-
PDF
- Split View
-
Views
-
Cite
Cite
Aaron Lerner, Yehuda Shoenfeld, Torsten Matthias, Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease, Nutrition Reviews, Volume 75, Issue 12, December 2017, Pages 1046–1058, https://doi.org/10.1093/nutrit/nux054
Close - Share Icon Share
Abstract
In light of the coincident surge in overall gluten intake and the incidence of autoimmune diseases, the possible biological adverse effects of gluten were explored. PubMed, MEDLINE, and the Cochrane Library databases were screened for reports published between 1964 and 2016 regarding the adverse effects of gluten as well as the effects of a gluten-free diet on autoimmune diseases. In vitro and in vivo studies describing gluten intake in animal models or cell lines and gluten-free diets in human autoimmune diseases were reviewed. Multiple detrimental aspects of gluten affect human health, including gluten-dependent digestive and extradigestive manifestations mediated by potentially immunological or toxic reactions that induce gastrointestinal inadequacy. Gluten affects the microbiome and increases intestinal permeability. It boosts oxidative stress and affects epigenetic behavior. It is also immunogenic, cytotoxic, and proinflammatory. Gluten intake increases apoptosis and decreases cell viability and differentiation. In certain nonceliac autoimmune diseases, gluten-free diets may help curtail the adverse effects of gluten. Additional in vivo studies are needed to unravel the puzzle of gluten effects in humans and to explore the potential beneficial effects of gluten-free diets in autoimmune diseases.
INTRODUCTION
Gluten is the main protein in wheat, constituting approximately 80% of the total proteins in this grain. The remaining 20%—albumins and globulins; multiple enzymes such as β-amylases, uridine diphosphate glucose pyrophosphorylases, peroxidases, and thioredoxins; amylase-trypsin inhibitors; puroindolines; heat-shock proteins; and proteins involved in response to stress— are outside the scope of this review. Wheat also contains nonprotein components like fermentable, oligo-, di-, and monosaccharides and polyols (FODMAPs). Not surprisingly, gluten peptides are also contained in other nonwheat cereals like barley, rye, and oats.1
Increased consumption of gluten
Wheat is an important food in human nutrition, and wheat- or gluten-containing foods are common in Western diets. Wheat grain is easy to stockpile and convenient to grind into flour, which improves its texture, and palatability. According to the Food and Agriculture Organization of the United Nations, worldwide production of wheat increased from 711.4 million metric tons in 2013 to 750.1 million metric tons in 2017 (as of October 5, 2017).2 Gluten is a major food additive in the processed food industry, with a net increase in usage of 1.8 ± 0.4% per year reported over the last 30 years1 Currently, both the prevalence and the incidence of gluten-related disorders are surging in many countries worldwide.3–7 The reasons most frequently suggested include the spread of the Western diet,1 the increased consumption of gluten-containing diets,8 the gradual shift toward increased production and consumption of wheat, which is replacing the traditional food of rice in Asia and Africa,9 and the evolutionary development of wheat with higher gluten content and cytotoxic properties.10,11 Even advanced food-processing technology, which reduces dough fermentation time, can increase gluten concentration in commercial bakery products.10,12 It is estimated that, in the 20th century, global wheat output increased 5-fold, with most of the increase occurring after 1955.4 In parallel, the prevalence of celiac disease increased 5-fold in the United States between 1974 and 1989.13 On the other hand, in a 2014 US Consumer Reports survey, 63% of responders felt that abstaining from bread and other gluten-containing diets would enhance their physical or mental health. Moreover, in a 2015 Gallup poll, 21% of Americans disclosed they tried to follow a gluten-free diet.14
Increased frequency of autoimmune disease
Increasing evidence demonstrates a continuous rise in the incidence of autoimmune diseases.3 The incidence of rheumatic, endocrinological, gastrointestinal, and neurological autoimmune diseases has increased annually, at rates ranging from 3.7% to 7.1%, between 1985 and 2015.3 These observations, together with the fact that only 1% to 3% of the 30% of the general population that carries the human leukocyte antigen DQ 2/8 allele will develop celiac disease, strengthen the hypothesis that the environment plays a more important role than genetics, in the development of autoimmune diseases, in the last decades.3,4,15 The increased incidence of celiac disease has been linked to increased gluten consumption.4,15 However, some evidence indicates that gluten might be related to the increased incidence of other, nonceliac autoimmune diseases. The parallel surge in gluten consumption3,15 and the increased incidence of autoimmune diseases prompted a review of the current knowledge about the disadvantages of dietary gluten and the role of gluten-free diets in the nutritional therapy of autoimmune diseases.
METHODS
The first part of this review addresses the effects of gluten on cell lines of human origin in vitro and on animal models in vivo, since in vivo studies in humans are scarce. The second part addresses the effects of gluten-free diets in various autoimmune diseases. The literature search covered the period 1964–2016 and included studies that describe the following: (1) effects of gluten intake by animals, (2) in vitro effects of gluten on cell lines, and (3) effects of gluten-free diets on nonceliac autoimmune diseases in humans.
Research studies, reviews, and case–control series were included, while case reports were excluded. The literature search was performed using the PubMed, MEDLINE, Embase, Scopus, and Cochrane Database of Systematic Reviews databases to identify the most relevant information.
The following search algorithm was used (“gluten side effect” or “gluten adverse effects” or “gluten treatment” or “gluten therapy” or “gluten application”) AND (“human” or “animal” or “cell-line”) AND (“gluten”) or (“gliadin”) AND (“autoimmune disease”).
Type 1 diabetes, multiple sclerosis, psoriasis, rheumatoid arthritis, autoimmune hepatitis, celiac hepatitis, autoimmune thyroiditis, and autoimmune diseases were searched separately for gluten or gluten-free diets. Additional studies were identified by examining the reference list of the retrieved articles. The search was limited to articles published in English. Altogether, 251 publications were identified. Relevant articles were selected for full-text review on the basis of screened titles and abstracts. Since primary data was not collected, ethical approval was not mandatory.
