-
PDF
- Split View
-
Views
-
Cite
Cite
Robert H. Devlin, L. Fredrik Sundström, Rosalind A. Leggatt, Assessing Ecological and Evolutionary Consequences of Growth-Accelerated Genetically Engineered Fishes, BioScience, Volume 65, Issue 7, 01 July 2015, Pages 685–700, https://doi.org/10.1093/biosci/biv068
Close - Share Icon Share
Abstract
Genetically engineered fish containing growth hormone (GH) transgenes have been synthesized for more than 25 years, now with modifications made in multiple aquacultured species. Despite significant improvements in production characteristics being realized, these fish have not yet entered commercial production. The very strong enhancement of growth rates that can arise from GH transgenesis in fish has generated public and scientific concern regarding ecological and food safety. Little ecological risk is anticipated from engineered strains kept in fully contained facilities, so the concern is largely directed toward the reliability of containment measures and determining whether robust ecological data, pertinent to nature, can be generated within research facilities to minimize uncertainty and allow reliable risk-assessment predictions. This article summarizes the growth, life history, and behavioral changes observed in GH-transgenic fish and discusses the environmental and evolutionary factors affecting the adaptation, plasticity, and fitness of transgenic fish and their potential consequences on natural ecosystems.
The modification of plant and animal phenotypes for agricultural purposes has been underway for millennia via processes of domestication and directed selection, resulting in organisms highly adapted to the farm environment. With the emergence and application of molecular biology in the last century and the expansion of genomics in this century, it has now become possible to direct phenotypic changes in animals by applying knowledge of specific gene functions. Whereas genetic engineering (or transgenesis) of domesticated crops has become commonplace in many parts of the world, extensive genetic modification of agricultural animals has not followed, and at this time, no transgenic animals have been approved for food use. However, one group of species, the fishes, has been subjected to a great deal of effort to engineer their characteristics to enhance production for the expanding aquaculture food production sector. Applications to allow the use of a growth hormone (GH)–transgenic Atlantic salmon (Salmo salar) strain for egg production for export or for food use have been under recent evaluations in Canada and the United States, with final decisions made (http://www.dfo-mpo.gc.ca/csas-sccs/Publications/ScR-RS/2013/2013_023-eng.pdf; http://gazette.gc.ca/rp-pr/p1/2013/2013-11-23/html/notice-avis-eng.html) or anticipated in the near future (http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/GeneticEngineering/GeneticallyEngineeredAnimals/ucm280853.htm). The potential application of transgenic fish technology has generated significant scientific and public interest surrounding the prospective economic benefits as well as the potential risks in the areas of food safety and environmental consequences should such animals enter nature or the human food supply. In this article, we examine how GH transgenesis in fish may influence potentials for persistence in nature, with a focus on efforts to estimate survival and reproductive success, and we also examine the potential ecological consequences for use within a risk-assessment context. The discussion in particular is focused on issues surrounding the reliability of data generated within contained laboratory facilities, and we examine the potential for transgenic fishes to modify their phenotype through plasticity and natural selection.
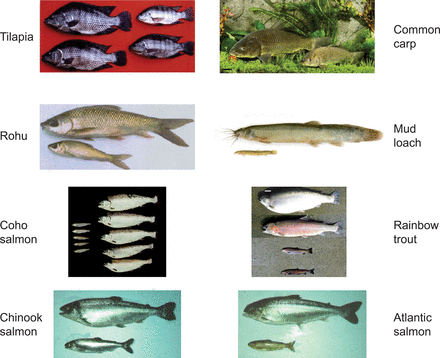
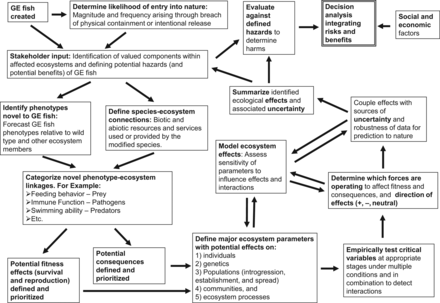
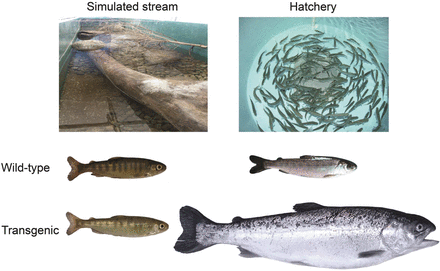
More than 35 species of fishes have been genetically modified with a range of gene constructs designed to alter their physiology for multiple objectives (see Devlin et al. 2006). The most common types of transgenic fish generated are those that have been engineered to overexpress growth hormone at elevated levels, which has resulted in very dramatic growth responses in several species (figure 1). The present article is focused on the data acquired for salmonids, common carp, and medaka, on which the greatest amount of study related to risk assessment has occurred. The assessment of the potential ecological effects of GH-transgenic fish in nature involves multiple considerations (see the example approach in figure 2). For example, the number of transgenic individuals in the ecosystem is affected by the frequency and magnitude of their introduction and by their subsequent survival and reproductive capabilities (overall fitness). The mere presence of transgenic fish in an ecosystem does not necessarily constitute harm, but rather it is their specific direct and indirect interactions with ecosystem components that may cause consequences on ecosystem functions and specific ecosystem members (Devlin et al. 2007, Kapuscinski et al. 2007). If such determinations can be made with reliability, this information can then be applied to determine whether transgenic fish constitute harms or benefits as previously defined through problem formulation and options analyses (PFOA, which incorporates social factors; Nelson et al. 2007).
(a) Examples of significant growth differences between nontransgenic and GH-transgenic fish in tilapia (Oreochromis niloticus, Rahman et al. 1998; photograph courtesy of Norman Maclean, University of Southampton, United Kingdom), triploid common carp (Cyprinus carpio, Yu et al. 2009), rohu (Labeo rohita, Venugopal et al. 1998), mud loach (Misgurnus mizolepis, Nam et al. 2001; photograph courtesy of Dong Soo Kim, Pukyong National University, Korea), coho salmon (Onchorhynchus kisutch, Devlin et al. 1994), mature rainbow trout (Onchorhynchus mykiss, Devlin et al. 2001), Chinook salmon (Onchorhynchus tshawytscha, Devlin et al. 1995), and Atlantic salmon (Salmo salar).
An example framework for empirical assessments of transgenic fish prior to entry into nature for use in risk-assessment processes (synthesized from information in Kapuscinski et al. 2007). The factors requiring assessment in the text boxes are examples only. Abbreviation: GE, genetically engineered.
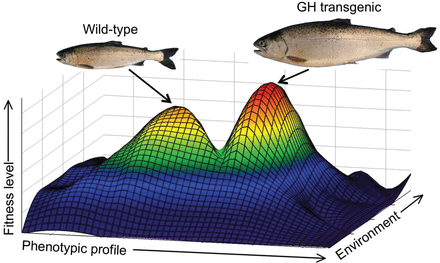
It is tempting to speculate that the fitness of a transgenic fish would be reduced if any of their characteristics have been altered from those seen in wild type. This assertion is derived from the assumption that wild-type fish are, for the genetic architectures available to them, near their peak of potential fitness in their environments. This may be true in many cases but is tautological without specific empirical evaluation (i.e., by their existence in nature, wild type are defined as being highly fit under existing environmental conditions). Given the large deviation in phenotype arising from GH transgenesis, it is possible that GH-transgenic fish could have been elevated to a higher fitness peak (Wright 1932) than that previously achieved by wild type. The interaction of a range of phenotypes with multiple environmental conditions is anticipated to generate variable fitness effects of transgenic fish relative to wild type. The latter may be restricted in elevating fitness further because of strong stabilizing selection or other evolutionary forces that prevent them from moving through surrounding fitness valleys (figure 3). Transgenic phenotypes of lower concern are those for which wild type have the capability to be selected to. In the case of growth, artificial selection can result in very high growth rates; however, this phenotype is not found in wild-type fish, suggesting it is not advantageous in nature. Clearly, barriers exist to elevating growth rate, presumably because of adaptive capacity or to trade-offs with other traits affecting fitness (Arendt 1997). Real-world fitness landscapes are likely much more complex and multidimensional (e.g., Gavrilets 1997), but simplistically, larger body size at a specific age in GH-transgenic fish may reduce season-dependent predation vulnerabilities relative to wild-type fish. It is also possible that GH-transgenic fish could be transformed to phenotypes that use new niches in the habitat but that do not significantly compete with conspecifics through much of their life history. In such a case, overall fitness could be impaired relative to wild type and only needs to be greater than that of other organisms within their new niche for them to persist. Therefore, although it is likely that transgenic fish would have impaired fitness in many cases, this should not be assumed and needs to be empirically demonstrated for risk-assessment processes. Certainly, many novel genotypes in the form of invasive species can successfully establish in new ecosystems even without having a specific evolutionary history in those locations (Fridley and Sax 2014).
