 41.1 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,896 | 31.3 | ||||
| Anesthesia during delivery (birth years 1993–2001) | ||||||||
| Epidural | 7 | 12.1 | 640 | 10.6 | 1.9 | 0.4, 13.7 | ||
| Spinal | 10 | 17.2 | 228 | 3.8 | 6.2 | 1.5, 43.4 | ||
| Local | 11 | 19.0 | 671 | 11.1 | 1.5 | 1.1, 30.7 | ||
| General | 2 | 3.4 | 82 | 1.4 | 2.0 | 0.2, 19.2 | ||
| None | 2 | 3.4 | 357 | 5.9 | 1 | Reference | ||
| Missing data | 26 | 44.8 | 4,078 | 67.3 | ||||
| Method of delivery | ||||||||
| Vaginal, with no assistance | 31 | 53.4 | 3,566 | 58.9 | 1 | Reference | ||
| Vaginal, with assistance† | 4 | 6.9 | 960 | 15.9 | 0.3 | 0.1, 0.9 | ||
| Cesarean | 23 | 39.7 | 1,530 | 58.9 | 1.1 | 0.6, 1.9 | ||
| Tocolysis (birth years 1988–2001) | ||||||||
| Yes | 3 | 5.2 | 66 | 1.1 | 1.0 | 0.2, 3.7 | ||
| No | 45 | 77.6 | 4,164 | 68.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,826 | 30.2 | ||||
| Abruptio placentae | ||||||||
| Yes | 2 | 3.4 | 54 | 0.9 | 1.1 | 0.2, 4.9 | ||
| No | 53 | 91.4 | 5,766 | 95.2 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Hydramnios or oligohydramnios (birth years 1988–2001) | ||||||||
| Yes | 4 | 6.9 | 37 | 0.6 | 2.7 | 0.6, 9.7 | ||
| No | 44 | 75.9 | 4,048 | 66.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | ||||
| Premature rupture of membranes | ||||||||
| Yes | 3 | 5.2 | 316 | 5.2 | 0.5 | 0.1, 1.6 | ||
| No | 52 | 89.7 | 5,504 | 90.9 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Fetal distress | ||||||||
| Yes | 4 | 6.9 | 348 | 5.7 | 0.7 | 0.2, 2.1 | ||
| No | 51 | 87.9 | 5,469 | 90.3 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 239 | 3.9 | ||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | ||
|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | ||
| Plurality of birth | ||||||||
| Singleton | 53 | 91.4 | 5,894 | 97.3 | 1 | Reference | ||
| Twin | 3 | 5.2 | 155 | 2.6 | 0.8 | 0.2, 3.3 | ||
| Triplet | 2 | 3.4 | 7 | 0.1 | 4.7 | 0.5, 44.9 | ||
| Birth position | ||||||||
| Vertex | 5 | 8.6 | 119 | 2.0 | 1 | Reference | ||
| Nonvertex | 51 | 87.9 | 5,665 | 93.5 | 1.0 | 0.3, 2.7 | ||
| Missing data | 2 | 3.4 | 272 | 4.5 | ||||
| Analgesia during labor (birth years 1988–2001) | ||||||||
| Yes | 16 | 27.6 | 1,670 | 27.6 | 1.0 | 0.5, 1.8 | ||
| No | 32 | 55.2 | 2,490 | 41.1 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,896 | 31.3 | ||||
| Anesthesia during delivery (birth years 1993–2001) | ||||||||
| Epidural | 7 | 12.1 | 640 | 10.6 | 1.9 | 0.4, 13.7 | ||
| Spinal | 10 | 17.2 | 228 | 3.8 | 6.2 | 1.5, 43.4 | ||
| Local | 11 | 19.0 | 671 | 11.1 | 1.5 | 1.1, 30.7 | ||
| General | 2 | 3.4 | 82 | 1.4 | 2.0 | 0.2, 19.2 | ||
| None | 2 | 3.4 | 357 | 5.9 | 1 | Reference | ||
| Missing data | 26 | 44.8 | 4,078 | 67.3 | ||||
| Method of delivery | ||||||||
| Vaginal, with no assistance | 31 | 53.4 | 3,566 | 58.9 | 1 | Reference | ||
| Vaginal, with assistance† | 4 | 6.9 | 960 | 15.9 | 0.3 | 0.1, 0.9 | ||
| Cesarean | 23 | 39.7 | 1,530 | 58.9 | 1.1 | 0.6, 1.9 | ||