DIRECT IMMUNE EFFECTS OF GLUTEN IN ANIMAL STUDIES
Direct effects of gluten on dendritic cells and innate immune systems in mice
The innate immune system plays a critical role in oral tolerance to nutritional antigens. In experiments with BALB/c and nonobese diabetic (NOD) mice, gluten-free diets increased both the percentage of macrophages in the spleen of BALB/c mice and the percentage of CD11c+ dendritic cells in the spleen of BALB/c and NOD mice. Moreover, gluten-free diets increased the percentage of CD103+ dendritic cells in BALB/c mice and decreased the percentage of CD11b+ dendritic cells in their mesenteric lymph nodes. Gluten-free diets in BALB/c mice attenuated the exposure of dendritic cells to CD40, CCR7, and major histocompatibility complex II in pancreatic lymph nodes. These results help to explain the low incidence of diabetes in gluten-free NOD mice, in which gluten might play a diabetogenic role. This pathway might be essential in the progression of type 1 diabetes (T1D), celiac disease, and nonceliac gluten sensitivity.16
Direct effects of gluten on cytokine production and cytotoxicity of natural killer cells
Larsen et al.17 have shown that gluten-containing diets drive an increased expression and cytotoxicity of natural killer cells toward pancreatic beta-cell line MIN6 cells, higher expression NKG2D and CD71 on NKp46(+) cells in all lymphoid organs of BALB/c and NOD mice. Gluten ingestion augments the secretion of both IFN-γ and IL-6 to a greater degree in BALB/c and NOD mice than in mice fed gluten-free diets, suggesting that gluten intake induces murine natural killer cell activity toward pancreatic beta cells. This may reinforce the progression of T1D and help explain the increased incidence of disease associated with gluten consumption in NOD mice.17
Direct effects of gluten on the Th17 pathway and T regulatory cells
Cytokine-secreting Th17 cells are associated with autoimmunity. The Th17 profile is substantially increased in the pancreatic lymph nodes of BALB/c gluten-fed mice, parallel to the increased populations of CD4+, CD45RBhigh+, and CD103+ T cells. This indicates that gluten intake impacts multiple subsets of T regulatory cells as well as Th17 cells in the mucosal lymphoid tissue.18
Closure of ATP-sensitive potassium channels and induction of insulin release
The incidence of diabetes is reduced by a gluten-free diet in the NOD murine model.19 Gluten peptides, such as gliadin, can cross the intestinal barrier and may directly affect the beta cells of the endocrine pancreas. In INS-1E cells and in isolated rat islets, the application of gliadin peptides induced a dose-related surge in insulin delivery and decreased electrical currents through ATP-sensitive potassium channels.19 This data may indicate gliadin fragments as modulators responsible for the activation of beta cells activity in the late stages of T1D.
Induction of neutrophil migration
Gliadin induces the secretion of interleukin (IL) 8, a powerful neutrophil-activating and chemoattractant chemokine. Recently, gliadin was shown to exhibit neutrophil chemoattractant capabilities similar to those of the classical neutrophil chemoattractant, N-formyl-methionyl-leucyl-phenylalanine (fMet-Leu-Phe), and likewise uses the formyl peptide receptor 1 to induce migration.20
Induction of expression of the activating receptor natural killer group 2D and its ligands
Enteric expression of the activating receptor natural killer group 2D (CD314) and its ligands is a marker of intestinal autoimmunity. In studies of enteric and pancreatic islets in BALB/c and NOD mice, gluten-free diets decreased the expression of natural killer group 2D and its ligands.21 This immunological response may lower T1D incidence associated with gluten-free diets in animals and, potentially, in humans.
Induction of inflammation through Toll-like receptor 4
Animal and cell culture models have shown that gluten peptides can induce inflammation by binding Toll-like receptor 4, thereby inducing the expression of proinflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor.22,23 Although these findings must still be corroborated in humans, they suggest an intriguing hypothesis, given the current trend toward increased use of gluten-free diets. Table 116–18,20,21,24–38 summarizes additional studies reporting in vivo and in vitro direct effects of gluten intake on the immune system. Taken together, the animal studies support the involvement of gluten peptides in the innate and the regulatory immune systems, on the Th17 and other proinflammatory pathways and neutrophil migration. There are some indications that gluten may be involved in the induction of diabetes and autoimmune disorders. The following section on adverse effects of gluten in humans might shed additional light on the subject.
Direct in vivo and in vitro effects of gluten intake on the immune system
| Reference . | Study type . | Cell type or animal model . | Effects of gluten intake . |
|---|---|---|---|
| Thomas et al. (2006),24 Tuckova et al. (2000),25 Tuckova (2002)26 | In vitro | Macrophages | Increases proinflammatory cytokine and nitric oxide production |
| Nikulina et al. (2004),27 Palova-Jelinkova et al. (2005),28 Ciccocioppo et al. (2008)29 | In vitro | Dendritic cells | Upregulates MHC II, costimulatory molecules, Toll-like receptor, cytokine, and chemokine production |
| Larsen et al. (2014)17 | In vitro | Lymphoid organ | Increases expression of NKG2D and CD71 on NKp46+ cells |
| Gujral et al. (2015)30 | In vitro | Caco-2 | Increases intestinal permeability, TNF-α, and IL-1β production |
| Flohe et al. (2003),31 Alam et al. (2010)32 | In vivo | NOD mice | Modifies Th1/Th2 intestinal cytokines |
| Alam et al. (2010)32 | In vivo | NOD mice | Increases counts of activated intestinal CD4+ T cells, dendritic cells, and Th17 cells |
| Scott et al. (2002)33 | In vivo | BB rats | Modifies Th1 intestinal and MLN cytokines |
| Scott et al. (1997)34 | In vivo | BB rats | Alters Th1 cytokine pattern in islet infiltrate |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Induces proportional changes in regulatory T-cell subsets |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts in peripheral lymph nodes |
| Antvorskov et al. (2013),35 Bernardo et al. (2007)36 | In vivo | BALB/c mice | Alters inflammatory cytokine pattern in FOX3− and FOXP3+ T cells, activates innate immune response |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts, increases proportions of CD4+CD45RBhigh+ cells, increases CD103+ T-cell counts |
| Larsen et al. (2015)16 | In vivo | BALB/c and NOD mice | Modulates dendritic cells and innate immune subsets in mice |
| Larsen et al. (2014)17 | In vivo | BALB/c and NOD mice | Increases natural killer cell cytotoxicity and cytokine secretion of IFN-γ and IL-6 |
| Adlercreutz et al. (2014)21 | In vivo | BALB/c and NOD mice | Induces expression of NKG2D and ligands |
| Palova-Jelinkova et al. (2013)37 | In vivo | NLRP−/− ASC−/− KO mice | Induces IL-1β production in bone-marrow-derived dendritic cells |
| Lammers et al. (2015)20 | In vivo | C57BL/6 and Lys-GFP mice | Induces neutrophil migration |
| Stĕpankova et al. (1996)38 | In vivo | AVN Wistar rats | Increases counts of pathogenic intraepithelial lymphocytes |
| Reference . | Study type . | Cell type or animal model . | Effects of gluten intake . |
|---|---|---|---|