Wright's (1932) fitness landscape depicting the theoretical positions of wild-type and growth hormone (GH)-transgenic fish for a range of phenotypic and environmental conditions. A key question regarding the phenotypic transformation of transgenic fish is whether they have been lifted to higher or different fitness peaks that wild type have not been able to access because of surrounding fitness valleys arising from limited adaptive capacity or the scope of phenotypic plasticity. The specific fitness peaks and valleys shown are hypothetical. The fish shown are coho salmon that were size matched at smolt and were subsequently grown in saltwater mesocosms.
The question of potential survival in and consequences on natural ecosystems by GH-transgenic fish is indeed highly analogous to invasive-species concerns (Devlin et al. 2006, Jeschke et al. 2013). Many analyses of invasive species use information from nature regarding the organism's ability to persist and compete under various biotic and abiotic environmental conditions, incorporating, for example, temperature tolerance or level of success in the presence of competitors. However, even with data from nature, predicting which species might eventually become invasive and what their consequences will be can only be achieved with partial success (Kumschick and Richardson 2013). A major difference for transgenic fish assessments is that the majority of informative data is not available from nature and must be obtained in artificial laboratory settings. Having data from nature for transgenic fish that integrates information from across the full life history of the animal (across multiple generations and environments) would be superior to the laboratory-derived estimates of invasiveness and consequences available today for some species (notably salmonids). This type of data, however, cannot be acquired from the wild without incurring significant uncertainty regarding potential environmental risk, because eradication of transgenic fish from many aquatic environments would be essentially impossible should a negative consequence arise (Britton et al. 2010). Therefore, there is a need to perform laboratory assessments of phenotype and ecological effects, which are subsequently extrapolated to effects as they may occur in nature. This process is subject to both measurement and interpretation uncertainty (Devlin et al. 2007, Kapuscinski et al. 2007); therefore, understanding the major factors that influence variability in assessment data is crucial to estimate uncertainty within quantitative risk assessments (Hayes et al. 2007).
The phenotypic effects of GH overexpression
The major objective of GH transgenesis is to accelerate growth rates in fish. This is achieved by driving unregulated overexpression of GH by circumventing negative feedback regulatory processes that serve to constrain and appropriately modulate GH production in normal animals in response to environmental and internal signals. GH is a high-level regulatory hormone that affects multiple pathways, and consequently, many phenotypic traits are modified in GH-transgenic fish, including developmental, morphological, physiological, and behavioral characteristics (see the examples for coho salmon, Oncorhynchus kisutch, in table 1). Many of these pleiotropic effects reflect the potential trade-offs arising from a forced alteration in growth physiology (Arendt 1997), and each has the potential to influence survival in—and consequences on—ecosystems by GH-transgenic fish. These alterations arise both as the direct effects of modification of GH-controlled pathways and as secondary consequences of rapid growth.
Example interpretations of the potential effects of altered traits of growth hormone (GH)–transgenic coho salmon compared with those of wild type on survival, reproduction, and ecosystem consequences, based on data available to date.
| Trait . | Survival . | Reproduction . | Ecosystem Consequence . | Stable among environments . |
|---|---|---|---|---|
| Life history effects | ||||
| arly hatching | +/– | N | P | No |
| arly smolting | +/– | + | P | No |
| aturation age and season | + | + | P | No |
| iurnal effects | – | n/a | n/a | n/a |
| igher fecundity, smaller egg size | – | +/– | P/NE | No |
| easonally uncoupled growth. | +/– | N | P | No |
| Genetics/cell biology | ||||
| ltered gene expression | n/a | n/a | n/a | No |
| ituitary function/structure altered | +/– | +/– | P | No |
| ltered growth pathway endocrinology | +/– | N | ? | No |
| ransgene structure stability | +/– | +/– | NE/P | n/a |
| Physiology/morphology | ||||
| lastic growth rate | + | n/a | n/a | No |
| cromegaly and altered allometry | – | – | n/a | No |
| ltered aerobic scope/osmoregulation | +/– | n/a | P | n/a |
| oorer swimming | – | – | P | n/a |
| educed innate immune function | – | – | P | n/a |
| cquired immune function normal | N | N | NE | n/a |
| ltered stress response | +/– | n/a | n/a | n/a |
| perm function | N | N | NE | n/a |
| ffects of triploidy | – | – | NE/P | n/a |
| nhanced feed-conversion efficiency | + | n/a | P | No |
| ltered metabolic pathways | +/– | N | n/a | No |
| ltered response to nutrition/dietary carbohydrate | +/– | n/a | n/a | No |
| ltered body coloration | – | – | n/a | No |
| Behavior | ||||
| nhanced feeding behavior | + | n/a | P | No |
| redator susceptibility | N/– | n/a | P | No |
| ltered spawning behavior | N | – | P | No |
| ncreased juvenile aggression | + | n/a | P | No |
| ecreased mature adult aggression | N | – | NE | No |
| ltered dispersal/migration | + | + | P | No |
| roader prey type selection | + | N | P | n/a |
| educed schooling/shoaling | – | – | NE/P | n/a |
| nhanced cannibalistic behavior | – | N | P | No |
| nvasion impacts | + | n/a | NE/P | n/a |
| Net effect | ? | ? | ? | ? |
| Trait . | Survival . | Reproduction . | Ecosystem Consequence . | Stable among environments . |
|---|---|---|---|---|
| Life history effects | ||||
| arly hatching | +/– | N | P | No |
| arly smolting | +/– | + | P | No |
| aturation age and season | + | + | P | No |
| iurnal effects | – | n/a | n/a | n/a |
| igher fecundity, smaller egg size | – | +/– | P/NE | No |
| easonally uncoupled growth. | +/– | N | P | No |
| Genetics/cell biology | ||||
| ltered gene expression | n/a | n/a | n/a | No |
| ituitary function/structure altered | +/– | +/– | P | No |
| ltered growth pathway endocrinology | +/– | N | ? | No |
| ransgene structure stability | +/– | +/– | NE/P | n/a |
| Physiology/morphology | ||||
| lastic growth rate | + | n/a | n/a | No |
| cromegaly and altered allometry | – | – | n/a | No |
| ltered aerobic scope/osmoregulation | +/– | n/a | P | n/a |
| oorer swimming | – | – | P | n/a |
| educed innate immune function | – | – | P | n/a |
| cquired immune function normal | N | N | NE | n/a |
| ltered stress response | +/– | n/a | n/a | n/a |
| perm function | N | N | NE | n/a |
| ffects of triploidy | – | – | NE/P | n/a |
| nhanced feed-conversion efficiency | + | n/a | P | No |
| ltered metabolic pathways | +/– | N | n/a | No |
| ltered response to nutrition/dietary carbohydrate | +/– | n/a | n/a | No |
| ltered body coloration | – | – | n/a | No |
| Behavior | ||||
| nhanced feeding behavior | + | n/a | P | No |
| redator susceptibility | N/– | n/a | P | No |
| ltered spawning behavior | N | – | P | No |
| ncreased juvenile aggression | + | n/a | P | No |
| ecreased mature adult aggression | N | – | NE | No |
| ltered dispersal/migration | + | + | P | No |
| roader prey type selection | + | N | P | n/a |
| educed schooling/shoaling | – | – | NE/P | n/a |
| nhanced cannibalistic behavior | – | N | P | No |
| nvasion impacts | + | n/a | NE/P | n/a |
| Net effect | ? | ? | ? | ? |
Note: Evidence for the stability of phenotypes among environments is also indicated where known. The summed individual phenotypic effects within columns (“Net effect”) indicated by a question mark simply reflect the difficulty in summarizing individual traits into a single overall influence. This summary table is intended to provide an example process toward determining the potential effects of the pleiotropic influences of a transgene on phenotype that may result in ecological consequences. A formal risk assessment would require a fulsome analysis by an expert panel to reach a consensus regarding the effects and associated uncertainty for each altered trait. Supporting references can be found in supplemental table S1. Symbols: +, increased/higher survival or reproduction due to a trait relative to wild type; –, reduced/lower survival or reproduction due to a trait relative to wild type; N, neutral or no effect on survival, reproduction, or ecosystem consequences due to a trait; n/a; unknown or no data available; NE, no expected ecological consequence; P, probable ecological consequence.
