-
PDF
- Split View
-
Views
-
Cite
Cite
Junichi Sakamoto, Chikuma Hamada, Mahbubur Rahman, Susumu Kodaira, Katsuki Ito, Hiroaki Nakazato, Yasuo Ohashi, Masayuki Yasutomi, An Individual Patient Data Meta-analysis of Adjuvant Therapy with Carmofur in Patients with Curatively Resected Colon Cancer, Japanese Journal of Clinical Oncology, Volume 35, Issue 9, September 2005, Pages 536–544, https://doi.org/10.1093/jjco/hyi147
Close - Share Icon Share
Abstract
Background: Oral carmofur, either as a single or in combination with other chemotherapeutic agents, has been used as adjuvant chemotherapy for curatively resected colon cancer patients. Past trials and meta-analyses indicate that it is somewhat effective in extending survival of patients with this cancer. The objective of this study was to perform a reappraisal of randomized clinical trials conducted in this regard.
Methods: We designed an individual patient-based meta-analysis of relevant clinical trials to examine the benefit of oral carmofur for curatively resected colon cancer in terms of overall survival (OS) and disease-free survival (DFS).
Results: We analyzed individual patient data of three randomized clinical trials, which met the predetermined inclusion criteria. These three trials had a combined total of 2152 patients, carmofur as adjuvant chemotherapy compared with surgery-alone, 5 years follow-up, intention-to-treat-based analytic strategy and similar end points (OS and DFS). In a pooled analysis, 5 year OS rates were 80.4 and 76.4%, and 5 year DFS rates 76.9 and 71.0%, respectively, in carmofur and surgery-alone group. Oral carmofur had significant advantage over surgery-alone in terms of both OS [pooled hazard ratio, 0.82; 95% confidence interval (CI) = 0.68–0.99; P = 0.043] and DFS (pooled hazard ratio, 0.77; 95% CI = 0.65–0.91; P = 0.003).
Conclusions: This individual patient-based meta-analysis demonstrated that oral carmofur significantly improves both OS and DFS in patients with curatively resected colon cancers.
INTRODUCTION
Colon cancer (along with rectum) is the third most common cancer in the world (9.4% of all new cancers) and two-thirds of which are in developed countries (1). In 2000, an estimated 945 000 new cases were diagnosed in all over the world, with 492 000 deaths (fourth most common after lung, stomach and liver). In Japan alone, in the same time, the number was 80 000 deaths, and it is expected to climb up to 110 000 in the year 2020 (1). Thus, the chemotherapeutic agents are expected to have a lot of things to do in terms of extending survival for the patients with colon cancer.
Extensive clinical studies that have been performed since 1980s have proved the efficacy of 5-fluorouracil (5-FU) in combination with leucovorin (LV) for patients with advanced colon cancer (2–4). In an adjuvant setting, this combination has also been shown to bring better prognosis in patients with colon cancer (5–7). However, these regimens have considerable hematological and non-hematological toxicities. Although continuous infusion of 5-FU/LV was shown to have better efficacy with lower toxicities (8,9), the need for implanted venous access and portable pump to release this combination have restricted the wide availability of this therapy.
The recent development of oral fluorinated pyrimidines have opened a new era for the treatment of colorectal cancer. These agents showed similar effectiveness as that of 5-FU with continuous infusion, but do not need special drug releasing device as needed for 5-FU/LV. In advanced colorectal cancers, the efficacy of uracil and tegafur (UFT), one of the oral fluorinated pyrimidines, in combination with oral folinic acid (10,11) or capecitabine (12) had similar efficacy like that of intravenous 5-FU/LV, but with less hematologic and non-hematologic toxicities. Meta-analyses that examined the efficacy of oral fluoropyrimidines (all together) as adjuvant therapies on colorectal cancer patients also showed that they improve overall survival (OS) and disease-free survival (DFS) after resection of early stage colorectal cancer (13,14). However, the efficacy of a particular oral fluoropyrimidine on colon cancer have not been examined separately using individual patient data meta-analysis technique.
1-Hexycarbamoyl-5-fluorouracil (carmofur, HCFU) is one of the oral fluorinated pyrimidines developed as a lipophilic masked compound of 5-FU, and have been widely used in Japan, Korea, Finland and in the mainland China for many years (15,16). Carmofur, administered orally, is reported to be converted either enzymatically or non-enzymatically into 5-FU in the human body and exert its antitumor effect (17,18). In a phase II study with colorectal cancer, carmofur demonstrated a response rate of 43% (19), which was superior to that of parenteral 5-FU (15%) and oral tegafur (11%) (20,21).
A preliminary appraisal of the effect of adjuvant therapy with carmofur was performed using individual patient data from three clinical trials conducted in the 1980s (22). In that meta-analysis, randomized trials comparing short induction therapy or ad libitum control with the same therapy with carmofur were analyzed. Although a significant benefit in terms of 7 year OS and 7 year DFS was shown in that study, total number of colon and rectal cancer patients analyzed was 614, and the results were not entirely convincing. Therefore, the actual benefit of carmofur regarding the prognosis of patients with non-metastatic colon cancers needs to be established reliably.
In this study, an individual patient-based meta-analysis of all eligible randomized trials that compared oral carmofur with surgery-alone was carried out to have more reliable assessment of the benefit of this therapy among patients with curatively resected colon cancer in terms of OS and DFS.
MATERIALS AND METHODS
Search Strategies and Clinical Trial Selection
In January 2002, a systematic search was conducted to identify all prospective trials performed with carmofur in curatively resected colon cancer patients. Eligible trials were searched through the MEDLINE database, Japanese publication database (Japanese MEDLINE) and presentations at meetings. Further inquiries were made through pharmaceutical industries to track down any trials since 1980 with an English or Japanese abstract. The primary source of the trials was contacted to obtain additional information on each identified trial. To be considered eligible for this meta-analysis, trials had to meet the following requirements: (i) randomized trial on colon cancer patients; (ii) randomization carried out centrally; (iii) curative tumor resection; (iv) carmofur adjuvant therapy given after tumor resection; (v) identical end points; (vi) surgery-alone as controlled arm; and (vii) trials having at least 5 year follow-up. Two investigators independently checked the selection criteria manually and discarded irrelevant studies. Any studies categorized as relevant by any of the two investigators were retrieved for further assessment. After assessment, they summarized the collected reports using a standardized form. Summaries of the reports were compared and disagreements were resolved by discussion without leaving any persisting differences.
Protocol for the Meta-analysis
In February 2002, a protocol for the meta-analysis describing its rationale, statistical methodology and rules for publication was distributed to the principal investigators of the eligible randomized trials. Principal investigators of each of the eligible trials were asked to provide individual data for every randomized patient, whether eligible or not, assessable or not, or properly followed or not.