| Tocolysis (birth years 1988–2001) | ||||||||
| Yes | 3 | 5.2 | 66 | 1.1 | 1.0 | 0.2, 3.7 | ||
| No | 45 | 77.6 | 4,164 | 68.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,826 | 30.2 | ||||
| Abruptio placentae | ||||||||
| Yes | 2 | 3.4 | 54 | 0.9 | 1.1 | 0.2, 4.9 | ||
| No | 53 | 91.4 | 5,766 | 95.2 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Hydramnios or oligohydramnios (birth years 1988–2001) | ||||||||
| Yes | 4 | 6.9 | 37 | 0.6 | 2.7 | 0.6, 9.7 | ||
| No | 44 | 75.9 | 4,048 | 66.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | ||||
| Premature rupture of membranes | ||||||||
| Yes | 3 | 5.2 | 316 | 5.2 | 0.5 | 0.1, 1.6 | ||
| No | 52 | 89.7 | 5,504 | 90.9 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Fetal distress | ||||||||
| Yes | 4 | 6.9 | 348 | 5.7 | 0.7 | 0.2, 2.1 | ||
| No | 51 | 87.9 | 5,469 | 90.3 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 239 | 3.9 | ||||
Relative risks were adjusted for birth year and birth weight.
Includes labor induction, labor augmentation, use of a vacuum, and use of forceps.
Delivery characteristics and risk of hepatoblastoma among children born in New York State, excluding New York City, 1985–2001
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | ||
|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | ||
| Plurality of birth | ||||||||
| Singleton | 53 | 91.4 | 5,894 | 97.3 | 1 | Reference | ||
| Twin | 3 | 5.2 | 155 | 2.6 | 0.8 | 0.2, 3.3 | ||
| Triplet | 2 | 3.4 | 7 | 0.1 | 4.7 | 0.5, 44.9 | ||
| Birth position | ||||||||
| Vertex | 5 | 8.6 | 119 | 2.0 | 1 | Reference | ||
| Nonvertex | 51 | 87.9 | 5,665 | 93.5 | 1.0 | 0.3, 2.7 | ||
| Missing data | 2 | 3.4 | 272 | 4.5 | ||||
| Analgesia during labor (birth years 1988–2001) | ||||||||
| Yes | 16 | 27.6 | 1,670 | 27.6 | 1.0 | 0.5, 1.8 | ||
| No | 32 | 55.2 | 2,490 | 41.1 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,896 | 31.3 | ||||
| Anesthesia during delivery (birth years 1993–2001) | ||||||||
| Epidural | 7 | 12.1 | 640 | 10.6 | 1.9 | 0.4, 13.7 | ||
| Spinal | 10 | 17.2 | 228 | 3.8 | 6.2 | 1.5, 43.4 | ||
| Local | 11 | 19.0 | 671 | 11.1 | 1.5 | 1.1, 30.7 | ||
| General | 2 | 3.4 | 82 | 1.4 | 2.0 | 0.2, 19.2 | ||
| None | 2 | 3.4 | 357 | 5.9 | 1 | Reference | ||
| Missing data | 26 | 44.8 | 4,078 | 67.3 | ||||
| Method of delivery | ||||||||
| Vaginal, with no assistance | 31 | 53.4 | 3,566 | 58.9 | 1 | Reference | ||
| Vaginal, with assistance† | 4 | 6.9 | 960 | 15.9 | 0.3 | 0.1, 0.9 | ||
| Cesarean | 23 | 39.7 | 1,530 | 58.9 | 1.1 | 0.6, 1.9 | ||
| Tocolysis (birth years 1988–2001) | ||||||||
| Yes | 3 | 5.2 | 66 | 1.1 | 1.0 | 0.2, 3.7 | ||
| No | 45 | 77.6 | 4,164 | 68.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,826 | 30.2 | ||||
| Abruptio placentae | ||||||||
| Yes | 2 | 3.4 | 54 | 0.9 | 1.1 | 0.2, 4.9 | ||
| No | 53 | 91.4 | 5,766 | 95.2 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Hydramnios or oligohydramnios (birth years 1988–2001) | ||||||||