Example interpretations of the potential effects of altered traits of growth hormone (GH)–transgenic coho salmon compared with those of wild type on survival, reproduction, and ecosystem consequences, based on data available to date.
| Trait . | Survival . | Reproduction . | Ecosystem Consequence . | Stable among environments . |
|---|---|---|---|---|
| Life history effects | ||||
| arly hatching | +/– | N | P | No |
| arly smolting | +/– | + | P | No |
| aturation age and season | + | + | P | No |
| iurnal effects | – | n/a | n/a | n/a |
| igher fecundity, smaller egg size | – | +/– | P/NE | No |
| easonally uncoupled growth. | +/– | N | P | No |
| Genetics/cell biology | ||||
| ltered gene expression | n/a | n/a | n/a | No |
| ituitary function/structure altered | +/– | +/– | P | No |
| ltered growth pathway endocrinology | +/– | N | ? | No |
| ransgene structure stability | +/– | +/– | NE/P | n/a |
| Physiology/morphology | ||||
| lastic growth rate | + | n/a | n/a | No |
| cromegaly and altered allometry | – | – | n/a | No |
| ltered aerobic scope/osmoregulation | +/– | n/a | P | n/a |
| oorer swimming | – | – | P | n/a |
| educed innate immune function | – | – | P | n/a |
| cquired immune function normal | N | N | NE | n/a |
| ltered stress response | +/– | n/a | n/a | n/a |
| perm function | N | N | NE | n/a |
| ffects of triploidy | – | – | NE/P | n/a |
| nhanced feed-conversion efficiency | + | n/a | P | No |
| ltered metabolic pathways | +/– | N | n/a | No |
| ltered response to nutrition/dietary carbohydrate | +/– | n/a | n/a | No |
| ltered body coloration | – | – | n/a | No |
| Behavior | ||||
| nhanced feeding behavior | + | n/a | P | No |
| redator susceptibility | N/– | n/a | P | No |
| ltered spawning behavior | N | – | P | No |
| ncreased juvenile aggression | + | n/a | P | No |
| ecreased mature adult aggression | N | – | NE | No |
| ltered dispersal/migration | + | + | P | No |
| roader prey type selection | + | N | P | n/a |
| educed schooling/shoaling | – | – | NE/P | n/a |
| nhanced cannibalistic behavior | – | N | P | No |
| nvasion impacts | + | n/a | NE/P | n/a |
| Net effect | ? | ? | ? | ? |
| Trait . | Survival . | Reproduction . | Ecosystem Consequence . | Stable among environments . |
|---|---|---|---|---|
| Life history effects | ||||
| arly hatching | +/– | N | P | No |
| arly smolting | +/– | + | P | No |
| aturation age and season | + | + | P | No |
| iurnal effects | – | n/a | n/a | n/a |
| igher fecundity, smaller egg size | – | +/– | P/NE | No |
| easonally uncoupled growth. | +/– | N | P | No |
| Genetics/cell biology | ||||
| ltered gene expression | n/a | n/a | n/a | No |
| ituitary function/structure altered | +/– | +/– | P | No |
| ltered growth pathway endocrinology | +/– | N | ? | No |
| ransgene structure stability | +/– | +/– | NE/P | n/a |
| Physiology/morphology | ||||
| lastic growth rate | + | n/a | n/a | No |
| cromegaly and altered allometry | – | – | n/a | No |
| ltered aerobic scope/osmoregulation | +/– | n/a | P | n/a |
| oorer swimming | – | – | P | n/a |
| educed innate immune function | – | – | P | n/a |
| cquired immune function normal | N | N | NE | n/a |
| ltered stress response | +/– | n/a | n/a | n/a |
| perm function | N | N | NE | n/a |
| ffects of triploidy | – | – | NE/P | n/a |
| nhanced feed-conversion efficiency | + | n/a | P | No |
| ltered metabolic pathways | +/– | N | n/a | No |
| ltered response to nutrition/dietary carbohydrate | +/– | n/a | n/a | No |
| ltered body coloration | – | – | n/a | No |
| Behavior | ||||
| nhanced feeding behavior | + | n/a | P | No |
| redator susceptibility | N/– | n/a | P | No |
| ltered spawning behavior | N | – | P | No |
| ncreased juvenile aggression | + | n/a | P | No |
| ecreased mature adult aggression | N | – | NE | No |
| ltered dispersal/migration | + | + | P | No |
| roader prey type selection | + | N | P | n/a |
| educed schooling/shoaling | – | – | NE/P | n/a |
| nhanced cannibalistic behavior | – | N | P | No |
| nvasion impacts | + | n/a | NE/P | n/a |
| Net effect | ? | ? | ? | ? |
Note: Evidence for the stability of phenotypes among environments is also indicated where known. The summed individual phenotypic effects within columns (“Net effect”) indicated by a question mark simply reflect the difficulty in summarizing individual traits into a single overall influence. This summary table is intended to provide an example process toward determining the potential effects of the pleiotropic influences of a transgene on phenotype that may result in ecological consequences. A formal risk assessment would require a fulsome analysis by an expert panel to reach a consensus regarding the effects and associated uncertainty for each altered trait. Supporting references can be found in supplemental table S1. Symbols: +, increased/higher survival or reproduction due to a trait relative to wild type; –, reduced/lower survival or reproduction due to a trait relative to wild type; N, neutral or no effect on survival, reproduction, or ecosystem consequences due to a trait; n/a; unknown or no data available; NE, no expected ecological consequence; P, probable ecological consequence.
Effects on growth and morphology affecting life-history traits
The overexpression of GH in transgenic fish has generated varied responses arising from transgene influences (promoter type, copy number, and site of insertion) and from species-specific responses to the same construct. The initial use of mammalian gene constructs resulted in either modest or no effect on growth, whereas the use of gene constructs derived at least partially from fish DNA sequences was found to significantly elevate growth (figure 1). For example, Atlantic salmon containing an “all-fish” GH construct were found to grow to approximately sixfold larger at age compared with controls (Du et al. 1992), and other salmonids using “all-salmon” constructs showed similar strong effects (Devlin et al. 2004a, Leggatt et al. 2012). Very strong growth responses have also been observed in Japanese loach, rohu, and tilapia (Martínez et al. 1996, Rahman et al. 1998, Venugopal et al. 1998, Nam et al. 2001, Lu et al. 2009), whereas other species or gene constructs generally have shown less than threefold differences in body size at a specific age due to GH transgenesis. For species that tend to mature at specific body sizes (unlike mammalian species, which tend to mature at specific ages), growth stimulation can result in a compression of the entire life cycle and thereby advance developmental milestones such as hatching (Devlin et al. 2004a, Lõhmus et al. 2010), age of smolting (Devlin et al. 1994, Saunders et al. 1998), and onset of sexual maturation (Bessey et al. 2004, Devlin et al. 2004a, Moreau and Fleming 2012, Leggatt et al. 2014). In contrast, for species or strains that mature at a specific age rather than size, developmental stages can occur at normal times but at larger body sizes (e.g., sexual maturation in rainbow trout; see figure 1; Devlin et al. 2001). It is important to note that the same gene constructs can display distinct influences on developmental effects among species and strains (e.g., Devlin et al. 1994, Rahman et al. 1998, Leggatt et al. 2012), therefore requiring individual evaluation.