Items requested for every patient were as follows: patient identification, date of surgery, eligibility, treatment assigned by randomization, age, sex, primary tumor site, stage of the disease, induction therapy, date of recurrence, death or last visit and cause of death, whichever occurred first. Survival was calculated from the date of surgery to the date of death, regardless of the cause of death.
Statistical Analysis
The method used for this meta-analysis and the format for the presentation of the results have been described in detail elsewhere (2,23). All analyses were based on individual patient data. Treatment effects on OS and DFS were first estimated within each trial and then combined using standard meta-analytical methods (23). Treatment effects were displayed as hazard ratios. These ratios can be estimated as relative risks of having an event in the carmofur group as compared with having the same event in the surgery-alone control group. A ratio less than unity indicates the benefit of oral carmofur, and this benefit is statistically significant when the 95% confidence interval (CI) of the ratio does not include unity. The overall effect of treatment was assessed through a chi-squared statistic with one degree of freedom and the heterogeneity between ‘n’ trials through a chi-squared statistic with ‘n − 1’degrees of freedom (23).
RESULTS
Review and Evaluation of Retrieved Reports
A total of 40 studies including unpublished data and protocol were found. Screening of these abstracts for relevant trials yielded 27 reports that were retrieved (24–51). Remaining 12 studies (52–63) were case reports, review articles, investigations based on patients with non-curative resection, carmofur in both arms of the trial, involved combination chemotherapy with other chemotherapeutic agents, preliminary reports of other trials, studies that had imbalanced background factors between the two arms, incomplete follow-up of patients and presented pooled data for the chemotherapy and immunochemotherapy arms.
The relevant retrieved reports are summarized in Table 1. The quality of the study design and the reporting of the results were evaluated, including the method of randomization, the definition of ineligible patients and the number of exclusions and subjects lost to follow-up. Three reports were published in English (24–26), and three reports were in Japanese (27–29) and among them one trial was included in the report of Japanese Ministry of Health and Welfare (28) and one remained unpublished (29). Some trials were excluded for the following reasons: non-curative resection (30,36,37), non-resectable advanced disease (37), carmofur in both arms (37,50), under 5 years follow-up (30,31), comparison with other anticancer drug (32,33), liver metastasis for colorectal cancer (34,35), neo-adjuvant chemotherapy (38,39) and not having central randomization (24).
Summary of the relevant trials retrieved for this meta-analysis
| Trial . | Randomization . | Patient accrual . | Stage of cancer . | Number of follow-up . | Protocol (number of colon cancer patients) . | . | Author's conclusion . | References . | |
|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | Arm (A) . | Arm (B) . | . | . | |
| Study Group of Tokai for HCFU | Envelope | 1981.7–1983.6 | Microscopically curative resection | 10 | MMC 2–4 mg 8 times (43) | HCFU 600 mg 12 weeks (45) | Effective (P < 0.05) | (25) | |
| Study Group of Tokai for HCFU | Centrally | 1987.10–1990.9 | Microscopically curative resection | 5 | Surgery-alone (43) | HCFU 8 mg/kg 12 months (44) | Effective (P < 0.05) | (26) | |
| Study Group of Kyushu for HCFU | Centrally | 1989.7–1991.6 | Curatively resected stage II–IV | 5 | 5-FU 10 mg/kg 6 times (71) | HCFU 300 mg 1 year (71) | NS | (27) | |
| JFMTC | Centrally | 1986.2–1988.12 | Curatively resected stage II–IV | 5 | Surgery-alone (464) | MMC 6 mg/m2 + HCFU 400 mg 1–2 weeks + HCFU 600 mg 1 year (462) | Effective (P < 0.05) for stage IIIb, IV | (28) | |
| JFMTC | Centrally | 1989–1990.12 | Curatively resected stage II–IV | 5 | Surgery-alone (414) | MMC 6 mg/m2 twice + 5-FU 250 mg civ 7 days + HCFU 300 mg 1 year (587) | Effective (P < 0.05) | (52) | |
| Study Group of Chugoku for HCFU | Centrally | 1993.10–1996.3 | Curatively resected stage IIIa and IIIb except N3(+) | 8 | 5-FU 333 mg/m2/24 h civ 3 days twice + 6 months later once (156) | HCFU 300 mg 2 years (157) | NS | (29) | |
| Chiba Mifurol Study Group | Centrally | 1990.6–1993.5 | Curative resection | 3 | Surgery-alone (−) | 5-FU 333 mg/m2/24 h civ 5 days + HCFU 300 mg (−) | Effective (P < 0.05) | (30) | |
| LH Study | 1994.9–1997.8 | Low grade malignancy | 3 | 5-FU 500 mg/24 h civ 7 days (40) | HCFU 300 mg 1 year (42) | NS | (31) | ||
| Study Group of Kanto for Mifurol | Envelope | 1982.1–1984.8 | Curative resection | 5 | MMC 0.2 mg/kg twice + Tegafur 12 mg/kg (45) | MMC 0.2 mg/kg twice + HCFU 6–12 mg/kg (46) | Log rank test (P < 0.05) | (32) | |
| Takano Hospital Colorectal Center | 1988.12–1992.6 | – | – | Five protocol (HCFU, UFT, 5-DFUR, Tegafur etc.) (−) | (−) | NS | (33) | ||
| Shimane Medical College | Envelope | 1987–1993 | Liver metastasis resection from colorectal cancer | 5 | Surgery-alone (8) | MMC 15 mg twice + HCFU 300 mg 1 year (10) | NS | (34) | |
| National Cancer research Center | Envelope | 1988.6–1990.5 | Liver metastasis resection from colorectal cancer | – | Surgery-alone (8) | MMC 0.1 mg/kg and 5-FU 15 mg/kg every 2 weeks + HCFU 400 mg/kg (8) | NS | (35) | |
| Cooperative Study Group of Kyushu and Chugoku for HCFU | Envelope | 1982.6–1984.5 | Non-curative resection | 5 | MMC total 100 mg (46) | HCFU 600 mg over 1 year (45) | Effective (P < 0.05) | (36) | |
| Trial . | Randomization . | Patient accrual . | Stage of cancer . | Number of follow-up . | Protocol (number of colon cancer patients) . | . | Author's conclusion . | References . | |
|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | Arm (A) . | Arm (B) . | . | . | |