| Yes | 4 | 6.9 | 37 | 0.6 | 2.7 | 0.6, 9.7 | ||
| No | 44 | 75.9 | 4,048 | 66.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | ||||
| Premature rupture of membranes | ||||||||
| Yes | 3 | 5.2 | 316 | 5.2 | 0.5 | 0.1, 1.6 | ||
| No | 52 | 89.7 | 5,504 | 90.9 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Fetal distress | ||||||||
| Yes | 4 | 6.9 | 348 | 5.7 | 0.7 | 0.2, 2.1 | ||
| No | 51 | 87.9 | 5,469 | 90.3 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 239 | 3.9 | ||||
. | Cases (n = 58) . | . | Controls (n = 6,056) . | . | Relative risk* . | 95% confidence interval . | ||
|---|---|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | . | . | ||
| Plurality of birth | ||||||||
| Singleton | 53 | 91.4 | 5,894 | 97.3 | 1 | Reference | ||
| Twin | 3 | 5.2 | 155 | 2.6 | 0.8 | 0.2, 3.3 | ||
| Triplet | 2 | 3.4 | 7 | 0.1 | 4.7 | 0.5, 44.9 | ||
| Birth position | ||||||||
| Vertex | 5 | 8.6 | 119 | 2.0 | 1 | Reference | ||
| Nonvertex | 51 | 87.9 | 5,665 | 93.5 | 1.0 | 0.3, 2.7 | ||
| Missing data | 2 | 3.4 | 272 | 4.5 | ||||
| Analgesia during labor (birth years 1988–2001) | ||||||||
| Yes | 16 | 27.6 | 1,670 | 27.6 | 1.0 | 0.5, 1.8 | ||
| No | 32 | 55.2 | 2,490 | 41.1 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,896 | 31.3 | ||||
| Anesthesia during delivery (birth years 1993–2001) | ||||||||
| Epidural | 7 | 12.1 | 640 | 10.6 | 1.9 | 0.4, 13.7 | ||
| Spinal | 10 | 17.2 | 228 | 3.8 | 6.2 | 1.5, 43.4 | ||
| Local | 11 | 19.0 | 671 | 11.1 | 1.5 | 1.1, 30.7 | ||
| General | 2 | 3.4 | 82 | 1.4 | 2.0 | 0.2, 19.2 | ||
| None | 2 | 3.4 | 357 | 5.9 | 1 | Reference | ||
| Missing data | 26 | 44.8 | 4,078 | 67.3 | ||||
| Method of delivery | ||||||||
| Vaginal, with no assistance | 31 | 53.4 | 3,566 | 58.9 | 1 | Reference | ||
| Vaginal, with assistance† | 4 | 6.9 | 960 | 15.9 | 0.3 | 0.1, 0.9 | ||
| Cesarean | 23 | 39.7 | 1,530 | 58.9 | 1.1 | 0.6, 1.9 | ||
| Tocolysis (birth years 1988–2001) | ||||||||
| Yes | 3 | 5.2 | 66 | 1.1 | 1.0 | 0.2, 3.7 | ||
| No | 45 | 77.6 | 4,164 | 68.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,826 | 30.2 | ||||
| Abruptio placentae | ||||||||
| Yes | 2 | 3.4 | 54 | 0.9 | 1.1 | 0.2, 4.9 | ||
| No | 53 | 91.4 | 5,766 | 95.2 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Hydramnios or oligohydramnios (birth years 1988–2001) | ||||||||
| Yes | 4 | 6.9 | 37 | 0.6 | 2.7 | 0.6, 9.7 | ||
| No | 44 | 75.9 | 4,048 | 66.8 | 1 | Reference | ||
| Missing data | 10 | 17.2 | 1,971 | 32.5 | ||||
| Premature rupture of membranes | ||||||||
| Yes | 3 | 5.2 | 316 | 5.2 | 0.5 | 0.1, 1.6 | ||
| No | 52 | 89.7 | 5,504 | 90.9 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 236 | 3.9 | ||||
| Fetal distress | ||||||||
| Yes | 4 | 6.9 | 348 | 5.7 | 0.7 | 0.2, 2.1 | ||
| No | 51 | 87.9 | 5,469 | 90.3 | 1 | Reference | ||
| Missing data | 3 | 5.2 | 239 | 3.9 | ||||
Relative risks were adjusted for birth year and birth weight.
Includes labor induction, labor augmentation, use of a vacuum, and use of forceps.