In many cases, significant pleiotropic effects on processes other than growth have been observed. The overexpression of GH can cause significant deviations from normal morphology (particularly so for salmon), altering cranial cartilage deposition and allometric relationships among organs in some strains (Devlin et al. 2012, Leggatt et al. 2012) that can lead to reduced viability. Significant changes also occur to physiological processes, including altered metabolic pathways, immune function, and respiratory and exercise capacity (see Devlin et al. 2006). Many of these deviations from wild type would be anticipated to alter the fitness and ecological effects of GH-transgenic fish (e.g., table 1), and the quantum magnitudes of change in the phenotypes in these organisms are anticipated to have multiple effects on life-history characteristics and cause the redirection of evolutionary trajectories. Fish that have been highly stimulated by GH transgenesis are more likely to have significant deviations from normal morphology that strongly affect their ability to compete and survive in nature, whereas animals with a less extreme phenotype may be much less affected and be better able to compete with wild-type fish in nature. Therefore, fish with moderate growth stimulation and the ability to compete with wild type may pose the greatest concern from an ecological risk-assessment perspective compared with those that are phenotypically transformed to a small extent (and therefore are likely to not cause effects much different from those caused by wild type) or to a large extent (that causes pathological consequences impairing persistence in nature).
Transformation of feeding behavior
A striking feature of many GH-transgenic fish is their markedly enhanced feeding motivation (see supplemental video S1). Enhanced growth requires increased feed intake and processing of energy, which can be achieved by increasing food intake at each meal (rate or duration) and/or by increasing the frequency of meals. GH-transgenic salmon display enhanced feeding motivation and enhanced proportion of fish feeding at each meal time, which can result in elevated daily feed intake (approximately two- to threefold during late presmolt stages in salmonids) and an increased ability to compete for food (Abrahams and Sutterlin 1999, Devlin et al. 1999, Sundström et al. 2004a, Vandersteen Tymchuk et al. 2005). Transgenic common carp (Cyprinus carpio) also show enhanced dominance and ability to acquire food (approximately twofold) and, like salmon, also display a higher level of movement and time spent in the feeding area (Sundström et al. 2007a, Duan et al. 2011). These influences can extend across broad periods of the animal's life history: On a seasonal level, GH-transgenic salmon do not show the normal suppression of growth and feed intake seen in wild-type fish during winter (Devlin et al. 1994, Lõhmus et al. 2008) but rather continue growing throughout the year at rates similar to those seen in warmer seasons. This lack of seasonality likely arises from the high expression of GH throughout the year, most clearly demonstrated for transgenic common carp (Zhong et al. 2009). A shift in feeding motivation by GH-transgenic fish to meet metabolic demands might be expected to provide a significant advantage during seasons when food is abundant, but importantly, no clear disadvantage was seen when food was scarce, perhaps because of the strong plasticity available to the animal to adjust its physiology and behavior in response to different resource availabilities (Sundström et al. 2007b, Sundström and Devlin 2011).
Antipredator behavior
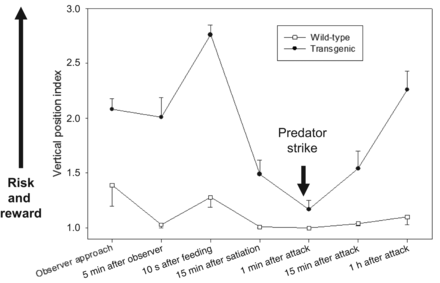
Elevated feeding motivation in GH-transgenic fish has several important implications for the animal related to other behaviors. Transgenic salmon reside higher in the water column in culture and naturalized environments (video S1). The surface is an entry point for new food resources in tanks and for some natural food sources in streams, but it is also closer in proximity to some predation threats (e.g., humans, surface penetrating predators). Transgenic salmon also show disrupted shoaling behavior (less group cohesion) with individuals inhabiting a larger proportion of their environmental space, particularly after a predation attack (Sundström et al. 2003, Sundström et al. 2007a). These dramatic behavior shifts result in GH-transgenic fish (common carp and salmon) tolerating higher levels of predation risk and resuming risky behavior more rapidly after a predator attack (see figure 4; Abrahams and Sutterlin 1999, Sundström et al. 2003, Duan et al. 2013). It is likely these behaviors in tank conditions are more easily established in GH-transgenic versus in wild-type fish because the higher feeding motivation in the former is continuously reinforced by the associative learning that surface dwelling is accompanied with food acquisition and not with actual predation.
The behavioral responses of genetically engineered with growth hormone (GH)-transgenic coho salmon to feeding and predation threat (reward versus risk, as measured by proximity to the water surface). Transgenic fish are more willing to expose themselves to predation risk than wild-type (nontransgenic) controls. Transgenic fish are still assessing predation threat because they will reduce their risk exposure after reaching satiety, and they further lower their exposure following a simulated bird predation attack (applied at the downward arrow). However, transgenic fish also then return to riskier behavior more rapidly than do normal fish. (Adapted from Sundström et al. 2003).
These behavioral tendencies can also be associated with higher actual mortality under naturalized environmental conditions with predators in several species (Dunham et al. 1999, Sundström et al. 2004b). In the case of salmon, higher mortality of GH-transgenic fry in the presence of predators was further exacerbated by low food–availability conditions that presumably forced GH-transgenic fish to forage to a greater degree (Sundström et al. 2004b). Similarly, higher risk-taking behavior and poorer survival of GH-transgenic common carp have been observed when housed with predators (Duan et al. 2013). The effects of GH transgenesis on predation sensitivity may be transitory during the life history and can be environment specific, because GH-transgenic salmon in some stream environments were not found to have greater predation mortality as older fry (2 weeks after first feeding) nor as parr, suggesting that the primary sensitivity for this susceptibility may be during very early feeding stages (Vandersteen Tymchuk et al. 2005). Therefore, GH transgenesis increases feeding behavior, which results in greater time spent in exposed conditions, shifting the balance between foraging and predation risk from that evolved in wild type.
Heterochronic, dominance, and exploratory behaviors
The precise seasonal timing of entry of an animal into required niches is a highly evolved characteristic in many species, allowing them to capitalize on dynamic resource opportunities. GH-transgenic salmon display an advanced development rate, which can begin in embryonic stages in some species and strains and have very strong growth effects through the presmolt (freshwater) phase, followed by a more modest growth stimulation throughout their life history (Devlin et al. 2004a, Moreau and Fleming 2012). Such shifts in the age when GH-transgenic fish achieve a specific developmental stage have the potential to create a mismatch with seasonal opportunities (for resources, mates, etc.). For example, GH-transgenic coho salmon emerge from their natal gravel redds sooner than wild type do, which, under low predator loads, affords the transgenic salmon a developmental advantage that allows them to outgrow nontransgenic siblings when food supplies are abundant (Sundström et al. 2005). However, the study also revealed that in the presence of predators, early emergence behavior causes GH-transgenic animals to suffer greater mortality in the absence of nontransgenic siblings that would otherwise share the predation risk. This example reveals how the same trait can provide either an advantage or a disadvantage to transgenic fish depending on the environmental conditions they experience.
A significant developmental milestone in the life history of many salmonids occurs when they transition as smolts from freshwater streams to oceanic environments. Typical smolt size is achieved sooner in cultured GH-transgenic salmon than in wild type (Devlin et al. 1994, Saunders et al. 1998), which could influence when transgenic salmon would make this migration and therefore determine whether their effects would occur in freshwater streams versus marine ecosystems. Sundström and colleagues (2010) found that the migration of GH-transgenic smolts occurred at approximately the normal time for wild type, even though GH-transgenic fish surpassed the crucial smolt size for migration to the ocean 8 months earlier. Therefore, it is anticipated that the ecological effects of GH-transgenic fish released into a stream environment would primarily be directed at the freshwater environment prior to them migrating normally to sea in the spring.