| Study Group of Tokai for HCFU | Envelope | 1981.7–1983.6 | Microscopically curative resection | 10 | MMC 2–4 mg 8 times (43) | HCFU 600 mg 12 weeks (45) | Effective (P < 0.05) | (25) | |
| Study Group of Tokai for HCFU | Centrally | 1987.10–1990.9 | Microscopically curative resection | 5 | Surgery-alone (43) | HCFU 8 mg/kg 12 months (44) | Effective (P < 0.05) | (26) | |
| Study Group of Kyushu for HCFU | Centrally | 1989.7–1991.6 | Curatively resected stage II–IV | 5 | 5-FU 10 mg/kg 6 times (71) | HCFU 300 mg 1 year (71) | NS | (27) | |
| JFMTC | Centrally | 1986.2–1988.12 | Curatively resected stage II–IV | 5 | Surgery-alone (464) | MMC 6 mg/m2 + HCFU 400 mg 1–2 weeks + HCFU 600 mg 1 year (462) | Effective (P < 0.05) for stage IIIb, IV | (28) | |
| JFMTC | Centrally | 1989–1990.12 | Curatively resected stage II–IV | 5 | Surgery-alone (414) | MMC 6 mg/m2 twice + 5-FU 250 mg civ 7 days + HCFU 300 mg 1 year (587) | Effective (P < 0.05) | (52) | |
| Study Group of Chugoku for HCFU | Centrally | 1993.10–1996.3 | Curatively resected stage IIIa and IIIb except N3(+) | 8 | 5-FU 333 mg/m2/24 h civ 3 days twice + 6 months later once (156) | HCFU 300 mg 2 years (157) | NS | (29) | |
| Chiba Mifurol Study Group | Centrally | 1990.6–1993.5 | Curative resection | 3 | Surgery-alone (−) | 5-FU 333 mg/m2/24 h civ 5 days + HCFU 300 mg (−) | Effective (P < 0.05) | (30) | |
| LH Study | 1994.9–1997.8 | Low grade malignancy | 3 | 5-FU 500 mg/24 h civ 7 days (40) | HCFU 300 mg 1 year (42) | NS | (31) | ||
| Study Group of Kanto for Mifurol | Envelope | 1982.1–1984.8 | Curative resection | 5 | MMC 0.2 mg/kg twice + Tegafur 12 mg/kg (45) | MMC 0.2 mg/kg twice + HCFU 6–12 mg/kg (46) | Log rank test (P < 0.05) | (32) | |
| Takano Hospital Colorectal Center | 1988.12–1992.6 | – | – | Five protocol (HCFU, UFT, 5-DFUR, Tegafur etc.) (−) | (−) | NS | (33) | ||
| Shimane Medical College | Envelope | 1987–1993 | Liver metastasis resection from colorectal cancer | 5 | Surgery-alone (8) | MMC 15 mg twice + HCFU 300 mg 1 year (10) | NS | (34) | |
| National Cancer research Center | Envelope | 1988.6–1990.5 | Liver metastasis resection from colorectal cancer | – | Surgery-alone (8) | MMC 0.1 mg/kg and 5-FU 15 mg/kg every 2 weeks + HCFU 400 mg/kg (8) | NS | (35) | |
| Cooperative Study Group of Kyushu and Chugoku for HCFU | Envelope | 1982.6–1984.5 | Non-curative resection | 5 | MMC total 100 mg (46) | HCFU 600 mg over 1 year (45) | Effective (P < 0.05) | (36) | |
NS, non-significant; (−), information not available; HCFU, Carmofur; Mifurol, Carmofur; JFMTC, Japanese Foundation for Multidisciplinary Treatment for Cancer; LH Study, Low and High Colorectal Malignancy Study Group; civ, continuous venous infusion.
Summary of the relevant trials retrieved for this meta-analysis
| Trial . | Randomization . | Patient accrual . | Stage of cancer . | Number of follow-up . | Protocol (number of colon cancer patients) . | . | Author's conclusion . | References . | |
|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | Arm (A) . | Arm (B) . | . | . | |
| Study Group of Tokai for HCFU | Envelope | 1981.7–1983.6 | Microscopically curative resection | 10 | MMC 2–4 mg 8 times (43) | HCFU 600 mg 12 weeks (45) | Effective (P < 0.05) | (25) | |
| Study Group of Tokai for HCFU | Centrally | 1987.10–1990.9 | Microscopically curative resection | 5 | Surgery-alone (43) | HCFU 8 mg/kg 12 months (44) | Effective (P < 0.05) | (26) | |
| Study Group of Kyushu for HCFU | Centrally | 1989.7–1991.6 | Curatively resected stage II–IV | 5 | 5-FU 10 mg/kg 6 times (71) | HCFU 300 mg 1 year (71) | NS | (27) | |
| JFMTC | Centrally | 1986.2–1988.12 | Curatively resected stage II–IV | 5 | Surgery-alone (464) | MMC 6 mg/m2 + HCFU 400 mg 1–2 weeks + HCFU 600 mg 1 year (462) | Effective (P < 0.05) for stage IIIb, IV | (28) | |
| JFMTC | Centrally | 1989–1990.12 | Curatively resected stage II–IV | 5 | Surgery-alone (414) | MMC 6 mg/m2 twice + 5-FU 250 mg civ 7 days + HCFU 300 mg 1 year (587) | Effective (P < 0.05) | (52) | |
| Study Group of Chugoku for HCFU | Centrally | 1993.10–1996.3 | Curatively resected stage IIIa and IIIb except N3(+) | 8 | 5-FU 333 mg/m2/24 h civ 3 days twice + 6 months later once (156) | HCFU 300 mg 2 years (157) | NS | (29) | |
| Chiba Mifurol Study Group | Centrally | 1990.6–1993.5 | Curative resection | 3 | Surgery-alone (−) | 5-FU 333 mg/m2/24 h civ 5 days + HCFU 300 mg (−) | Effective (P < 0.05) | (30) | |
| LH Study | 1994.9–1997.8 | Low grade malignancy | 3 | 5-FU 500 mg/24 h civ 7 days (40) | HCFU 300 mg 1 year (42) | NS | (31) | ||
| Study Group of Kanto for Mifurol | Envelope | 1982.1–1984.8 | Curative resection | 5 | MMC 0.2 mg/kg twice + Tegafur 12 mg/kg (45) | MMC 0.2 mg/kg twice + HCFU 6–12 mg/kg (46) | Log rank test (P < 0.05) | (32) | |
| Takano Hospital Colorectal Center | 1988.12–1992.6 | – | – | Five protocol (HCFU, UFT, 5-DFUR, Tegafur etc.) (−) | (−) | NS | (33) | ||
| Shimane Medical College | Envelope | 1987–1993 | Liver metastasis resection from colorectal cancer | 5 | Surgery-alone (8) | MMC 15 mg twice + HCFU 300 mg 1 year (10) | NS | (34) | |
| National Cancer research Center | Envelope | 1988.6–1990.5 | Liver metastasis resection from colorectal cancer | – | Surgery-alone (8) | MMC 0.1 mg/kg and 5-FU 15 mg/kg every 2 weeks + HCFU 400 mg/kg (8) | NS | (35) | |
| Cooperative Study Group of Kyushu and Chugoku for HCFU | Envelope | 1982.6–1984.5 | Non-curative resection | 5 | MMC total 100 mg (46) | HCFU 600 mg over 1 year (45) | Effective (P < 0.05) | (36) | |
| Trial . | Randomization . | Patient accrual . | Stage of cancer . | Number of follow-up . | Protocol (number of colon cancer patients) . | . | Author's conclusion . | References . | |
|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | Arm (A) . | Arm (B) . | . | . | |
| Study Group of Tokai for HCFU | Envelope | 1981.7–1983.6 | Microscopically curative resection | 10 | MMC 2–4 mg 8 times (43) | HCFU 600 mg 12 weeks (45) | Effective (P < 0.05) | (25) | |