A higher percentage of cases were born to mothers with a history of prior fetal loss, although prior fetal loss was not associated with increased risk of hepatoblastoma after adjustment for birth year and birth weight (table 3). The only delivery characteristic that was associated with hepatoblastoma risk was use of anesthesia during delivery (table 4). Information on anesthesia was available only for births occurring in 1993 or later. Both spinal anesthesia and local anesthesia were associated with statistically significantly increased risk, although only two cases were unexposed to any anesthesia.
DISCUSSION
In this study, we observed an increased risk of hepatoblastoma with extremely low birth weight, younger maternal age, maternal smoking, greater maternal weight, and use of infertility treatment. The elevated relative risk of hepatoblastoma with very low birth weight observed in this study is similar to the relative risks reported by Reynolds et al. (22) in California (RR = 40.8, 95 percent CI: 4.21, 395.5) and Ansell et al. (23) in the United Kingdom Childhood Cancer Study (RR = 69.0, 95 percent CI: 12.0, 118.2). In addition, the birth weight distribution of the California cases followed a pattern very similar to that seen in figure 1, although a larger percentage of hepatoblastoma cases in New York had extremely low birth weights (11 out of 58 cases (19 percent)). Of the 99 hepatoblastoma cases in the California study, 13 had birth weights under 1,500 g, and of these, 10 had birth weights under 1,000 g (22). Reynolds et al. (22) did not report a relative risk for birth weight under 1,000 g, probably because only two controls had very low birth weights in their matched study.
Our results suggest that the strongly increased risk may be limited to the very smallest infants, since the birth weight range of 1,000–1,500 g was associated with a relatively smaller and nonsignificantly increased risk in New York. However, combining the results from California and New York shows that the relative risk of hepatoblastoma is approximately doubled for low birth weight children relative to normal birth weight children (1,500–2,499 g vs. 2,500–3,999 g: pooled RR = 2.1, 95 percent CI: 4.1, 4.2; adjusted for study site). In the smaller study from the United Kingdom (23), two out of 24 hepatoblastoma cases had birth weights under 1,000 g, and one additional case had a birth weight under 1,500 g. In a Japanese case-control study that included birth-weight-matched controls, Maruyama et al. (27) and Ikeda et al. (28) found that oxygen therapy, furosemide treatment, and slower postnatal growth were risk factors for hepatoblastoma among extremely low birth weight infants. Using some of the cases from that case series, Ikeda et al. (29) showed that children with hepatoblastoma were not more likely to have evidence of oxidative damage to the liver than children with other nonneoplastic liver disease. One alternative hypothesis that has been proposed is increased exposure to plasticizers for very low birth weight infants (30).
In this study, maternal smoking was associated with a twofold increased risk of hepatoblastoma that was not accounted for by birth weight. There was evidence that the increased risk was limited to children with normal birth weight and that risk was stronger for later age at diagnosis. In the United Kingdom Childhood Cancer Study, Pang et al. (31) reported relative risks for maternal preconceptional smoking of 2.7 (95 percent CI: 1.2, 6.2) for developing hepatoblastoma at any age and 12.0 (95 percent CI: 2.5, 56.7) for developing hepatoblastoma at an age older than the median. These associations were not confounded by birth weight, although Pang et al. (31) did not report on a possible interaction with birth weight (32). They also reported that having both parents smoke preconceptionally increased risk more than fourfold (RR = 4.7, 95 percent CI: 1.7, 13.4). Hepatoblastoma was the only form of childhood cancer that was significantly associated with maternal smoking in the United Kingdom Childhood Cancer Study (31). Elevated risk of death from hepatoblastoma was also associated with both parents' smoking in the Oxford Survey of Childhood Cancer (RR = 2.28, 95 percent CI: 1.02, 5.09), although maternal smoking alone was associated with a nonsignificantly elevated risk (RR = 1.73, 95 percent CI: 0.93, 3.21) (33).
Information on paternal smoking status is not collected on the New York State birth certificate, so the joint effects of maternal and paternal smoking cannot be assessed. Since maternal smoking and paternal smoking are correlated, it is possible that the effects seen in this study are actually due to either paternal smoking or the combined effects of maternal and paternal smoking. Parental preconceptional and prenatal smoking is also correlated with parental smoking after birth and therefore potentially continued exposure of the child to sidestream smoke. The potential mechanisms of action for parental smoking include exposure to carcinogens across the placenta or preconceptional oxidative damage to sperm (33–36). In contrast to these findings, an early study from the Children's Cancer Study Group found similar smoking prevalences between case and control mothers on the basis of maternal interviews (37).