| Thomas et al. (2006),24 Tuckova et al. (2000),25 Tuckova (2002)26 | In vitro | Macrophages | Increases proinflammatory cytokine and nitric oxide production |
| Nikulina et al. (2004),27 Palova-Jelinkova et al. (2005),28 Ciccocioppo et al. (2008)29 | In vitro | Dendritic cells | Upregulates MHC II, costimulatory molecules, Toll-like receptor, cytokine, and chemokine production |
| Larsen et al. (2014)17 | In vitro | Lymphoid organ | Increases expression of NKG2D and CD71 on NKp46+ cells |
| Gujral et al. (2015)30 | In vitro | Caco-2 | Increases intestinal permeability, TNF-α, and IL-1β production |
| Flohe et al. (2003),31 Alam et al. (2010)32 | In vivo | NOD mice | Modifies Th1/Th2 intestinal cytokines |
| Alam et al. (2010)32 | In vivo | NOD mice | Increases counts of activated intestinal CD4+ T cells, dendritic cells, and Th17 cells |
| Scott et al. (2002)33 | In vivo | BB rats | Modifies Th1 intestinal and MLN cytokines |
| Scott et al. (1997)34 | In vivo | BB rats | Alters Th1 cytokine pattern in islet infiltrate |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Induces proportional changes in regulatory T-cell subsets |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts in peripheral lymph nodes |
| Antvorskov et al. (2013),35 Bernardo et al. (2007)36 | In vivo | BALB/c mice | Alters inflammatory cytokine pattern in FOX3− and FOXP3+ T cells, activates innate immune response |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts, increases proportions of CD4+CD45RBhigh+ cells, increases CD103+ T-cell counts |
| Larsen et al. (2015)16 | In vivo | BALB/c and NOD mice | Modulates dendritic cells and innate immune subsets in mice |
| Larsen et al. (2014)17 | In vivo | BALB/c and NOD mice | Increases natural killer cell cytotoxicity and cytokine secretion of IFN-γ and IL-6 |
| Adlercreutz et al. (2014)21 | In vivo | BALB/c and NOD mice | Induces expression of NKG2D and ligands |
| Palova-Jelinkova et al. (2013)37 | In vivo | NLRP−/− ASC−/− KO mice | Induces IL-1β production in bone-marrow-derived dendritic cells |
| Lammers et al. (2015)20 | In vivo | C57BL/6 and Lys-GFP mice | Induces neutrophil migration |
| Stĕpankova et al. (1996)38 | In vivo | AVN Wistar rats | Increases counts of pathogenic intraepithelial lymphocytes |
Abbreviations: IL, interleukin; IFN, interferon; KO, knockout; MHC, major histocompatibility complex; MLN, mesenteric lymph node; Th, T helper; TNF, tumor necrosis factor.
Direct in vivo and in vitro effects of gluten intake on the immune system
| Reference . | Study type . | Cell type or animal model . | Effects of gluten intake . |
|---|---|---|---|
| Thomas et al. (2006),24 Tuckova et al. (2000),25 Tuckova (2002)26 | In vitro | Macrophages | Increases proinflammatory cytokine and nitric oxide production |
| Nikulina et al. (2004),27 Palova-Jelinkova et al. (2005),28 Ciccocioppo et al. (2008)29 | In vitro | Dendritic cells | Upregulates MHC II, costimulatory molecules, Toll-like receptor, cytokine, and chemokine production |
| Larsen et al. (2014)17 | In vitro | Lymphoid organ | Increases expression of NKG2D and CD71 on NKp46+ cells |
| Gujral et al. (2015)30 | In vitro | Caco-2 | Increases intestinal permeability, TNF-α, and IL-1β production |
| Flohe et al. (2003),31 Alam et al. (2010)32 | In vivo | NOD mice | Modifies Th1/Th2 intestinal cytokines |
| Alam et al. (2010)32 | In vivo | NOD mice | Increases counts of activated intestinal CD4+ T cells, dendritic cells, and Th17 cells |
| Scott et al. (2002)33 | In vivo | BB rats | Modifies Th1 intestinal and MLN cytokines |
| Scott et al. (1997)34 | In vivo | BB rats | Alters Th1 cytokine pattern in islet infiltrate |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Induces proportional changes in regulatory T-cell subsets |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts in peripheral lymph nodes |
| Antvorskov et al. (2013),35 Bernardo et al. (2007)36 | In vivo | BALB/c mice | Alters inflammatory cytokine pattern in FOX3− and FOXP3+ T cells, activates innate immune response |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts, increases proportions of CD4+CD45RBhigh+ cells, increases CD103+ T-cell counts |
| Larsen et al. (2015)16 | In vivo | BALB/c and NOD mice | Modulates dendritic cells and innate immune subsets in mice |
| Larsen et al. (2014)17 | In vivo | BALB/c and NOD mice | Increases natural killer cell cytotoxicity and cytokine secretion of IFN-γ and IL-6 |
| Adlercreutz et al. (2014)21 | In vivo | BALB/c and NOD mice | Induces expression of NKG2D and ligands |
| Palova-Jelinkova et al. (2013)37 | In vivo | NLRP−/− ASC−/− KO mice | Induces IL-1β production in bone-marrow-derived dendritic cells |
| Lammers et al. (2015)20 | In vivo | C57BL/6 and Lys-GFP mice | Induces neutrophil migration |
| Stĕpankova et al. (1996)38 | In vivo | AVN Wistar rats | Increases counts of pathogenic intraepithelial lymphocytes |
| Reference . | Study type . | Cell type or animal model . | Effects of gluten intake . |
|---|---|---|---|
| Thomas et al. (2006),24 Tuckova et al. (2000),25 Tuckova (2002)26 | In vitro | Macrophages | Increases proinflammatory cytokine and nitric oxide production |
| Nikulina et al. (2004),27 Palova-Jelinkova et al. (2005),28 Ciccocioppo et al. (2008)29 | In vitro | Dendritic cells | Upregulates MHC II, costimulatory molecules, Toll-like receptor, cytokine, and chemokine production |
| Larsen et al. (2014)17 | In vitro | Lymphoid organ | Increases expression of NKG2D and CD71 on NKp46+ cells |
| Gujral et al. (2015)30 | In vitro | Caco-2 | Increases intestinal permeability, TNF-α, and IL-1β production |
| Flohe et al. (2003),31 Alam et al. (2010)32 | In vivo | NOD mice | Modifies Th1/Th2 intestinal cytokines |
| Alam et al. (2010)32 | In vivo | NOD mice | Increases counts of activated intestinal CD4+ T cells, dendritic cells, and Th17 cells |
| Scott et al. (2002)33 | In vivo | BB rats | Modifies Th1 intestinal and MLN cytokines |
| Scott et al. (1997)34 | In vivo | BB rats | Alters Th1 cytokine pattern in islet infiltrate |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Induces proportional changes in regulatory T-cell subsets |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts in peripheral lymph nodes |
| Antvorskov et al. (2013),35 Bernardo et al. (2007)36 | In vivo | BALB/c mice | Alters inflammatory cytokine pattern in FOX3− and FOXP3+ T cells, activates innate immune response |
| Antvorskov et al. (2012)18 | In vivo | BALB/c mice | Increases Th17 cell counts, increases proportions of CD4+CD45RBhigh+ cells, increases CD103+ T-cell counts |
| Larsen et al. (2015)16 | In vivo | BALB/c and NOD mice | Modulates dendritic cells and innate immune subsets in mice |
| Larsen et al. (2014)17 | In vivo | BALB/c and NOD mice | Increases natural killer cell cytotoxicity and cytokine secretion of IFN-γ and IL-6 |
| Adlercreutz et al. (2014)21 | In vivo | BALB/c and NOD mice | Induces expression of NKG2D and ligands |
| Palova-Jelinkova et al. (2013)37 | In vivo | NLRP−/− ASC−/− KO mice | Induces IL-1β production in bone-marrow-derived dendritic cells |
| Lammers et al. (2015)20 | In vivo | C57BL/6 and Lys-GFP mice | Induces neutrophil migration |
| Stĕpankova et al. (1996)38 | In vivo | AVN Wistar rats | Increases counts of pathogenic intraepithelial lymphocytes |
Abbreviations: IL, interleukin; IFN, interferon; KO, knockout; MHC, major histocompatibility complex; MLN, mesenteric lymph node; Th, T helper; TNF, tumor necrosis factor.