| Study Group of Tokai for HCFU | Centrally | 1987.10–1990.9 | Microscopically curative resection | 5 | Surgery-alone (43) | HCFU 8 mg/kg 12 months (44) | Effective (P < 0.05) | (26) | |
| Study Group of Kyushu for HCFU | Centrally | 1989.7–1991.6 | Curatively resected stage II–IV | 5 | 5-FU 10 mg/kg 6 times (71) | HCFU 300 mg 1 year (71) | NS | (27) | |
| JFMTC | Centrally | 1986.2–1988.12 | Curatively resected stage II–IV | 5 | Surgery-alone (464) | MMC 6 mg/m2 + HCFU 400 mg 1–2 weeks + HCFU 600 mg 1 year (462) | Effective (P < 0.05) for stage IIIb, IV | (28) | |
| JFMTC | Centrally | 1989–1990.12 | Curatively resected stage II–IV | 5 | Surgery-alone (414) | MMC 6 mg/m2 twice + 5-FU 250 mg civ 7 days + HCFU 300 mg 1 year (587) | Effective (P < 0.05) | (52) | |
| Study Group of Chugoku for HCFU | Centrally | 1993.10–1996.3 | Curatively resected stage IIIa and IIIb except N3(+) | 8 | 5-FU 333 mg/m2/24 h civ 3 days twice + 6 months later once (156) | HCFU 300 mg 2 years (157) | NS | (29) | |
| Chiba Mifurol Study Group | Centrally | 1990.6–1993.5 | Curative resection | 3 | Surgery-alone (−) | 5-FU 333 mg/m2/24 h civ 5 days + HCFU 300 mg (−) | Effective (P < 0.05) | (30) | |
| LH Study | 1994.9–1997.8 | Low grade malignancy | 3 | 5-FU 500 mg/24 h civ 7 days (40) | HCFU 300 mg 1 year (42) | NS | (31) | ||
| Study Group of Kanto for Mifurol | Envelope | 1982.1–1984.8 | Curative resection | 5 | MMC 0.2 mg/kg twice + Tegafur 12 mg/kg (45) | MMC 0.2 mg/kg twice + HCFU 6–12 mg/kg (46) | Log rank test (P < 0.05) | (32) | |
| Takano Hospital Colorectal Center | 1988.12–1992.6 | – | – | Five protocol (HCFU, UFT, 5-DFUR, Tegafur etc.) (−) | (−) | NS | (33) | ||
| Shimane Medical College | Envelope | 1987–1993 | Liver metastasis resection from colorectal cancer | 5 | Surgery-alone (8) | MMC 15 mg twice + HCFU 300 mg 1 year (10) | NS | (34) | |
| National Cancer research Center | Envelope | 1988.6–1990.5 | Liver metastasis resection from colorectal cancer | – | Surgery-alone (8) | MMC 0.1 mg/kg and 5-FU 15 mg/kg every 2 weeks + HCFU 400 mg/kg (8) | NS | (35) | |
| Cooperative Study Group of Kyushu and Chugoku for HCFU | Envelope | 1982.6–1984.5 | Non-curative resection | 5 | MMC total 100 mg (46) | HCFU 600 mg over 1 year (45) | Effective (P < 0.05) | (36) | |
NS, non-significant; (−), information not available; HCFU, Carmofur; Mifurol, Carmofur; JFMTC, Japanese Foundation for Multidisciplinary Treatment for Cancer; LH Study, Low and High Colorectal Malignancy Study Group; civ, continuous venous infusion.
Relevant Trials
As a result of the above selection process, three trials (25,27,51) covering a total of 2152 patients were analyzed. In each of these trials, carmofur and surgery-alone arms were balanced with regard to the number of patients. Although there were slight differences of the induction chemotherapy regimen in the chemotherapy arm among the trials, chemotherapy usually consisted of induction with mitomycin C and long-term carmofur. In each of the trials, the effect of chemotherapy with carmofur was compared with that of surgery-alone treatment. Details of the trials included in this individual patient data meta-analysis are presented in Table 2. In one trial (51), the authors analyzed their data separately due to the fact that OK-432 (picibanil, Chugai Pharmaceutical Co., Tokyo, Japan), a drug previously indicated for colorectal cancer, had been used for 259 out of 587 patients in the treatment group. After December 1989, when OK-432 had been excluded from the guidelines for colon cancer treatment, rest of the patients registered during January–December 1990 period (328 out of 587) in the treatment group did not have this drug. We also divided this trial into two parts and analyzed them as separate entities in this meta-analysis.
Details of the randomized controlled trials included in the individual patient data meta-analysis
| Category . | TSGHCFU . | JFMTC7-2 . | JFMTC15-A* . | JFMTC15-B* . | ||||
|---|---|---|---|---|---|---|---|---|
| Subject | Colon cancer | Colon cancer | Colon cancer | Colon cancer | ||||
| Induction therapy | None | Mitomycin C | Mitomycin C | Mitomycin C | ||||
| Randomization | Telephoning | Telephoning | Telephoning | Telephoning | ||||
| Accrual time | 1987–90 | 1986–88 | 1989 | 1990 | ||||
| Follow-up time | 5 years | 5 years | 5 years | 5 years | ||||
| Eligibility criteria | ||||||||
| Operation | Curative resection | Same | Same | Same | ||||
| Stage of cancer | Depth of invasion from PM to Si/Ai | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | ||||
| Age | <75 years | Same | Same | Same | ||||
| Previous CMX/RX | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Double cancers | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Others | No severe complications | Same | Same | Same | ||||
| Category . | TSGHCFU . | JFMTC7-2 . | JFMTC15-A* . | JFMTC15-B* . | ||||
|---|---|---|---|---|---|---|---|---|
| Subject | Colon cancer | Colon cancer | Colon cancer | Colon cancer | ||||
| Induction therapy | None | Mitomycin C | Mitomycin C | Mitomycin C | ||||
| Randomization | Telephoning | Telephoning | Telephoning | Telephoning | ||||
| Accrual time | 1987–90 | 1986–88 | 1989 | 1990 | ||||
| Follow-up time | 5 years | 5 years | 5 years | 5 years | ||||
| Eligibility criteria | ||||||||
| Operation | Curative resection | Same | Same | Same | ||||
| Stage of cancer | Depth of invasion from PM to Si/Ai | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | ||||
| Age | <75 years | Same | Same | Same | ||||
| Previous CMX/RX | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Double cancers | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Others | No severe complications | Same | Same | Same | ||||
TSGHCFU, Study Group of Tokai for HCFU the third Study; JFMTC, Japanese Foundation for Multidisciplinary Treatment for Cancer; 7-2, Special Study #7-2; 15-1-A, Special Study #15-1-A; 15-1-B, Special Study #15-1B.