The establishment of territories and the migration of salmon fry in streams are normal and significant factors influencing survival. In naturalized streams, GH-transgenic salmon fry dispersed differently from wild type (Sundström et al. 2005, Sundström et al. 2007a), and this was also dependent on experimental conditions. Movement levels and exploratory behavior were found to be enhanced in GH-transgenic salmon and common carp (Sundström et al. 2007a, Duan et al. 2011)—behaviors that could facilitate more rapid access to new territory. Whether these effects reflect a motivation to explore new habitats or simply a response to greater overall mobility of GH-transgenic fish remains unknown but would have important ramifications for their ability to be successful as invading organisms.
Spawning behavior and reproductive parameters
A crucial fitness component for transgenic fish to sustain themselves in natural environments would be their ability to mate and transmit the transgene to the next generation. Reproductive success has been examined in one or both sexes of several GH-transgenic fish strains, involving measures of mate selection, gamete quality and quantity, and F1 progeny production. Dunham and colleagues (1992) found that GH-transgenic catfish had spawning success comparable to that of wild type, and similar conclusions were drawn for the reproductive capability of GH-transgenic common carp males in naturalized ponds (Lian et al. 2013). In contrast, for salmon, both Bessey and colleagues (2004) and Moreau and colleagues (2011) found significant reproductive impairment compared to wild-type fish. Bessey and colleagues (2004) also reported a strong effect of culture, in which wild-type salmon raised for their full life history in the laboratory culture environments (as required for transgenic salmon) had poor spawning success in competition relative to wild-reared fish. This impairment reflects phenotypic changes that can arise from culture rearing conditions (i.e., tanks) affecting both body size and spawning behavior (Leggatt et al. 2014). Therefore, certainly for some salmon species, their plastic reproductive responses to environmental conditions can complicate the determination of effects specific to the transgene. To overcome culture effects in salmon to a degree, Leggatt and colleagues (2014) raised GH-transgenic and wild-type salmon in large-volume seawater mesocosms in conditions more similar to those in nature than to those in standard tank rearing. These experiments demonstrated that the spawning success of all genotypes from mesocosms was improved and that wild-type and GH-transgenic coho salmon reared in mesocosms had comparable spawning success. The study also revealed that reproductive success differed among culture environments, indicating that genotype-by-environment (GxE) interactions were acting to influence the relationship between genotypes. In a model species, Howard and colleagues (2004) found that GH-transgenic medaka (Oryzias latipes) possessed a male mating advantage over wild type, whereas other experiments showed that transgenic males possessed a reduced mating advantage, or no difference, when compared with wild type (Pennington et al. 2010, Pennington and Kapuscinski 2011), suggesting that there may be significant environmental differences among laboratories or strain effects that can influence reproductive phenotypes in this model organism as well. Interestingly, Howard and colleagues (2004) discovered that wild-type medaka can adopt alternate mating tactics in an effort to counter the advantage possessed by GH-transgenic males. The progeny of matings involving the transgenic medaka, however, displayed reduced survival, which—in combination with the enhanced mating success of the parents—was theorized to be capable of causing population extinction (see the “Modeling” section below). In no experiments to date has compelling evidence been presented to support assortative mating for GH-transgenic and nontransgenic strains. However, such effects might arise if large differences in body size at maturation were present and could redirect the evolutionary responses of GH-transgenic and wild-type animals by restricting the exchange of genetic information.
First age of maturity and longevity are also significant effectors of reproductive contributions (Muir and Howard 2001). In coho salmon, GH transgenesis accelerates development such that sexual maturation is reached in 2 years rather than in the normal 3–4 years for wild-type strains in laboratory culture conditions (Devlin et al. 1995, Bessey et al. 2004, Devlin et al. 2004a). This advancement in maturity could allow this strain to propagate its transgenes up to twofold faster than in wild-type salmon. Age of maturation for GH-transgenic medaka was found not to differ from wild type in two studies (Pennington et al. 2010, Pennington and Kapuscinski 2011), whereas Muir and Howard (2001) found an earlier age of maturation in a different GH-transgenic strain. Pennington and colleagues (2010) also found that longevity relative to wild type was greater in one of two transgenic strains. The age of maturity and span of gamete production are therefore affected by GH transgenesis but appear also to be dependent on strain genetic background (see below) and conditions experienced.
Physical and biological containment
In most proposed applications of transgenic fish, the plan is to use containment methods to reduce the risk of exposure of the animals to nature or, if such escape occurs, to reduce their capacity to breed and establish in natural populations (Devlin and Donaldson 1992, Kapuscinski et al. 2007, Wong and Van Eenennaam 2008). These measures have been proposed because in most cases, the eradication of introduced individuals or subsequent generations of transgenic fish is seen as not feasible (Britton et al. 2010) and because the level of uncertainty associated with estimating the environmental consequences is often high (see below). Although the scale of the facilities is an important risk factor to consider when estimating the probability of the escape of transgenic fish, physical containment methods can be highly effective when appropriate site-specific engineering and human resource operations are established (e.g., multiple screen systems, maintenance, security). To date, no record of a GH-transgenic fish escaping to nature has been reported worldwide. However, in a case in which a breach in containment had occurred or in which a purposeful introduction is planned (e.g., to control invasive species), the survival and reproductive capacity of the transgenic animals will play a key role in determining the duration and magnitude of any ensuing impacts. Therefore, having a second line of defense in the form of biological containment is desirable. Obvious approaches to limit the effects of escapees are, first, to locate the facilities in areas where escapees cannot survive in surrounding waters and, second, to control reproductive capability, either by using single-sex (monosex) strains in situations in which no conspecifics exist or by directly sterilizing transgenic animals (Devlin and Donaldson 1992). The simplest sterilization method to apply is to induce a triploid condition (Benfey et al. 2015), which for many fish species prevents the development of functional gametes in both sexes. In the case of sterilization, as for monosex containment approaches, impacts would be limited to the single generation associated with escaped animals.
The utility of any containment system is dependent on its efficacy, and for triploidy, farm-scale tests have shown that high levels of sterility can be induced routinely at more than 98% and as high as 99.8% when specific induction and screening measures are used (Devlin et al. 2010). In cases in which only small numbers of escapees are anticipated or other impairments are expected (e.g., lack of a suitable mate in nature, impaired survival), a failure rate that yields only 0.2% diploids may provide sufficient containment strength. However, if large escapes occur into an ecosystem with suitable mates, even a low failure rate might allow the introduction of sufficient fertile transgenic fish to nature that could initiate an invasion. Because of these factors, research to develop alternate methods of reproductive containment have been explored, including transgenic approaches that are now showing promise (Wong and Van Eenennaam 2008). Once developed, these approaches to containment will require large-scale assessments of efficacy similar to those conducted for the more traditional approaches described above.
The degree to which transgenic fish might be used in aquaculture in the future will depend on their production characteristics. Therefore, containment measures that impair performance would influence the degree to which they may be used in aquaculture, which in turn will affect the potential exposure rates of these organisms to nature. For many GH-transgenic fish, triploidy has been seen to reduce growth rates relative to diploid animals (Razak et al. 1999, Nam et al. 2001, Devlin et al. 2004a, Yu et al. 2009, Leggatt et al. 2012, Tibbetts et al. 2013). Triploid and GH-transgenic animals can also show impaired disease resistance in some cases (Jhingan et al. 2003, Kim et al. 2013), which could reduce their utility for application and could alter their capacity as disease vectors in nature.