ADVERSE EFFECTS OF GLUTEN ON HUMAN HEALTH
The role of gluten in celiac disease is well established and will not be described in this review. Yet gluten also plays a role, though indirectly, in other nonceliac health conditions.
Gliadin is an ideal substrate for endogenous tissue transglutaminase and exogenous microbial transglutaminase, both of which belong to the extended transglutaminase family.39 Gluten is abundant in glutamine and lysine and thus is a very attractive substrate for posttranslational modification of protein by both endogenous tissue transglutaminase and exogenous microbial transglutaminase. Both enzymes are able to deamidate or cross-link gluten peptides to numerous other peptides, thus turning naive, self-proteins into immunogenic ones.40–44 Importantly, luminal microbial transglutaminase, derived from processed food or from the microbiome or, more specifically, from the dysbiome (microbial imbalance), is a major cause of post-translational modification of gliadin or other naive proteins.45 Recently, it was hypothesized that the luminal transglutaminases, which originate in the dysbiome, are potential drivers of end-organ or systemic autoimmunity.46
Immunogenicity of gluten
Antigliadin antibodies are age dependent and are found in the normal population well as in patients with intestinal and extraintestinal diseases.47–50 Antigliadin antibodies may represent an epiphenomenon whereby the immune system reacts against gluten peptides. Even in the PreventCD intervention study, 30% of healthy infants produced antibodies against gluten/gliadin without anti–tissue transglutaminase antibodies shortly after gluten introduction.51 In patients with rheumatoid arthritis, a non–gluten-related condition, the intestinal fluid and sera were significantly enriched with immunoglobulin M (IgM) antibodies against gliadin.44,52 When the immunogenicity of processed food products vs their raw constituents was checked in normal sera, titers of the specific corresponding immunoglobulins were significantly higher against the processed products. When pizza, pasta, cereals, or bakery products from supermarket shelves were checked, products containing processed wheat provoked much higher immunoglobulin G (IgG), immunoglobulin A (IgA), and IgM antibody responses than products containing raw wheat. It is suggested that industrial processing of wheat may enhance the immunogenicity of the protein (gluten).53
Microbial transglutaminase docked by gluten peptides is immunogenic in celiac disease.40 Microbial transglutaminase–modified gluten proteins react with IgA antigliadin antibodies in the sera of patients with celiac disease. Microbial transglutaminase–treated gluten peptides cultured with intestinal biopsies from patients with celiac disease induced a substantial release of interferon-ϒ accompanied by a surge in anti–tissue transglutaminase and antiendomysial autoantibodies, suggesting that those gluten peptides are proinflammatory and immunogenic, at least ex vivo. Interestingly, in sera from celiac patients, IgA antibodies were higher against wheat from microbial-transglutaminase-treated breads than against gluten-free breads not treated with microbial transglutaminase. Moreover, microbial-transglutaminase-deamidated gluten peptides react with gluten-selected T cells, thus boosting the immunogenicity of gluten.40 It appears that commercially available products contain certain quantities of microbial transglutaminase, implying that microbial transglutaminase used during food processing is eaten by consumers and eventually finds its way to the gut lumen.54
Immunogenicity of gliadin when docked on tissue transglutaminase or microbial transglutaminase
Even in celiac disease, neoepitopes generated by the docking of gliadin onto tissue transglutaminase and microbial transglutaminase are more immunogenic than undocked gliadin. Compared with conventional tissue transglutaminase isotypes, the tissue transglutaminase neoepitope IgA + IgG isotype (detected by AESKULISA, CeliCheck New Generation, Wendelsheim, Germany) has a higher optical density value, more accurately reflects intestinal injury, has higher sensitivity and specificity, and targets different autoantigens.55,56 The same was found for the microbial transglutaminase neoepitope.57
Pathogenicity of gluten in HCT116 cells
When applied to HCT116 cells, gliadin reduced cell viability by 50% (P < 0.05), induced lactic dehydrogenase secretion over time (P < 0.01), and increased apoptosis significantly (A.L. and T.M., unpublished data, 2016). These effects of gliadin are supported by multiple studies in cell lines of human origin. Decreased viability,58,59 necrosis and/or lactic dehydrogenase release,59 and induction of apoptosis59–61 have been described.