Actually these two trials are nested in one trial. The authors analyzed the data separately due to the fact that OK-432, a drug previously indicated for colorectal cancer, had been used for 259 out of 587 patients in the treatment group up to December 1989. And after December 1989, when OK-432 had been excluded from the guidelines for colon cancer treatment, patients were without this drug. Thus, the first slot was regarded as JFMTC15-A and the rest as JFMTC15-B.
Details of the randomized controlled trials included in the individual patient data meta-analysis
| Category . | TSGHCFU . | JFMTC7-2 . | JFMTC15-A* . | JFMTC15-B* . | ||||
|---|---|---|---|---|---|---|---|---|
| Subject | Colon cancer | Colon cancer | Colon cancer | Colon cancer | ||||
| Induction therapy | None | Mitomycin C | Mitomycin C | Mitomycin C | ||||
| Randomization | Telephoning | Telephoning | Telephoning | Telephoning | ||||
| Accrual time | 1987–90 | 1986–88 | 1989 | 1990 | ||||
| Follow-up time | 5 years | 5 years | 5 years | 5 years | ||||
| Eligibility criteria | ||||||||
| Operation | Curative resection | Same | Same | Same | ||||
| Stage of cancer | Depth of invasion from PM to Si/Ai | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | ||||
| Age | <75 years | Same | Same | Same | ||||
| Previous CMX/RX | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Double cancers | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Others | No severe complications | Same | Same | Same | ||||
| Category . | TSGHCFU . | JFMTC7-2 . | JFMTC15-A* . | JFMTC15-B* . | ||||
|---|---|---|---|---|---|---|---|---|
| Subject | Colon cancer | Colon cancer | Colon cancer | Colon cancer | ||||
| Induction therapy | None | Mitomycin C | Mitomycin C | Mitomycin C | ||||
| Randomization | Telephoning | Telephoning | Telephoning | Telephoning | ||||
| Accrual time | 1987–90 | 1986–88 | 1989 | 1990 | ||||
| Follow-up time | 5 years | 5 years | 5 years | 5 years | ||||
| Eligibility criteria | ||||||||
| Operation | Curative resection | Same | Same | Same | ||||
| Stage of cancer | Depth of invasion from PM to Si/Ai | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | Macroscopic Dukes' B, C | ||||
| Age | <75 years | Same | Same | Same | ||||
| Previous CMX/RX | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Double cancers | Ineligible | Ineligible | Ineligible | Ineligible | ||||
| Others | No severe complications | Same | Same | Same | ||||
TSGHCFU, Study Group of Tokai for HCFU the third Study; JFMTC, Japanese Foundation for Multidisciplinary Treatment for Cancer; 7-2, Special Study #7-2; 15-1-A, Special Study #15-1-A; 15-1-B, Special Study #15-1B.
Actually these two trials are nested in one trial. The authors analyzed the data separately due to the fact that OK-432, a drug previously indicated for colorectal cancer, had been used for 259 out of 587 patients in the treatment group up to December 1989. And after December 1989, when OK-432 had been excluded from the guidelines for colon cancer treatment, patients were without this drug. Thus, the first slot was regarded as JFMTC15-A and the rest as JFMTC15-B.
Overall Survival
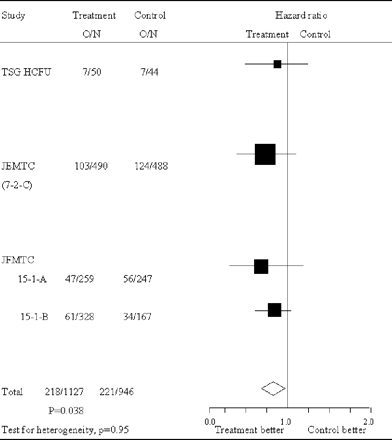
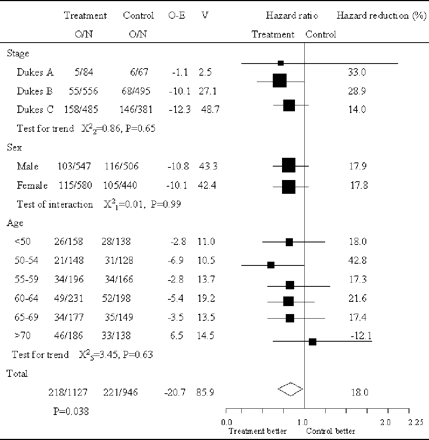
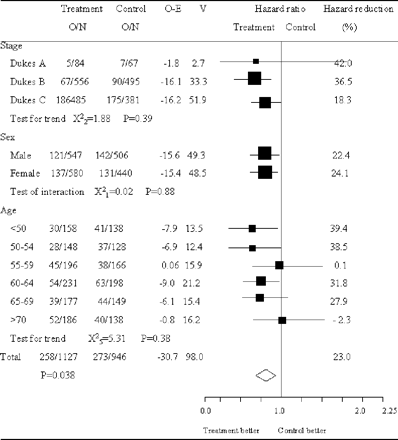
OS time calculation was based on the date of colon cancer resection in each of the trials. Therefore, we believe that the annexation of the survival hazard ratio of the eligible three trials would be well synchronized and appropriate. Survival hazard ratios are presented in Fig. 1 for all the trials separately along with pooled estimate. The pooled hazard ratio was 0.82 (95% CI = 0.68–0.99; P = 0.038). No significant heterogeneity was observed (χ2 for heterogeneity = 0.34 with three degrees of freedom; P = 0.95). Analysis stratified by Dukes' stage showed some kind of trend suggesting the efficacy of carmofur for both Dukes' B and Dukes' C patients where survival hazard ratios were 0.73 (95% CI = 0.51–1.05, P = 0.086) and 0.83 (95% CI = 0.66–1.04; P = 0.11), respectively (Fig. 2).