Modeling
Modeling transgenic fish in populations has revealed the importance of understanding multiple biological and environmental factors and their interactions that could affect the persistence of a transgene in populations, their ecosystem effects, and the reliability of predictions. The frequency and magnitude of introduction, the number of transgene sites in the genome, and the inclusion of stochasticity in population dynamics have all been found to affect transgene frequencies in modeled populations (Davis et al. 1999, Muir and Howard 1999, Maclean and Laight 2000). Knowing the magnitudes and frequencies of transgenic fish introductions into nature, coupled with phenotypic information, can provide an estimate of short-term effects, but for longer-term predictions, it is also important to know their fitness (survival and reproductive output) relative to that of competing ecosystem members. Therefore, the effects of GH-transgenic fish life history and population characteristics have also been modeled, with behavior (e.g., scramble versus contest competition), reproductive success, stage-dependent survival, age of maturity, alternate mating strategies, lifespan, and density dependence being seen to influence modeling outcomes (Muir and Howard 1999, 2001, 2002, Aikio et al. 2008). Muir and Howard (2001) found that they could fully parameterize transgenic fish fitness models with values for just six life-history characteristics to make predictions of transgene prevalence in populations. Muir and Howard (1999) also found that combinations of fitness characteristics (such as elevated mating success and reduced survival) could cause model population declines resulting from transgene introgression into a wild-type population (termed a Trojan gene effect). The genetic and evolutionary effects of GH-transgenic fish have also been examined (Ahrens and Devlin 2010), in which selection on background genetics allowed shifts in phenotype and compensating adaptation that was found to have strong effects on model outcomes.
More recently, the application of food-web ecosystem modeling (Ecopath with Ecosim) has explored the response of multiple trophic levels (predators and prey and interacting species) to the theoretical introduction of GH-transgenic coho salmon into an ecosystem. Few effects on ecosystem components were detected at biomass levels of transgenic salmon similar to that of nontransgenic salmon currently in the ecosystem (Li et al. 2014). However, some effects unique to GH-transgenic fish were noted when biomass was elevated to extreme—perhaps unrealistic—levels. Ecosystem modeling is perhaps one of the few ways that potential consequences to large, complex ecosystems can be explored short of an actual introduction experiment. Future modeling objectives would benefit from merging fitness-based and ecosystem-effect modeling approaches.
Bringing nature and transgenic fish together for environmental risk assessments
The majority of phenotypic data for GH-transgenic fish has been collected using animals reared and studied in artificial tank or aquarium environments. As the study of GH-transgenic fish in nature is problematic, two alternate approaches to estimating GH-transgenic fitness and consequences in nature are to recreate natural conditions within the laboratory, and to use surrogate models for GH-transgenic fish in nature.
Developing nature in the lab
To achieve more realistic data applicable to the wild, researchers have developed naturalized environments within physically contained laboratories designed to prevent the escape of transgenic fish. These naturalized environments often contain complex physical structure (e.g., rocks, logs, plant life), natural abiotic influences (light, water type, temperature), access to natural food items only, and, in some cases, the presence of live predators (e.g., Sundström et al. 2007b). For species such as salmon, this has allowed more realistic experimentation at specific developmental stages and the examination of effects of significant variables (e.g., predator load, food supply). Such studies in salmon have been important for identifying the forces acting to influence survival and reproduction in freshwater environments (e.g., see naturalized stream studies of juvenile coho salmon, as was discussed in the “Phenotypic effects” section above). Recently, the fitness of GH-transgenic common carp has been examined in a large manmade pond (Lian et al. 2013) that simulates nature to a much greater degree than culture facilities do. In these studies, the calculation of lifetime reproductive output coupled with reductions in survival were suggested to be sufficient such that transgenic common carp would be prevented from displacing wild common carp in populations in nature. For salmon in marine conditions, mimicking the spatial, abiotic, and biotic scope of the marine habitat may not be feasible, because recent experiments in which GH-transgenic and wild-type coho salmon were reared in large marine mesocosms only partially normalized the mature phenotype of nontransgenic fish relative to nature-reared wild-type salmon (Leggatt et al. 2014). These data indicate that very large environmental facilities would likely be required to mimic nature realistically.
For smaller (model) species, it is logistically feasible to construct mesocosm-scale habitats that provide a closer representation of the environmental conditions the fish would experience in nature. For example, Pennington and colleagues (2010) performed invasion experiments with GH-transgenic medaka in naturalized mesocosms that supported both growth and reproduction but were competitive and associated with overall declines in population size. The survival of two strains of GH-transgenic medaka was found to be lower than that of a related wild-type strain. Such data can provide a fuller life-cycle estimate of fitness across all stages of the life history simultaneously (i.e., net fitness; Muir and Howard 2002) than approaches that assess individual fitness components or subcomponents (Devlin et al. 2006, Devlin et al. 2007).
Assessing surrogates for GH-transgenic fish in nature
Researchers have introduced to nature nontransgenic “surrogate” fish with phenotypes similar to GH-transgenic animals in an effort to provide some relevant real-world data but without the risk of releasing a transgene outside of containment. The treatment of fish with long-lasting GH protein formulations, although not mimicking a GH-transgenic phenotype fully (Raven et al. 2012), does provide an opportunity to assess the consequences of elevated GH levels on fish in nature. In field studies using brown trout, GH-treated trout grew and used body energy reserves faster over winter than did controls but had equal over-winter mortality (Johnsson et al. 2000). Alternatively, in another study, GH-treated brown trout had an elevated growth rate compared with that of controls in the summer when food abundance was presumably higher, but no effect on weight gain or loss was seen during winter (Johnsson and Björnsson 2001). GH treatment was also found to affect movement patterns, causing fish to remain in original-release areas more than controls did. In contrast, Sundt-Hansen and colleagues (2009) found that GH-treated trout moved significantly more in stream environments, suggesting GH may cause elevated foraging but also improve the ability to retain prime territories. Sundt-Hansen and colleagues (2012) also examined younger stages of Atlantic salmon fry in natural streams, but in this case, they found that GH treatment actually suppressed growth, without affecting dispersal or overall survival. Together, these studies examining fish with elevated GH in nature have demonstrated that responses are subject to influences from both the environment and the developmental stage.
Another class of fish that has a growth-elevated phenotype as compared with wild type includes strains that have undergone domestication selection. Such fish show many of the same behavioral (appetite and predation sensitivity), endocrinological (e.g., elevated GH and insulin-like growth factor 1), and physiological changes that GH-transgenic fish do, albeit at a more modest magnitude (Fleming et al. 2002, Tymchuk et al. 2009, Devlin et al. 2013). Introductions of domesticated salmonids to nature have revealed altered survival relative to wild-type fish depending on environmental conditions and the degree of introgression (Fleming et al. 2000, McGinnity et al. 2003). Such data may be an indicator of how GH-transgenic strains would also fare in nature, and using domesticated fish to assess ecological consequences could provide an approach that would allow measures to be made in the wild without the genetic risks associated with transgenic strains. However, extrapolations to long-term ecological effects should include the differences in the genetic basis generating phenotype between transgenic and domesticated fish as compared with wild type (i.e., a single transgene locus that generates a relatively stable phenotype through generations versus domesticated strains with polygenic influences that halve their phenotypic divergence from wild type with each generation following introgression). Therefore, domesticated fish may best represent the effects of GH-transgenic fish in their first generation following entry into nature.