Ability of gliadin to increase intestinal permeability
Tight-junction integrity is breached in multiple autoimmune diseases, including celiac disease.62,63 Numerous in vitro, ex vivo, and in vivo studies have confirmed that gliadin, the main antigen of gluten, increases intestinal permeability.64,65 After binding to its chemokine receptor CXCR3 on epithelial cells, gliadin activates the MyD88-pathway, resulting in the release of zonulin. Gliadin has agglutinating activity, polymerizes soluble G- and F-actins, inhibits cell proliferation, is proapoptotic, decreases redox potential, affects the rearrangement of the enterocyte cytoskeleton, and induces zonulin secretion, resulting in increased gut leakage.63,65
Most recently, studies of epithelial transit of immunogenic and toxic gliadin peptides have shown enhanced luminal degradation of the peptides, highlighting the importance of the epithelial barrier against shorter gliadin peptides.66 In summary, gluten and gliadin induce multiple adverse effects, owing to their capacity as substrates for enzymatic post translational modification of proteins their immunogenicity (whether alone or complexed), their ability to reduce cell viability, their necrotogenic and apoptotic properties, and their ability to breach tight-junction integrity.
Effects of gluten on the microbiome
Human beings are host to a multiform but host-specific gut microbial population along the proximal-to-distal axis of the intestine. Analysis of the microbiota in the large intestine has shown that nutrition affects the luminal bacteria, and together affect the host’s well-being. Does gluten affect the intestinal ecosystem? The answer is yes.
When NOD mice were fed a gluten-free diet, numbers of cecal microbes and gram-positive bacteria were lower than those in mice fed a standard gluten-containing diet.67,68 Recently, Antvorskov et al.67 and Marietta et al.69 showed that gluten-containing diets increased the numbers of Bifidobacterium, Tannerella, and Barnesiella species in the intestinal flora of NOD mice, whereas gluten-free diets resulted in increased counts of Akkermansia species. It appears that the microbiome is altered in celiac disease, as a richer, more diverse microbiome has been observed.70 As stated recently by Sanz,70 “disease-associated alterations in microbiome-derived metabolites could not only reflect intestinal dysbiosis but also contribute to dysfunction of the immunoregulatory mechanism.” However, in celiac disease, the situation is more complex, since crosstalk between the host genome and members of the microbiota related to the development of celiac disease was recently observed.44,71
The precise pathomechanisms that determine how gluten impacts the intestinal ecosystem are not well understood, but several potential mechanisms, described below, have been offered.
Gluten-induced dysbiosis is accompanied by a breach in the tight-junction barrier and the induction of several inflammation-associated microRNAs.72 In addition, the crosstalk between the enteric microbiota and the intestinal epithelium is mediated by receptors shared with the innate immune system. Thus, gluten-induced dysbiosis may contribute to the activation of this inflammatory pathway. Patients with celiac disease are known to have fewer beneficial gut species and a greater number of potentially pathogenic ones compared with healthy controls. Gluten-free diets reduce this dysbiosis, although it is not eliminated.73 Additionally, dietary factors, such as meat-containing or vegetarian diets or the presence or absence of gluten in the diet, can affect a number of taxa in the lumen and influence multiple bacterial pathways, especially those involved specifically in carbohydrate and starch metabolism. Variation in the diet, including the introduction of gluten-free diets, could affect the composition of the microbiome, even in normal volunteers.74
There are also other potential mechanisms by which gluten might impact luminal conditions. Compared with healthy controls, untreated and treated patients with celiac disease showed evidence of gluten-induced dysbiosis at the duodenal and/or the fecal level.74,75 The ratio of protective antiinflammatory microbes such as Lactobacillus and Bifidobacterium to potentially harmful microbes like Bacteroides and Enterobacteriaceae was lower in untreated children than in children treated with a gluten-free diet. Similar to observations in dysbiotic microbiota, serum, fecal, and urinary metabolomes from patients with celiac disease showed abnormal levels of free amino acids and volatile organic substances that may convey messages to the mucosal wall. There is no consensus across studies with regard to the specific microbes and/or metabolites in individuals with untreated or treated celiac disease.75 Finally, it should be emphasized gluten affects the microbiome, but the microbiome also affects gluten and might play a role in the breakdown and immunogenicity of gluten.76
Induction of oxidative stress by gluten
Hudson et al.77 were the first to report the suppressed growth and morphological alterations induced by application of gliadin peptides to cell lines of human origin. Gliadin induces agglutination of K562 cells, decreases F-actin expression in intestine 407 cells, attenuates cell viability and growth, is proapoptotic and affects redox homeostasis in Caco-2 cells, alters the morphology of LoVo cells and of cells in 2- and 3-dimensional cultures, and induces restructuring of the cytoskeleton in intestinal epithelial cell line 6.59 Glutathione is a crucial low-molecular-weight molecule that affects redox status. Its depletion perturbs cell homeostasis, triggering cytotoxicity. In fact, the oxidative stress induced by gliadin was demonstrated in multiple cell lines, including Caco-2, HT29, SH-SY5Y, T84, and LoVo, and was reviewed extensively.59,78–80 The compromised antioxidant defense mechanisms may exacerbate mucosal inflammation, potentiating tissue damage and delaying the return of epithelial cell layer integrity, not only in celiac disease but also in other inflammatory conditions.
Effects of gluten on epigenetic programming
Epigenetic programming, including methylation and histone modification, is affected by numerous environmental factors, including nutrients.78,79 Altered expression of genes involving methylation homeostasis, induced by gluten-derived peptides, was recently observed.78,79 Several aspects of the relation between gliadins and epigenetics were observed not only in celiac disease but also in nonceliac cells like SH-SY5Y, a cell line of neuroblastoma origin, and Caco-2 cells.79 These results indicate the potential of gliadin peptides to trigger antioxidant and epigenetic alterations that may be crucial during the postnatal transition from breastfeeding to gluten consumption.78,79 Most recently, the involvement of the proinflammatory cytokine interferon-γ and the microbiota metabolome in switching epigenetic behavior was described in celiac disease.81 Reduced antioxidant capacity and the epigenetic consequences of the gliadin-induced opioid activities may predispose genetically susceptible individuals to inflammation or autoimmunity, partially explaining the benefits of gluten-free diets. In a more holistic way, the oxidative stress, gene expression, and inflammation induced by gluten-derived peptides during progression of the autoimmune and inflammatory cascade are closely related and interconnected.
Effects of gluten on cellular metabolism
In the 1990s, wheat-derived peptides were shown to affect cell metabolism in Caco-2 cell lines. One study reported that cell proliferation was inhibited by 50%, colony-forming ability was decreased by 20%, and alkaline phosphatase activity during cell differentiation was decreased.82 Furthermore, peptic/tryptic digests from wheat inhibited DNA and RNA synthesis by almost 80% and glycoprotein synthesis by close to 60%.83 Apoptosis induced by gliadin peptides was demonstrated in several cell lines, including Caco-2, LoVo, Hep-2, and MRC-5.59,61,84 The effects of prolamin-derived antinutritional gliadin peptides, which include antiproliferation, antidifferentiation, cellular agglutination, and synthesis of nucleic acids, can disturb cellular metabolism and intestinal recovery in various enteric inflammatory conditions and gluten-related diseases.