Survival hazard ratios of colon cancer patients individual trials and overall. Large squares indicate trials that provide more information and thus have narrower 95% CI. If the square is to the left of the vertical line, then OS is better in the group allocated carmofur, but if the CI crosses this line then the result is not statistically significant (P < 0.05). The overall total hazard ratio is represented as a diamond center of which is an estimate of hazard ratio, with 95% CI shown by the width of the diamond. O/N, observed events (death)/total number of patients.
OS of colon cancer patients in trials of carmofur versus surgery-alone based on different stratification. Large squares indicate trials that provide more information and thus have narrower 95% CI. If the square is to the left of the vertical line, then OS is better in the group allocated carmofur, but if the CI crosses this line then the result is not statistically significant (P < 0.05). The overall total hazard ratio is represented as a diamond center of which is an estimate of hazard ratio, with 95% CI shown by the width of the diamond. The hazard reduction is also given as a percentage for each subcategory. O/N, observed events (death)/total number of patients; O − E, observed minus expected events; V, variance of O − E.
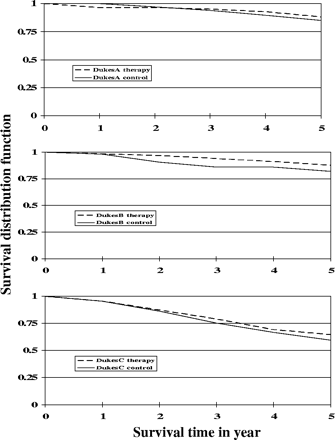
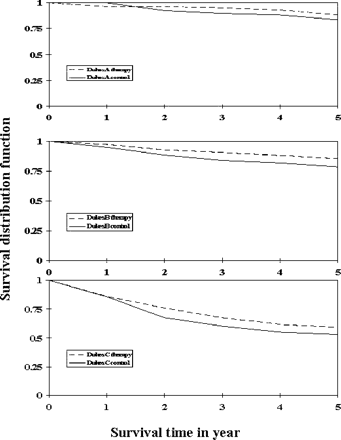
Figure 3 shows survival (OS) curves by treatment. The 5 year OS rate was 80.5% for the treatment group and 76.4% for the control group.
OS distribution function of colon cancer patients for Dukes' A, B and C stages. Therapy, - - -; control, ——.
Disease-free Survival
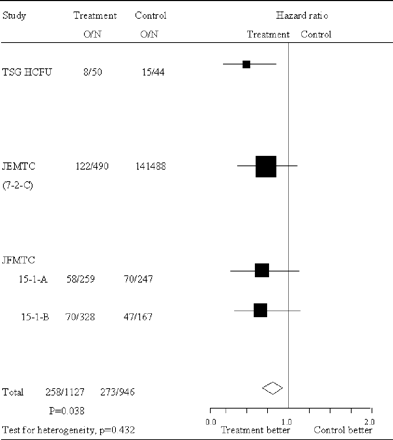
DFS hazard ratios are presented in Fig. 4 for all the trials along with the pooled estimate. This figure shows somewhat larger effect of treatment on DFS than on OS, with an overall hazard ratio of 0.77 (95% CI = 0.67–0.91; P = 0.025). Again no significant heterogeneity was observed (χ2 for heterogeneity = 2.75 with three degrees of freedom; P = 0.43). Analysis stratified by Dukes' stage demonstrated a significant effect of carmofur for Dukes' B and for Dukes' C patients where survival risk ratios were 0.64 (95% CI = 0.47–0.89; P = 0.008) and 0.80 (95% CI = 0.65–0.098, P = 0.032), respectively (Fig. 5).
DFS hazard ratios of colon cancer patients in individual trials and overall. Large squares indicate trial that provide more information and thus have narrower 95% CI. If the square is to the left of the vertical line, then OS is better in the group allocated carmofur, but if the CI crosses this line then the result is not statistically significant (P < 0.05). The overall total hazard ratio is represented as a diamond center of which is an estimate of hazard ratio, with 95% CI shown by the width of the diamond. O/N, observed events (death and not disease-free)/total number of patients.
DFS hazard ratios of colon cancer patients in trials of carmofur versus surgery-alone. Large squares indicate trial that provide more information and thus have narrower 95% CI. If the square is to the left of the vertical line, then OS is better in the group allocated carmofur, but if the CI crosses this line then the result is not statistically significant (P < 0.05). The overall total hazard ratio is represented as a diamond center of which is an estimate of hazard ratio, with 95% CI shown by the width of the diamond. The hazard reduction is also given as a percentage for each of subcategories. O/N, observed events (death and not disease-free)/total number of patients; O − E, observed minus expected events; V, variance of O − E.
Figure 6 shows survival (DFS) curves by treatment. The 5 year DFS rate was 77.0% for the treatment group and 71.0% for the control group.
DFS distribution function of colon cancer patients for Dukes' A, B and C stage. Therapy, - - -; control, ——.
DISCUSSION
By pooling individual data from three clinical trials, we have been able to show significant differences between carmofur users and surgery-alone procedure in terms of OS and DFS among curatively resected colon cancer patients. A significant 4.0% relative increase in OS (5 year) was observed in favor of carmofur. Also, a 5.9% significant relative increase in 5 year DFS was observed. In spite of the fact that two of the trials (27,51) had induction therapy along with the carmofur, no heterogeneity was found among the results, which means that there was no effect of induction component. These results are highly reliable, because the end points of this meta-analysis are the same as they are in individual trials. Thus, carmofur prevents early death and increase DFS for curatively resected colorectal cancer patients and, therefore, can be recommended for these patients. Since analyses stratified by Dukes' stage showed that carmofur was most effective in Dukes' B followed by Dukes' C (Figs 2 and 5), it might be better to recommend this drug for those two groups particularly.
To avoid publication and selection bias, each trial was examined in terms of standard practice of conducting individual patient data meta-analysis. By looking at the individual patient data, we were able to examine whether proper randomization was performed or not, whether intention-to-treat analysis was possible and the quality of the data (deletion, lost to follow-up and improper exclusion). Our present meta-analysis was performed by using the Medical Research Council and Meta-Analysis Group in Cancer criteria, which are well-known in the relevant scientific community. Similar criteria have been followed in our previous study as well (13,14).