Phenotypic plasticity and GxE interaction effects
The influences of environments and their interaction with genotype are well known to strongly affect the final phenotype of an organism, particularly so for many fish species that show a high degree of phenotypic plasticity (Hutchings 2004). It is therefore important for reliable assessments to determine whether the phenotypic relationships among transgenic fish and other ecosystem members under study remain stable across environmental conditions and, if that is not the case, then to clearly understand reaction norms for significant traits. A clear finding associated with assessments of transgenic and wild-type fish is that their physiological and behavioral characteristics can change depending on the conditions they are both raised and studied in (e.g., table 1). Furthermore, transgenic and wild-type fish can show different plastic responses, causing the relative phenotypic relationship between them to differ among environments. Such GxE–interaction effects on phenotype are also expected to alter their relative fitness and their consequences on ecosystem components (box 1). Stability in phenotypic relationships means that the effects of transgenic fish may be predicted from relatively few data, whereas dynamic phenotypes will require more complex assessments to allow robust estimates to be made. A remarkable example of GxE–interaction effects can be seen for GH-transgenic coho salmon reared in tanks versus those raised in naturalized streams (Sundström et al. 2007b). The extreme growth enhancement of GH-transgenic coho salmon prior to smolt in culture conditions was not found when fish were raised in naturalized stream environments (figure 5), with transgenic salmon growing less than twofold heavier than wild type in stream conditions compared with more than 25 times as heavy in culture. These data suggest that the GH transgene has, at least for growth, extended the scope of phenotypic plasticity compared with that available for wild-type fish and, as such, may provide an altered capacity to perform in varied environmental conditions. Furthermore, the high-food, low-risk environmental conditions seen in culture can play a large role in allowing the fast-growth phenotype of transgenic salmon to emerge, whereas wild-type salmon grow similarly in culture, naturalized lab conditions, and nature. Therefore, the major influence of the GH transgene on growth difference appears to be associated with transgenic animals acquiring a growth rate (and therefore body size at age) in culture that would be remarkable to see arise in nature. The significantly reduced growth of GH-transgenic salmon seen in naturalized environments likely would alter the effects they may have on ecosystems compared with those of their tank-reared counterparts. The latter can have strong effects (e.g., on prey consumption; Sundström et al. 2007b, 2009, 2014), but such fish also show extreme changes in their physiology and morphology and may not be as fit as GH-transgenic fish that are from nature-like environments and that appear more normal phenotypically. Therefore, cultured transgenic salmon may have strong but short-term effects, whereas those from naturalized environments may have weaker effects but a more sustained capacity to survive and reproduce in populations.
The environmental effects on the growth rate and phenotype in growth hormone (GH)–transgenic and wild-type coho salmon. The capacity for growth in transgenic fish seen in tank conditions is not realized in naturalized stream conditions capable of supporting normal growth of wild-type salmon. This enhanced scope for phenotypic plasticity for growth also affects the magnitude of impacts on prey in naturalized streams (based on Sundström et al. 2007b).
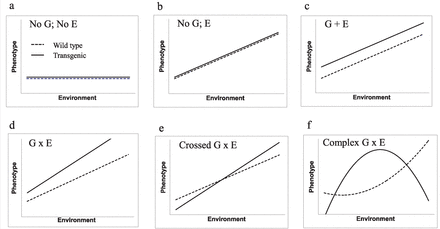
Genotype-by-environment (GxE) interactions could cause the miscalculation of the magnitude of effects of transgenic animals relative to wild type, and if reaction norms cross (thereby reversing the relative characteristics of two genotypes), erroneous conclusions regarding effects (both directional and magnitude) may arise if an incomplete data set is used for assessment. To provide a robust assessment of potential effects, it is therefore desirable to study animals under multiple environmental conditions—ideally including extremes—to allow an understanding of their plastic responses to the range of conditions that they may be exposed to in nature. Ideally, the use of simulated nature environments over tank (culture) conditions is desirable. If wild-type fish from naturalized facilities are near equivalent to fish from nature, then the data for transgenic fish from those same experimental conditions also may be more similar to the data for transgenic fish from nature. However, the absence of transgenic fish from nature unfortunately precludes a formal validation of the true relationship of wild-type and transgenic fish as would exist in nature. Accurate risk predictions with minimal data are likely in scenarios a, b, and c, whereas scenarios d, e, and f could lead to incorrect risk predictions when insufficient data is available.
Environmental conditions differentially affect not only the phenotype of transgenic and wild-type fish but also their consequences on prey within their habitats. For example, a strong effect on prey mortality could be exacted by GH-transgenic fish that had been reared under culture conditions, but this was not seen for transgenic and wild-type salmon that had been reared in naturalized conditions (Sundström et al. 2007b). Furthermore, in stream environments, tank-reared GH-transgenic coho salmon invading stream populations of nontransgenic salmonid fry (i.e., conspecifics, steelhead trout, or Chinook salmon) had a greater effect than wild-type invaders that had been reared in culture conditions (Sundström et al. 2014). However, no difference between the two genotypes was seen in their effects on recipient populations if the invaders were derived from stream-reared populations. These observations suggest a degree of caution should be applied when using data generated from some artificial tank conditions for risk assessments.
The interpretation of data for risk assessments
Interpreting the data regarding potential invasiveness, interactions with, and consequences on ecosystem components based on data from nonnatural conditions has several significant complications (Devlin et al. 2006, 2007, Kapuscinski et al. 2007, Niemelä and Dingemanse 2014) which have been recently empirically documented. Perhaps most important is that information relating to fitness and the consequences of transgenic fish is collected under limited experimental conditions and developmental stages that only partially mimic nature and full life histories (see above). Specific experiments can likely detect whether a particular force affects transgenic versus wild-type fish under specific circumstances, but the actual magnitude of the effects obtained from laboratory and naturalized conditions is unlikely to quantitatively equate to that of the effects that would occur in nature. A further complication arises when a particular trait of transgenic fish provides an advantage under one set of conditions and a disadvantage under other circumstances. For example, elevated feeding motivation in GH-transgenic salmon can provide a competitive advantage in high-food, low-predator environments that gives them a growth and potentially a survival advantage, whereas this same trait can also cause them to forage more and suffer greater mortality under low-food conditions in the presence of predators (Sundström et al. 2004b). Such antagonistic pleiotropic effects with opposing signs of influence on fitness complicate the determination of the effects on lifetime fitness and consequences when attempts are made to combine data from relatively few simple experiments under specific conditions (as is the case currently for many GH-transgenic fish).
Variability among data sets can also generate uncertainty regarding the potential effects—or lack thereof—of transgenic organisms. For example, in initial studies, greater predation mortality was found for GH-transgenic fish under naturalized conditions (Sundström et al. 2004b, 2005). However, subsequent research revealed no differences in fry survival under moderate food–availability conditions and using a different predator species (Sundström and Devlin 2011), nor at the same stage under slightly different conditions, nor at an older developmental stage with yet another predator species (Vandersteen Tymchuk et al. 2005). Therefore, different conclusions could be drawn regarding fitness if only one of the data sets was available and was extrapolated to a lifetime effect. A second case of incongruent data is associated with population-level effects among environments. When GH-transgenic and wild-type coho salmon were cohabitated in simple tank environments with limited food, crashes were observed for all populations containing transgenic fish (Devlin et al. 2004b). In contrast, when a similar experiment was conducted in complex stream environments, populations did not experience drastic reductions in numbers (Sundström and Devlin 2011), suggesting that the complex habitats afforded fish an opportunity to avoid cannibalism and aggressive social conflicts that wasted energy and caused mortal injury. Therefore, concluding that transgenic fish can cause population effects is highly condition specific. Summing data from multiple experiments examining only a few variables and experiments (table 1) is not certain to yield an accurate net determination of effects of phenotype over a full lifetime—and could even generate the wrong direction of predicted effects depending on how the specific experiments were conducted (e.g., if experimental predator loads were higher than actually exist in nature, an underestimate of net fitness could arise).
In contrast, lifetime estimates of survival and reproductive components of fitness (net fitness) provide an approach to integrate multiple effects into fewer measures (Muir and Howard 2002). Condensing all influences into measures of juvenile viability, adult viability, age at sexual maturity, female fecundity, male fertility, and mating advantage can provide an estimate of lifetime fitness. This approach is particularly applicable for estimating fitness when parameter values can be accurately determined and are likely to represent those as would occur in nature, such as for small species when nature can be mimicked or for species with simple habitats. However, even for such species, predictions can be challenging: Muir and Howard (2001) predicted an increase in transgene frequency on the basis of the use of laboratory-derived data for life history parameter values, but different results were observed for other GH-transgenic medaka strains in populations studied in naturalized mesocosms (Pennington et al. 2010). These differences reveal that we have an incomplete understanding of the forces acting to modify phenotype and affect fitness among environments. Although still conceptually very useful for large species with complex life cycles and geographic ranges (e.g., rivers and oceans), it has proven to be empirically very challenging to measure net fitness by accurately mimicking in the lab the range of conditions that would be encountered in nature. Significant difficulties also exist for attempts to summarize multiple individual traits into effects on fitness or ecological consequences (see table 1). Therefore, a net fitness approach (Muir and Howard 2001) naturally integrates influences from multiple traits and may therefore be advantageous if it can be applied with reliability across broad portions of an animal's life history under realistic conditions. Notwithstanding these limitations, the more data available from multiple environments approximating nature, the more likely phenotypic ranges and developmental differences will be understood to overcome limitations in predictive capacity. The incorporation of such information with formal uncertainty analysis (Hayes et al. 2007) can provide a reliable framework for developing robust risk-assessment outcomes.