Effects of gluten on cognitive function
Gluten initiates an immune reaction and decomposes, during luminal digestion, to peptides with opioid activity, which has multiple consequences on mental function and behavior in humans.85 Gluten-derived peptides that exhibit strong opioid activities are called exorphins. Exorphin receptors are widely scattered throughout the body, at locations that include the gut, the brain, and the nervous system. If the intestinal and blood–brain barriers are disrupted by stress, dietary components,40,63 alcohol, or over-the-counter drugs, gluten-derived exorphins can impact cognitive function.85
The ability of gluten to affect mental function is not merely a hypothesis. Multiple studies have shown a high prevalence of antigliadin antibodies in patients with schizophrenia.86 Patients with autistic spectrum disorders are affected by gluten intake. Gluten-free diets are efficient in modulating both gastrointestinal symptoms and behavior in patients with autistic spectrum disorder.87 Various risk factors for the progression of schizophrenia can be related to a common pathway in the intestinal tract. It is increasingly recognized that bidirectional communication, involving neural, hormonal, and immunological routes, exists between the brain and the gut. An increased incidence of gastrointestinal barrier dysfunction, food antigen sensitivity, inflammation, and the metabolic syndrome is seen in patients with schizophrenia.86 These findings may be influenced by the composition of the gut microbiota. A significant subgroup of patients may benefit from adherence to a gluten-free diet. Antimicrobial agents and probiotics have therapeutic potential to attenuate the metabolic abnormalities and immune dysfunction encountered in patients with schizophrenia.88 A form of gluten psychosis—a clinical psychosis clearly related to gluten consumption, which completely resolved after a gluten-free diet was implemented—has been described.89 Food proteins, including gluten, affect the rates of synthesis of amino acids and brain neurotransmitters in rats.90 Prolonged gluten intake impairs cognitive performance in elderly patients with celiac disease,91 many of whom benefit from gluten-free diets.92 Multiple behavioral and peripheral or central neurological manifestations have been described in patients with celiac disease, some of whom respond to gluten-free diets.93–96
Gluten exorphin B5 is an opioid peptide identified after wheat gluten digestion. It stimulates prolactin secretion and affects brain neurotransmitter discharge, even without crossing the blood–brain barrier, in rats.97
The scientific and medical communities are witnessing a major era in which the relationship between the gut and the remote organs, including the gut–brain axis, is under intense investigation.98 The beneficial or partial response to gluten withdrawal suggests some degree of cause-and-effect relationship between gluten and neurological manifestations.
To provide support that gluten intake may have adverse effects in patients with nonceliac autoimmune disease, the next section describes various autoimmune diseases in which gluten-free diets had some beneficial effect.
ROLE OF GLUTEN-FREE DIET IN NONCELIAC AUTOIMMUNE DISEASES
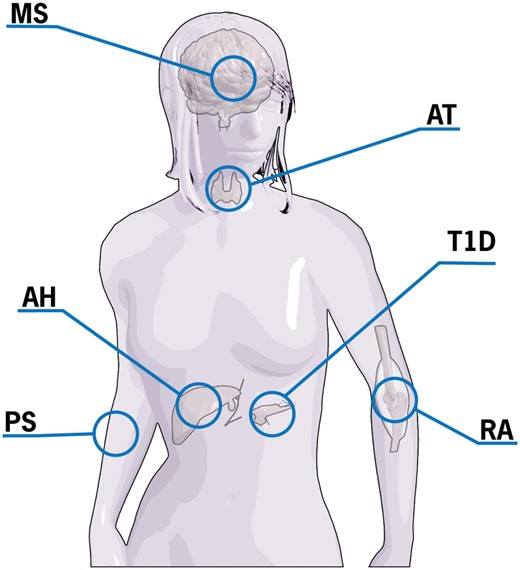
Since celiac disease and many autoimmune diseases share some human leukocyte antigen genes and multiple non–human leukocyte antigen genes, it is conceivable that this genetic load transfers some gluten-dependent toxicity to other autoimmune diseases.99–104 Since celiac disease and other autoimmune diseases share common genetic, environmental, and immunological pathways, it would be interesting to investigate the possible therapeutic effects of gluten-free diets in patients with nonceliac autoimmune conditions. At least in celiac disease, the additive risk of subsequent autoimmune disease was decreased in patients who adhered to a gluten-free diet vs those who did not.105 It was concluded that a gluten-free diet has a protective effect. The effects of gluten in non–gluten-sensitive populations were recently reviewed.106,107 The protective effect of gluten withdrawal has been observed in several autoimmune diseases (Figure 1). Moreover, the ingestion of gluten seems to be related to disease progression. It should be stressed, however, that the Speculation about the benefits of gluten-free diets is based on a limited number of clinical trials that investigated gluten-free diets as either a causal factor in the pathogenesis of disease or in association with the improvement of symptoms.
Autoimmune diseases that improve following gluten withdrawal. Abbreviations: AT, autoimmune thyroiditis; AH, autoimmune hepatitis; MS, multiple sclerosis; PS, psoriasis; RA, rheumatoid arthritis; T1D, type 1 diabetes.
Type 1 diabetes mellitus
Multiple findings suggest a connection between T1D and gluten or celiac disease: shared genes, coexistence of TID and celiac disease in some patients, increased celiac disease prevalence in patients with T1D, increased selective T-cell response and high concentration of wheat antibodies in both T1D and celiac disease, the dependency of animal models of T1D on diet, with gluten being a major potent diabetes-inducing protein, and, recently, the identification of T1D-related proteins from wheat, showing that cDNA clones of diverse wheat storage globulins were associated with islet damage.108,109 Moreover, a partial depletion of regulatory T cells before gliadin sensitization induced severe insulitis in a mice model. The presence of gliadin-responsive T cells in the pancreatic lymph nodes of mice that develop insulitis suggests that gliadin-specific T-cell clones have a role in the induction of insulitis in this animal model.110
Gluten-free diets delayed and prevented diabetes in NOD mice that had never ingested gluten.111 The intestinal immune system plays a primary role in the pathogenesis of T1D, since diabetogenic T cells are initially primed locally in the gut, islet-infiltrating T cells express gut-associated homing receptors, and mesenteric lymphocytes passively transfer diabetes from NOD mice to mice with NOD or severe combined immunodeficiency.67 During infancy, the amount, timing, and mode of gluten introduction have been shown to affect the diabetogenic potential of gluten, and it is now suggested that a gluten-free diet may preserve beta cell function.67 A subset of patients with T1D had an abnormal immune reaction to gluten, manifested by a positive reaction to rectal gluten challenge that resulted in lymphocyte infiltration of the rectal mucosa, increased proliferative T-cell response to wheat proteins, with production of proinflammatory cytokines, and an elevated T-cell response to gluten in one-fourth of patients with newly diagnosed T1D. All of these effects occurred in patients without celiac disease–associated human leukocyte antigen haplotype, suggesting a direct, diabetes-specific effect of gluten.67
Notably, the benefits of a gluten-free diet in patients with symptomatic celiac disease and T1D are supported by the literature, though it is unclear whether these benefits occur in asymptomatic patients. In a 6-month study of a gluten-free diet in individuals at high risk for T1D, autoantibody levels did not change, but insulin secretion was reduced.112 Other studies did not substantiate the benefit of gluten-free diets.113,114 Taken together, gluten consumption may play a substantial role in the progression of T1D, but additional studies are warranted to explore whether gluten withdrawal could prevent disease in susceptible individuals or be applied in newly diagnosed patients to stop or attenuate disease progression.