The increased risk of hepatoblastoma with infertility treatment is interesting in light of the potential association between Beckwith-Wiedemann syndrome and use of assisted reproductive technology (38–41). Beckwith-Wiedemann syndrome is also associated with hepatoblastoma. Beckwith-Wiedemann syndrome is a rare disorder associated with imprinting disorders of chromosome 11p15; its estimated prevalence is 13 cases per million births (42). Similar imprinting defects have also been observed in hepatoblastomas not associated with Beckwith-Wiedemann syndrome (11–13). On the basis of the data available in the cancer registry and the electronic birth records, we were unable to determine whether any of the children with hepatoblastoma also had Beckwith-Wiedemann syndrome, but it was presumably a small number. In a study conducted in the United States in the early 1980s, only four hepatoblastoma cases out of 75 had either Beckwith-Wiedemann syndrome or hemihypertrophy (37).
There have also been several isolated case reports of hepatoblastoma among children conceived by means of assisted reproductive technology, including one case each from the United Kingdom Childhood Cancer Study and the Japanese case series (23, 43–45). Cohort studies of children born following use of assisted reproductive technology have not shown increased risks of cancer in the offspring, although the statistical power of these studies has been low (42, 46–51). In this study, use of infertility treatment was measured as an indication on the birth certificate of in vitro fertilization, other fertilization, and, in a separate analysis, those two measures plus triplet births. Approximately 82 percent of triplet or higher-order births are estimated to be due to fertility treatment (52). Note that although triplet birth was used here as a surrogate for infertility treatment, twinning is also associated with Beckwith-Wiedemann syndrome. In addition, the increased risk of hepatoblastoma may be due to either the treatment or the couple's underlying infertility (53). Finally, the small number of study subjects exposed to infertility treatment precluded multivariate analysis, other than adjustment for birth weight.
Unlike the current study, neither the California study nor the United Kingdom study found an increased risk of hepatoblastoma with older or younger maternal age. Neither study reported on maternal weight. These findings are exploratory in nature. Although the relative risks were elevated, we did not observe a linear dose response with either maternal age or maternal weight, although risk may not be expected to increase linearly with these measures. Investigators in the United Kingdom Childhood Cancer Study observed large increased risks with polyhydramnios (two exposed cases) and severe maternal preeclampsia and eclampsia (three exposed cases). In the current study, only two children with hepatoblastoma had been exposed to any form of maternal hypertension (one with preeclampsia and one with chronic hypertension), and maternal hypertension was associated with decreased risk overall. On the New York State birth certificate, data on hydramnios and oligohydramnios are collected as one data element, so a separate effect of polyhydramnios could not be examined. The combination of hydramnios and oligohydramnios was associated with an increased crude risk of hepatoblastoma, but the relation was confounded by birth weight; after adjustment for birth weight (two cases with extremely low birth weight also had hydramnios or oligohydramnios), the relative risk was elevated but not statistically significant. There was no overlap in the United Kingdom study between the two cases with polyhydramnios and three cases with very low birth weight. Children with Beckwith-Wiedemann syndrome may also have a high prevalence of polyhydramnios during gestation, which may also be associated with preterm delivery (54, 55).
Since this was a case-cohort study, lack of follow-up of controls creates a potential selection bias. The potential loss to follow-up in this study arises from the fact that controls were ascertained from existing records with no subsequent follow-up (other than eliminating potential controls who died within the first month of life). By sampling the controls from the birth cohorts, we are assuming that the controls, had they developed cancer, would have been ascertained as cases in the study. It is unlikely that cases are unreported to the cancer registry, which is estimated to be over 95 percent complete for all cancers in New York State and is probably more complete for childhood cancer because of high levels of hospitalization of preschoolers with cancer (56, 57). On the other hand, some children may have moved out of state prior to diagnosis or may have been excluded from the study because their cancer records did not match the appropriate birth record. It is unlikely that loss to follow-up due to out-migration or failure to match was related to the risk factors being studied, but out-migration may be related to some unmeasured confounders, such as socioeconomic status.
In addition, using the case-cohort design implies that the relative risk is an estimator of the cumulative incidence ratio, and therefore assumes that no controls have left the risk set because of death (25). Childhood mortality is associated, either directly or indirectly, with several of the perinatal factors being studied, including infant birth weight and maternal age. After the immediate postnatal period, childhood mortality is low (58). Competing causes of death may be a particular concern, however, for extremely low birth weight infants. If extremely low birth weight infants died after the first month of life but before they had a chance to develop hepatoblastoma, their person-time at risk would be overestimated, and the risk of hepatoblastoma among extremely low birth weight infants would be overestimated.
Despite these limitations, the use of a case-cohort study design based on a cancer registry and birth records has several advantages over a case-control study design based on interviews with respect to selection bias. By using administrative record data instead of interview data, there is less potential for self-selection bias related to refusal of cases or controls to participate in the study or selection bias related to the inability to trace and contact study subjects.
Use of birth certificate data for ascertaining perinatal exposures reduces the potential for differential misclassification, since all measurements are made prior to diagnosis and measurement does not rely on parental recall of pregnancy history. However, use of birth certificates in epidemiologic studies may be problematic because of problems with completeness and validity (59). In a study of the validity of electronic birth certificate data for New York State, excluding New York City, Roohan et al. (60) observed that the sensitivity of birth certificate data on maternal medical risk factors varied but specificity was high. For example, specificity was 99 percent or higher for in vitro fertilization, other fertilization, tobacco use, and very low birth weight. This high specificity would lead us to believe that the relative risks estimated in this study are attenuated. Further, given the high prevalence of overweight and obesity, the possible association with hepatoblastoma should be examined in other cohorts.
Partial support was received from the Centers for Disease Control and Prevention's National Program of Cancer Registries, through cooperative agreement U55/CCU222012-03 awarded to the New York State Department of Health.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
References
Kingston JE, Herbert A, Draper GJ, et al. Association between hepatoblastoma and polyposis coli.
Krush AJ, Traboulsi EI, Offerhaus JA, et al. Hepatoblastoma, pigmented ocular fundus lesions and jaw lesions in Gardner syndrome.
Koch A, Denkhaus D, Albrecht S, et al. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene.
Koch A, Waha A, Hartmann W, et al. Elevated expression of Wnt antagonists is a common event in hepatoblastomas.
Jeng YM, Wu MZ, Mao TL, et al. Somatic mutations of beta-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma.
Udatsu Y, Kusafuka T, Kuroda S, et al. High frequency of beta-catenin mutations in hepatoblastoma.
Debaun MR, Tucker MA. Risk of cancer during the first four years of life in children from the Beckwith-Wiedemann Syndrome Registry.
Fraumeni JF Jr, Miller RW, Hill JA. Primary carcinoma of the liver in childhood: an epidemiologic study.
Rahman N. Mechanisms predisposing to childhood overgrowth and cancer.
Martin RA, Grange DK, Zehnbauer B, et al. LIT1 and H19 methylation defects in isolated hemihyperplasia.
Albrecht S, Hartmann W, Houshdaran F, et al. Allelic loss but absence of mutations in the polyspecific transporter gene BWR1A on 11p15.5 in hepatoblastoma.
Albrecht S, Von SD, Waha A, et al. Loss of maternal alleles on chromosome arm 11p in hepatoblastoma.
Ross JA, Radloff GA, Davies SM. H19 and IGF-2 allele-specific expression in hepatoblastoma.
Feusner J, Plaschkes J. Hepatoblastoma and low birth weight: a trend or chance observation?
Oue T, Kubota A, Okuyama H, et al. Hepatoblastoma in children of extremely low birth weight: a report from a single perinatal center.
Feusner JH, Ortega J, Hass J, et al. Hepatoblastoma in premature infants. (Abstract 1892). In: Proceedings of the American Society of Clinical Oncology, vol 16. Alexandria, VA: American Society of Clinical Oncology,
Ikeda H, Matsuyama S, Tanimura M. Association between hepatoblastoma and very low birth weight: a trend or a chance?
Jaing TH, Hung IJ, Lin JN, et al. Hepatoblastoma in a child of extremely low birth weight.
Tanimura M, Matsui I, Abe J, et al. Increased risk of hepatoblastoma among immature children with a lower birth weight.