Our meta-analysis is individual patient data meta-analysis (IPD). In contrast to meta-analyses that use summary results of studies from journal articles, IPD uses individual patient data obtained from the original group and the data analyzed as if they are nested in one study. The minimum data required are patient identifier, treatment allocation, outcome(s), date of randomization and date of outcome if time to event is to be calculated. Methodologically, individual data from different groups are accumulated and considered as components of a single study to calculate the effect size (odds ratio, hazard ratio, etc.). Strictly speaking, a meta-analysis based on individual patient data is more comprehensive, scientifically more authentic and complete when compared with literature-based meta-analysis (64). The advantages for the former category are: reduction of publication bias, better control for confounding factors and statistical reanalysis based on common definitions of variables, more complete identification of relevant trials, better compliance with providing missing data, more balanced interpretation of results, wider endorsement and dissemination of results and better clarification of further research (65). However, they are much more time consuming to perform and require more resource than literature-based meta-analysis, with time and costs proportional to the number of studies included (66,67). This study also included one unpublished study, which would definitely reduce publication bias.
Advance in the treatment of colorectal cancer is really rapid. In the last few years, novel oral fluorinated pyrimidines have been approved. Carmofur was proved to be superior compared with surgery-alone like 5-FU/LV did, but no trials have yet been conducted comparing carmofur with 5-FU/LV or the new agents. We still do not know whether carmofur is superior, equal or inferior to the new standards like capecitabine and UFT/LV. Although capecitabine and UFT/LV are considered to be the standard for adjuvant therapy for colon cancer, carmofur can also be regarded as an alternative option for the similar purpose since no evidence have been generated against its use.
Oral fluorinated pyrimidines have long been utilized as standard chemotherapeutic agents for curatively resected colorectal cancers. Our previous meta-analysis, based on all oral fluorinated pyrimidines, showed relative advantage of them in terms of survival and DFS for colorectal cancers. However, no meta-analysis have been conducted based on individual fluorinated pyrimidines. To our knowledge, our study is the first meta-analysis, which examined the efficacy of individual fluorinated pyrimidines. Our study showed that carmofur is significantly effective as adjuvant chemotherapy for curatively resected colon cancer in Japan. On the one hand, this study attests the fact that the use of carmofur for colorectal cancer patients in the 1980s and 1990s was scientifically right. And on the other hand, its use can be recommended for the same purpose, unless it is proved inferior to other alternatives in a well-designed randomized clinical trial.
There are some limitations to this study. Firstly, the number of trials found eligible for this study was only three. Future individual patient-based meta-analysis by including more trials, which are currently ongoing, could reveal broader view of effectiveness of carmofur for colorectal cancer. Secondly, the results generated from this study may not be generalized to the similar subjects in other countries as all the studies included in this study have been conducted in Japan. Further study based on individual patient data from different countries is needed to address this issue globally. Thirdly, risk–benefit ratios were not available for the trials included in this study. Thus, we do not know whether carmofur was really favorable in terms of risk–benefit ratio. However, by looking at the toxicity results from each of the trials, we do not see any severe or specific toxicity, and the reports of the trials were consistent with the independent patient data we have obtained from the original investigators.
In view of the results of this meta-analysis, we conclude that carmofur have been proved to be effective in improving both OS and DFS in curatively resected colorectal cancer patients.
References
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture.
Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate.
Meta-Analysis Group In Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer.
Meta-Analysis Group In Cancer. Toxicity of 5-fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors.
Wolmark N, Rockette H, Mamovnas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovolin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes'B and C carcinoma of the colon: Results from national surgical adjuvant breast and bowel project C-04.
O'Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dfose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer.
Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators.
De Gramont A, Bosset J-F, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer.
Andre T, Colin P, Louvet C, et al. Phase III trial (GERCOR C96.1) comparing bimonthly LV5FU2 to monthly 5FU-leucovorin high-dose (LV hd) in patients with Dukes' B2 and C colon cancer.
Schilsky RL, Levin J, West WH, Wong A, Colwell B, Thirlwell MP, et al. Randomized, open-label, phase III study of a 28-day oral regimen of eniluracil plus fluorouracil versus intravenous fluorouracil plus leucovorin as first-line therapy in patients with metastatic/advanced colorectal cancer.
Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A, et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer.
Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study.
Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials.
Sakamoto J, Hamada C, Kodaira S, Nakazato H, Ohashi Y. Adjuvant therapy with oral fluoropyrimidines as main chemotherapeutic agents after curative resection for colorectal cancer: individual patient data meta-analysis of randomized trials.
Hoshi A, Iigo M, Yoshida M, Kuretani K. Antitumor activity of carbamoyl derivatives of 5-fluorouracil by oral administration.
Hoshi A, Iigo M, Nakamura A, Yoshida M, Kuretani K. Antitumor activity of 1-hexylcarbamoyl-5-fluorouracil in a variety of experimental tumors.
HCFU Clinical Study Group. Absorption and excretion of new oral antitumor drug, 1-hexylcarbamoyl-5-fluorouracil (HCFU), in cancer patients.
Iigo M, Hoshi A, Nakamura A, Kuretake K. Antitumor activity of 1-alkylcarbamoyl derivatives of 5-fluorouracil in a variety mouse tumors.
Koyama Y. 1-hexylcarbamoyl-5-fluorouracil (HCFU): a masked 5-fluorinated pyrimidine.
Buroker T, Padilla F, Groppe C, Guy G, Quagliana J, McCracken J, et al. Phase II evaluation of ftorafur in previously untreated colorectal cancer.
Sakamoto J, Kodaira S, Hamda C, et al. An individual patient data meta-analysis of long supported adjuvant chemotherapy with oral carmofur in patients with curatively resected colorectal cancer.
Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomized trials.
Ito K, Yamaguchi A, Mura K, et al. Prospective adjuvant therapy with mitomycin C and carmofur (HCFU) for colorectal cancer, 10-year follow-up: Tokai HCFU Study Group, the First Study for Colorectal Cancer.
Ito K, Yamaguchi A, Miura K, et al. Oral adjuvant chemotherapy with carmofur (HCFU) for colorectal cancer: five-year follow-up. Tokai HCFU study Group-Third Study on Colorectal Cancer.
Sugimachi K, Maehara Y, Ogawa M, et al. Postoperative chemotherapy for colorectal cancer by combining 5-fluorouracil infusion and 1-hexylcarbamoy1-5-fluorouracil administration after curative resection.
Yasutomi M, Takahashi T, Kodaira S, et al. Prospective controlled study on the usefulness of carmofur as a postoperative adjuvant chemotherapy of colorectal cancer.