Rheumatoid arthritis
Multiple studies have described shared domains between rheumatoid arthritis and celiac disease.44 Thus, the partial response of celiac disease–associated rheumatic manifestations to gluten-free diets is logical. Additionally, there is a protective effect of gluten withdrawal on the cumulative incidence of additional autoimmune diseases in patients with celiac disease.78,105 In fact, dietary modifications, like gluten-free vegan diets, had clinical benefits in certain patients with rheumatoid arthritis that may result from reduced immunoreactivity to food antigens that are eliminated by a restrictive diet.115 A gluten-free vegan diet in patients with rheumatoid arthritis drives changes that are potentially atheroprotective and antiinflammatory.116 Of note, human leukocyte antigen DQ2/8-restricted gluten-specific T cells were noticed to migrate from the enteric mucosa into peripheral blood upon gluten challenge, thus indicating an additional mechanism of articular pathology in celiac disease and suggesting a basis for the benefit of gluten-free diets.
Multiple sclerosis
Numerous neurological manifestations that mimic multiple sclerosis have been described in patients with celiac disease.93,94 A certain percentage of patients with multiple sclerosis show high levels of IgA anti–tissue transglutaminase autoantibodies.117 It has been recommended that gluten-free diets be considered in patients who test positive for antigliadin antibodies. More recently, an increased prevalence of antibodies associated with celiac disease was found in a population of patients with relapsing-remitting multiple sclerosis (odd ratio, 5.33),118 and 11% of that population was diagnosed as having celiac disease. This is 5 to 10 times greater than the prevalence in the normal population. Furthermore, 32% of first-degree relatives of this population had positive serology for celiac disease. More relevant to the present review, however, is the finding that “the response of the gluten-free diets was excellent in all of them, both from the digestive and the neurologic point of view in the average follow-up period of three years long.” Several other small series and case reports observed clinical improvement in patients on gluten-free diets, but further research is needed to evaluate the response of patients with multiple sclerosis to nutritional changes.119,120 Many immunopathogenic pathways and various celiac disease–associated antibodies have been described. These antibodies can cross the blood–brain barrier and deposit at the level of the Purkinje cells, producing a remarkable inflammatory reaction followed by neuronal damage and cerebellar atrophy.118 The isoenzyme of tissue transglutaminase subtype 6 was demonstrated in the cerebellum of patients with celiac disease ataxia. Nutritional factors and positive anti–transglutaminase 6 autoantibodies might explain the pathogenesis of the gut–brain axis.121
Psoriasis
Psoriasis and celiac disease share genetic and inflammatory features. The connection between psoriasis and celiac disease was examined in a review of studies of gluten-free diets in patients with psoriasis.122 Of 33 patients with psoriasis who were positive for antigliadin antibodies and were treated with a gluten-free diet, 24 showed a reduction in their psoriatic severity index when compared with patients who had psoriasis and were negative for antigliadin antibodies.123 In another 28 patients, gluten withdrawal was shown to reduce the expression of tissue transglutaminase in patients with psoriasis who were positive for antigliadin antibodies.124 Several additional case reports were published in which patients with psoriasis experience rapid lesion resolution following adherence to a gluten-free diet.125–127 There is limited evidence to suggest that gluten restriction may benefit some patients with psoriasis, but additional controlled studies in defined patient populations are needed.
Autoimmune hepatitis and celiac hepatitis
Forty percent of individuals have abnormal liver tests upon the diagnosis of celiac disease, but tests will normalize in the majority who adhere to a gluten-free diet.128 Celiac disease is twice as common in patients with cirrhosis as in the normal population, and a gluten-free diet is known to improve liver function tests. Abnormally high levels of antiendomysial antibodies and high levels of tissue transglutaminase raise suspicion of celiac disease in patients with cirrhosis and may prompt physicians to recommend a gluten-free diet.129 Several hepatic and biliary diseases are associated with celiac disease: autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cirrhosis. These liver diseases are less prevalent than celiac hepatitis and are associated with increased morbidity and mortality, which requires they be treated with appropriate immunosuppressive therapy, rather than just gluten-free diets.130 However, a significantly increased proportion of patients with coexisting autoimmune hepatitis and celiac disease show sustained remission when compared with patients with autoimmune hepatitis without celiac disease. The improvement in liver function following dietary intervention suggests a possible long-term beneficial effect of a gluten-free diet.131,132
Autoimmune thyroiditis
In an Italian multicenter study, thyroid function was studied in 241 consecutive untreated patients with celiac disease and 212 controls. Thyroid disease was 3-fold higher in patients with celiac disease than in controls. In most patients who adhered to gluten-free diet for 1 year, a normalization of subclinical hypothyroidism was noticed,133 suggesting that, in some patients, a gluten-free diet may, by itself, reverse abnormal thyroid levels. Recently, however, an additional study could not repeat those results.134 In thyroid-associated orbitopathy, celiac disease is the only autoimmune disease in which symptoms and tissue transglutaminase levels usually improve if patients adhere to a gluten-free diet.135 In a study of the role of gluten in the induction of antiendocrine autoantibodies and organ dysfunction in adolescent patients with celiac disease, at least 1 antibody was positive in 10 of 19 untreated patients but in only 5 of 25 patients treated with gluten-free diets.136 A recent review described the similarities and differences between celiac disease and Hashimoto thyroiditis as well as the potential gut–thyroid interrelated pathways.137 The concept of a gut–thyroid relationship was supported by the fact that these 2 autoimmune diseases are related and that tissue transglutaminase antibodies were described to bind to thyroid follicles and to the extracellular matrix in patients with celiac disease. Furthermore, anti–tissue transglutaminase titers correlate with anti–thyroid peroxidase antibody titers. These findings suggest that celiac-associated autoantibodies could contribute to the progression of thyroid dysfunction in celiac disease, thus establishing some logical basis for the beneficial effects of gluten withdrawal in patients with autoimmune thyroid disease.137
Immunoglobulin A nephropathy
Like other autoimmune diseases, IgA nephropathy shares multiple characteristics with celiac disease,138 and several animal and human studies documented a beneficial effect of gluten-free diets in this disease.139,140
DISCUSSION
This is the first comprehensive review to address the adverse effects of gluten application or ingestion, in vitro or in vivo, and the effect of gluten-free diets in multiple autoimmune diseases: T1D, rheumatoid arthritis, multiple sclerosis, psoriasis, autoimmune hepatitis, and autoimmune thyroiditis. Basic science and translational and clinical research were reviewed to clarify the adverse effects of gluten and the mechanisms underlying the beneficial effects of gluten withdrawal. Summarizing the evidence from studies that explore the adverse effects of gluten in vitro or of uptake in vivo can help guide the decision to recommend restriction of dietary gluten to improve the well-being or clinical management of patients with nonceliac chronic conditions. However, it should be stressed that the lack of studies in normal human populations devoid of any inflammatory or autoimmune condition limits any recommendation to attenuate or prevent gluten consumption worldwide.
Since wheat is the most important staple crop in the West, and wheat consumption in countries undergoing urbanization, industrialization, and Westernization is steadily increasing,3,4,15,63 further investigations are needed to clarify the drawbacks and benefits of gluten ingestion. The enigma is further reinforced by the gains attributed to gluten, being a major source for starch, energy, proteins, vitamins, fiber, and phytochemicals. It should be clearly stated that the above-described response to gluten-free diets in nonceliac autoimmune diseases does not replace the corresponding disease-specific therapies of these disease. The mechanisms of gluten-free diets as nutritional therapy are far from being elucidated, but this review sheds new light that might clarify some of the pathways underlying the reduction in adverse effects sometimes observed after gluten withdrawal.
There are, however, some limitations to this review. The quality of evidence may be limited because of the small number of randomized clinical studies and animal studies, or because studies may have included patients with high heterogeneity and varying medical phenotypes, disease activities, and morbidity. There is always the possibility that some studies were not captured by the keywords used in the literature search. Since the search screened for autoimmune diseases, and not nonceliac gluten sensitivity, some information could have been missed. Furthermore, the gluten used in the studies might have been contaminated with other soluble proteins, such as amylase-trypsin inhibitors or yeasts used in commercial bakeries, which could have affected the results. Finally, evidence of the adverse effects of gluten was generated mainly in in vitro cell lines and animal models. There are not yet enough in vivo animal studies or human studies to support a recommendation of gluten withdrawal in clinical practice.
CONCLUSION
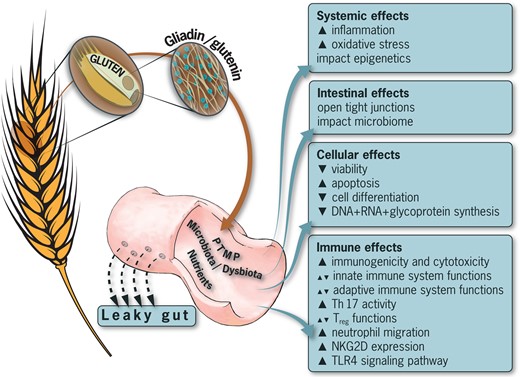
Wheat, along with its main storage protein, gluten, is a staple nutrient. Increasing evidence shows that gluten may have multiple detrimental effects on human health. Figure 2 summarizes the possible detrimental effects of gluten on the intestinal luminal compartment, on the immunological pathways, and on cellular as well as systemic domains. A word of caution is needed before conclusions are drawn and recommendations are suggested. Evidence of the detrimental effects of gluten is limited to several in vitro and animal studies. Well-designed, controlled studies in humans are lacking. Thus, no practical clinical recommendations can be suggested until further clinical trials are performed. Implementing gluten-free diets in certain individuals affected by nonceliac autoimmune disease might be instrumental in minimizing the adverse effects of gluten in humans, but for now, the use of such nutritional therapy is not based on adequate human investigations. Considering the effects of gluten on the microbiome, the role of gluten in oxidative stress and epigenetics, and the relationship of gluten to the induction and progression of autoimmunity, a gluten-free diet might have multiple indirect consequences.42,63,141–143
Adverse effects of gluten on the human cellular, immune, intestinal, and systemic compartments. Abbreviations and symbols: NKG2D, natural killer group 2D costimulatory molecule; PTMP, post-translational modification of proteins; TH-17, T-helper 17 lymphocytes; TLR4, Toll-like receptor 4 signaling pathway; Treg, regulatory T cells; ▲, increased; ▼, decreased.
Currently, there is no clear indication to withdraw gluten in non–gluten-related conditions. However, since there is an increased prevalence of celiac disease in multiple autoimmune diseases and vice versa, it is suggested that patients with autoimmune disease be screened for antibiodies associated with celiac disease. In view of the central role played by the human intestine in inducing autoimmunity in peripheral organs,44,98,99,137,138,144,145 there is a need to elucidate the pathways, the molecules, or the messengers involved in the interplay between gluten and nonceliac autoimmune conditions. It is hoped that the detrimental effects of gluten summarized here will encourage the clinical and the scientific communities to explore the underlying mechanistic pathways and verify whether observations in animals and ex vivo can be substantiated in humans.
Acknowledgments
The authors would like to acknowledge Mr Neu Alf, medical illustrator, for providing the figures and Dr. Ramesh Ajay for editing the manuscript.
Author contributions A.L. wrote the manuscript and was responsible for the study concept, the study design, and the literature review. Y.S. supervised and was responsible for the study concept, the interpretation of data, and the editing and critical revision of the manuscript. T.M. was responsible for the study design and the critical revision of the manuscript and provided administrative, technical, and material support. All authors approved the final version of the manuscript, including the list of authors.
Funding/support. No external funds supported this work.
Declaration of interest. The authors have no relevant interests to declare.
References
Food and Agriculture Organization of the United Nations: FAO cereal supply and demand brief: summary tables (world wheat market). http://www.fao.org/worldfoodsituation/csdb/en/. Updated October 5, 2017. Accessed October 5, 2017.