It should be mentioned that, in some cases, the effects on phenotype by a GH transgene could be so extremely large (e.g., strongly impaired swimming performance or disease resistance) and not subject to environmental influences that extrapolating effects to nature might be performed with significantly less risk of error. Similarly, if all observed phenotypic effects are found to be detrimental under a range of laboratory and naturalized conditions, then it is more likely that such a strain would show similar poor fitness in nature. A key objective for future research will be to well define the reaction norms for key traits affecting fitness and ecological consequences, which will thereby enhance our ability to extrapolate among environments with confidence (box 1).
The evolution of transgenic fish in nature
The capacity for transgenic fish to adapt to new environmental conditions has important implications for the stability of risk predictions over longer time periods (Devlin et al. 2006, Kapuscinski et al. 2007). Rollo (2014) has recently discussed how a range of evolutionary forces (including regulatory changes, modifier selection, sexual selection, hybridization, and recombination effects) could act on GH-transgenic mice and fish in populations under natural conditions. Although there are as yet no multigeneration experiments allowing natural selection to be assessed, for transgenic salmon and model fish (Muir and Howard 1999, Devlin et al. 2001, Pennington and Kapuscinski 2011, Devlin et al. 2013), genetic background has been recognized as an influence that can interact with a transgene to modify phenotype. From a risk-assessment perspective, it is therefore important to understand the effects arising from the transgene versus the genetic background, which are anticipated to have very different genetic and ecological consequences on populations. Studying the combined effects of both transgene and strain background genetic influences is most useful for assessing the effects in the early stage of introgression of transgenic fish into wild populations. A transgenic strain should be maintained in a wild-type genetic background (by crossing to wild-type animals from nature) to ensure, to the greatest degree possible, that domestication effects are being limited and that an appropriate comparison to wild fish as would occur in nature can be made. In cases in which this is not possible, a stable reference strain should be employed to calibrate the magnitude of domestication that may have arisen during laboratory culture.
It is unlikely that many anthropogenically generated transgenic mutations will have optimum fitness in nature (even if it that fitness is greater than in wild type). Therefore, selection will be expected to act on genetic backgrounds to improve the fitness of transgenic individuals and, in doing so, will modify phenotypes from that measured in the progenitor strain used for risk assessments. Indeed, even relatively weak selection is likely to override many of the population effects predicted by modeling exercises (Ahrens and Devlin 2010). A secondary consequence arising from the selection of background genetic variation in transgenic fish could be the retention of variation in populations that is not optimal for wild-type animals. For example, if GH-transgenic fish have too high a growth rate for optimal fitness, counteracting slow-growth alleles could accumulate in populations. This, in turn, may reduce the growth of wild type below the naturally selected rate, reduce their fitness, and cause them to counter select fast-growth alleles. Such opposite, conflicting selection of background variation between transgenic and wild-type fish in populations could reduce the fitness of both types until one prevails or an equilibrium is established (Ahrens and Devlin 2010). Alternatively, transgenic fish may be directed to fitness optima different from those acquired by wild type, in which case disruptive selection of background variation may arise that in the long term could lead to further phenotypic divergence and ultimately sympatric speciation if assortative mating between transgenic and nontransgenic animals prevailed (e.g., if large-bodied versus small-bodied individuals prefer their respective kind—see above). It is also important to note that adaptation across multidimensional fitness landscapes (Gavrilets 1997) may provide alternative pathways for the evolution of transgenic fish compared with those available for wild type.
The significant phenotypic plasticity and extended phenotypic range seen for traits in transgenic fish may afford them opportunities to shift to new fitness optima among environments that are not achievable solely through selection of genetic variation present in natural populations. However, it is currently unclear for GH transgenes in fish what proportion of variation among individuals is due to genetic or epigenetic influences. Some transgenes are known to have variable penetrance and expressivity in fish (Martin and McGowan 1995) because of epigenetic influences which could cause the same genotypes to have different selection responses and fitness optima among environmental conditions. Furthermore, although persistent across developmental stages within a generation (Sundström et al. 2009), it is not known whether the plastic effects on phenotype in transgenic fish can be transmitted to offspring in the same or different ways than in wild-type fish (Evans et al. 2014). Therefore, understanding how epigenetics and plasticity may alter the phenotype of transgenic and wild-type fish and how this responds to genetic, environmental, and transgenerational influences are important evolutionary considerations required for predicting potential ecological effects.
Conclusions
With transgenic fish in the pipeline for commercial production, a challenge faces society with respect to assessing their potential benefits and risks. Transgenic-fish technology in general holds significant potential for enhancing production efficiency in various socioeconomic and environmental settings and has the promise to improve and diversify product quality for the end user. Indeed, the highly efficient growth and metabolism characteristics of some GH-transgenic fish make them attractive options for contained land-based aquaculture, perhaps moreso even than existing domesticated strains. Avoiding the environmental impacts from this technology is a universal goal of developers, but history has shown that fish in culture facilities often do escape to nature. Therefore, understanding the fate and actions of transgenic fish in nature is an important consideration and provides a significant challenge for regulators and decisionmakers charged with meeting legislative mandates to protect the environment but without unnecessarily blocking a technology with future potential. Compared with risk assessments of transgenic plants (often generated from long-domesticated species with known and often limited capacity for establishment in nature), transgenic fish are usually made in strains of near-wild origin with an excellent capacity for interbreeding with themselves or wild relatives and therefore possess a significant scope for establishment in nature should they escape biotic or abiotic containment measures. In many—perhaps most—cases, GH-transgenic strains are expected in theory to have equivalent or reduced fitness when compared with wild type, because the dramatically altered phenotypes they possess are not those that have evolved because of natural selection. Scientifically based ecological risk-assessment processes require empirical information to best estimate fitness components and the consequences on ecosystem members and also must evaluate the uncertainty associated with those data which, to date, have been generated in artificial environments rather than in nature. Currently, a high degree of uncertainty remains regarding the potential for GH-transgenic fish to survive in nature and cause ecosystem consequences. Primarily, this is due to the remarkable phenotypic plasticity displayed for life history traits by these organisms, which generates variable experimental data in a range of developmental stages and environmental conditions. We do not assert that generating sufficient risk-assessment data is not possible, but rather we recommend that adequate and appropriate empirical data be acquired to well understand the environmental effects on phenotype and the potential for the evolution of GH-transgenic strains. Certainly, the variable empirical data gathered to date supports the need to assess each GH-transgenic strain on a case-by-case basis with respect to specific traits affecting fitness and consequences, at least until generalizations emerge regarding their predictability among strains and environments. However, using general principles (e.g., GxE effects) illuminated from studies with other transgenic strains and species should be considered broadly among risk-assessment processes to provide the most fulsome understanding possible of the potential outcomes of transgenic fish in the full complexity of nature. Whether such an understanding can, a priori, reduce uncertainty sufficiently to prevent the ecological consequences of transgenic fish should they enter nature remains a significant objective for further research.
The authors appreciate the helpful comments of the three anonymous reviewers. Funding was provided by the Canadian Regulatory System for Biotechnology to RHD and the Swedish Research Council to LFS.
Supplemental material
The supplemental material is available online at Supplementary Data.
References cited