Ross JA, Gurney JG. Hepatoblastoma incidence in the United States from 1973 to 1992.
Reynolds P, Urayama KY, Von Behren J, et al. Birth characteristics and hepatoblastoma risk in young children.
Ansell P, Mitchell CD, Roman E, et al. Relationships between perinatal and maternal characteristics and hepatoblastoma: a report from the UKCCS.
McLaughlin CC. Perinatal risk factors for childhood cancer. (PhD dissertation). Albany, NY: School of Public Health, State University of New York at Albany,
Rothman KJ, Greenland S, eds. Modern epidemiology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins,
Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation.
Maruyama K, Ikeda H, Koizumi T, et al. Case-control study of perinatal factors and hepatoblastoma in children with an extremely low birthweight.
Ikeda H, Hachitanda Y, Tanimura M, et al. Development of unfavorable hepatoblastoma in children of very low birth weight: results of a surgical and pathologic review.
Ikeda H, Hirato J, Suzuki N, et al. Detection of hepatic oxidative DNA damage in patients with hepatoblastoma and children with non-neoplastic disease.
Latini G, Gallo F, De Felice C. Birth characteristics and hepatoblastoma risk in young children. (Letter).
Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study.
Pang D, Birch JM. Reply: smoking and hepatoblastoma: confounding by birth weight? (Letter).
Sorahan T, Lancashire RJ. Parental cigarette smoking and childhood risks of hepatoblastoma: OSCC data.
Fraga CG, Motchnik PA, Wyrobek AJ, et al. Smoking and low antioxidant levels increase oxidative damage to sperm DNA.
DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review.
Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos.
Buckley JD, Sather H, Ruccione K, et al. A case-control study of risk factors for hepatoblastoma. A report from the Children's Cancer Study Group.
Chang AS, Moley KH, Wangler M, et al. Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients.
Debaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19.
Gicquel C, Gaston V, Mandelbaum J, et al. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCNQ1OT gene. (Letter).
Maher ER. Imprinting and assisted reproductive technology.
Lightfoot T, Bunch K, Ansell P, et al. Ovulation induction, assisted conception and childhood cancer.
Toren A, Sharon N, Mandel M, et al. Two embryonal cancers after in vitro fertilization.
Maruyama K, Ikeda H, Koizumi T, et al. Prenatal and postnatal histories of very low birthweight infants who developed hepatoblastoma.
Melamed I, Bujanover Y, Hammer J, et al. Hepatoblastoma in an infant born to a mother after hormonal treatment for sterility. (Letter).
Klip H, Burger CW, de Kraker J, et al. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF.
Lerner-Geva L, Toren A, Chetrit A, et al. The risk for cancer among children of women who underwent in vitro fertilization.
Bruinsma F, Venn A, Lancaster P, et al. Incidence of cancer in children born after in-vitro fertilization.
Doyle P, Bunch KJ, Beral V, et al. Cancer incidence in children conceived with assisted reproduction technology.
Bergh T, Ericson A, Hillensjo T, et al. Deliveries and children born after in-vitro fertilisation in Sweden 1982–95: a retrospective cohort study.
Brinton LA, Kruger KS, Thomsen BL, et al. Childhood tumor risk after treatment with ovulation-stimulating drugs.
Reynolds MA, Schieve LA, Martin JA, et al. Trends in multiple births conceived using assisted reproductive technology, United States, 1997–2000.
Buck Louis GM, Schisterman EF, Dukic VM, et al. Research hurdles complicating the analysis of infertility treatment and child health.
Wangler MF, Chang AS, Moley KH, et al. Factors associated with preterm delivery in mothers of children with Beckwith-Wiedemann syndrome: a case cohort study from the BWS registry.
Ranzini AC, Day-Salvatore D, Turner T, et al. Intrauterine growth and ultrasound findings in fetuses with Beckwith-Wiedemann syndrome.
Liu L, Krailo M, Reaman GH, et al. Childhood cancer patients' access to cooperative group cancer programs: a population-based study.
Ross JA, Severson RK, Pollock BH, et al. Childhood cancer in the United States. A geographical analysis of cases from the Pediatric Cooperative Clinical Trials groups.
National Center for Health Statistics. Vital statistics of the United States,
Kirby RS. Invited commentary: using vital statistics databases for perinatal epidemiology: does the quality go in before the name goes on?