Japanese foundation for multidisciplinary treatment of cancer. Report of Project #15, Tokyo, Japan
Iwagaki H. Consideration of Post-operative Adjuvant Chemotherapy for Colorectal Cancer with 5-Fluorouracil (5-FU) Infusion Combined with 1-Hexylcarbamoyl-5-fluorouracil (HCFU) Oral Administration after Curative Resection. Proceedings of the Japan Society of Coloproctology, Yokohama
Matsubara H, Okuyama K, Koide Y, et al. Consideration of Postoperative Adjuvant Chemotherapy in Combination Study. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Ito T, Morita T, Sasaki M, et al. Postoperative Adjuvant Chemotherapy when Histologic Grade of Malignancy for Colorectal Cancer was Preclassified. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Morioka Y, Muto T, Toyama J, et al. Postoperative adjuvant chemotherapy with HCFU (Carmofur) for colorectal cancer.
Takagi K, Fujimoto N, Fukushima Y, et al. Effect of Postoperative Adjuvant Chemotherapy for Colorectal Cancer. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Kouno H, Tahara H, Sudou I, et al. Signification of Adjuvant Chemotherapy After Liver Metastasis of Colorectal Cancer Resection. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Noda N, Sugihara K, Moriya N, et al. Consideration of Effect of Adjuvant Chemotherapy after Liver Metastasis of Colorectal Cancer Resection Curatively. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Cooperative Study Group of Kyushu and Chugoku for HCFU Adjuvant Chemotherapy. Mitomycin C plus HCFU adjuvant chemotherapy for noncuratively resected cases of colorectal cartinoma (Second report): 5-year survival rate.
Nishida O, Osawa S, Omori K, et al. Assessment of Immuno-chemotherapy for Noncuratively Resected and Nonresected Patients for Colorectal Cancer. Proceedings of the Japan Society of Clinical Oncology, Niigata
Fujii H, Inaji N, Nakajima Y, et al. Comparative Study of the Effect of Preoperative Chemotherapy for Colorectal Cancer. Proceedings of the Japan Society of Clinical Oncology, Hiroshima
Kotake K, Oyama Y, Shida S, et al. Neo-adjuvant chemotherapy with carmofur for colorectal cancer—a multi-institutional randomized controlled study.
Fujii Y, Akiyama N, Eriguchi M, et al. Efficacy of carmofur (HCFU) for colorectal carcinoma—comparison of continues and intermittent administration.
Takano S, Ogawa M. Consideration of Optimum Administration Period for HCFU in Postoperative Adjuvant Chemotherapy for Colorectal Cancer. Proceedings of the Japanese Society for Cancer of the Colon and Rectum, Tokyo
Ito K, Okushiba T, Ohno K, et al. Comparative study of administration period in postoperative adjuvant chemotherapy with HCFU for colorectal cancer.
Ito K, Kato T, Koeke A, et al. Optimum duration of oral adjuvant chemotherapy of HCFU for colorectal cancer; review of 5-year follow-up.
Nakamura T, Ohno M, Tabuchi Y, et al. Optimum duration of oral adjuvant chemotherapy with Carmofur in the colorectal cancer patients: the Kansai Carmofur Study Group trial III.
Watanabe T, Takagi H, Ichihashi, et al. Postoperative Adjuvant Chemotherapy with HCFU (Carmofur) and PSK for Colorectal Cancer, 6-year Follow-up. Proceedings of the Japan Society of Clinical Oncology, Niigata
Iwagaki H, Tanaka N, Esato K, et al. Randomized controlled trial of 5-fluorouracil (5-FU) infusion combined with 1-hexylcalbamoil-5-fluorouracil (HCFU) oral administration and HCFU alone as postoperative adjuvant chemotherapy for colorectal cancer.
Iwagaki H, Tanaka N, Esato K, et al. Post-operative adjuvant chemotherapy for colorectal cancer with 5-fluorouracil (5-FU) infusion combined with 1-hexylcarbamoyl-5-fluorouracil (HCFU) oral administration after curative resection.
Kobayashi S, Kameyama M, Imaoka M, et al. Comparative Study of the Combined Effect of HCFU + Dipyridamole (DP) in Colorectal Carcinoma. Proceedings of the Japanese society for Cancer of the Colon and Rectum
Iwamoto K, Saito T, Kunii Y, et al. Effect of Postoperative Adjuvant Chemotherapy with HCFU, MMC and 5FU for Colorectal Cancer. Proceedings of the Japanese Society for Cancer of the Colon and Rectum
Kobashi S, Sato Y, Kondo M, et al. Consideration of Postoperative Adjuvant Chemotherapy for Colorectal Cancer, Cur A and B with HCFU or UFT Mono-therapy vs. Combined of 5FU Continuous Infusion. Proceedings of the Japanese Society for Cancer of the Colon and Rectum
Japanese foundation for multidisciplinary treatment of cancer. Report of Project #7. Tokyo, Japan
Ito K, Yamaguchi A, Miura K, et al. Effect of oral adjuvant therapy with Carmofur (HCFU) for distant metastasis of colorectal cancer.
Maehara Y, Sugimachi K, Ogawa M, et al. Postoperative adjuvant chemotherapy with 1-hexylcalbamoil-5-fluorouracil in patients in colorectal cancer and at a high risk for recurrence.
Kanno H, Yoden Y, Ohashi S, et al. Comparative study of the combined effect of HCFU and dipyridamole (DP) in colorectal carcinoma—TS inhibition rate. Kinki Cooperative Study Group of Chemotherapy for Colorectal Carcinoma.
Koyama Y. (Cooperative Study Group of HCFU) Phase II Study of a new fluorinated pyrimidine, 1-hexylcarbamoyl-5-fluorouracil (HCFU).
Nakanishi M, et al. The experience of using HCFU, a new anticancer drug.
Yokoyama M, Okei A. Combination Therapy with Mitomycin C(MMC)/Carmofur(HCFU) for Progressive Gastrointestinal Cancer – Report of Tohoku Study Group. Proceedings of the Japan Society of Clinical Oncology
Takashima S, Kinami Y, Kiriyama M, et al. Distribution of 1-hezycarbamoyl-5-fluorouracil in rats and human patients, and clinical results in patients with colorectal cancer.
Iwahiro K, Inoue K, Akasaka S, et al. Immuno-chemotherapy with the combined of rentinum, HCFU and MMC.
Rino Y, Joujima T, Abe M, et al. A case of sigmoid colon cancer with lung metastases effectively treated with HCFU and PSK.
Matsuda T, Mabuchi K, Kitaoka H, et al. A case of multiple liver metastases showing good response by administration of carmofur alone in an aged patient with colorectal cancer.
Miyauchi M, Yamamoto N, Matsumoto M, et al. Comparative clinical study on 5-FU concentrations for oral HCFU and I.V. 5-FU.
Mori T. Review of comparative studies of postoperative adjuvant chemotherapy after curativety resected colorectal cancer in Japan.
Oxman AD, Clarke MJ, Stewart LA. From science to practice. Meta-analyses using individual patient data are needed.
Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group.
Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology.