-
PDF
- Split View
-
Views
-
Cite
Cite
Karsten Seidelmann, Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa, Behavioral Ecology, Volume 17, Issue 5, September/October 2006, Pages 839–848, https://doi.org/10.1093/beheco/arl017
Close - Share Icon Share
Abstract
Brood cell parasitism inflicts high fitness costs on solitary, nest-constructing bees. Many of these parasites enter open cells during its provisioning, when the mother bee is absent. Therefore, females can reduce the risk of open-cell parasitism by limiting the time they are away from the nest. However, provisioning efficiency (provisioning time per unit of progeny body mass) decreases due to aging. To limit the increasing risk of open-cell parasitism as the nesting season progresses, female bees could optimize their maternal investment strategy by shifting the sex ratio and the body size of offspring during the nesting season. This prediction was tested in the Red Mason bee Osmia rufa (O. bicornis), a stem- or hole-nesting, polylectic, univoltine megachilid bee. In O. rufa, the risk of open-cell parasitism was found to be correlated with cell provisioning time. Additionally, the provisioning efficiency of females declined during the nesting season to one-fourth of the initial value. However, cell-provisioning time did not increase correspondingly. Bees dealt with their decreasing provisioning efficiency by reducing the amount of stored larval food, leading to a reduction of offspring size and a seasonal shift toward males in the offspring sex ratio. The influence of provisioning efficiency and risk of open-cell parasitism on optimal offspring size was analyzed by means of a statistical model. The observed maternal investment pattern of Red Mason bees is an adaptive strategy to reduce open-cell parasitism.
Nest-constructing solitary bees are central place mass provisioners with exclusively maternal investment in brood care. Females construct nests for their brood, and provision brood cells therein with pollen and nectar. Due to the high investment costs of brood cell construction and provisioning, fecundity is low and depends on the female's capacity to build and provision brood cells. However, the stored pollen and nectar or the larva itself is a desirable resource for various kleptoparasites and parasitoids. In most bee species, parasitism of the brood is an important mortality factor (Krombein 1967; Brechtel 1986; Westrich 1989). When a brood cell is parasitized, the occupant usually dies, reducing the fitness of the nesting female. Females should therefore minimize or at least reduce the risk of parasitic attacks. Completed nests can be protected by structures that prevent enemies from parasitizing the cells, for example, by a solid plug sealing the nest entrance. However, parasites can enter open nests while the mother bee is absent on provisioning trips. One way to guard the nest against open-cell parasites is the closure of the entrance during provisioning flights, as seen in some Andrena species (Gebhardt and Röhr 1987). But this behavior is rare among solitary bees (Westrich 1989). If the nest remains unsealed, the probability that a cell will become parasitized is a function of the time that the cell is open and unguarded by the bee (Brockmann and Grafen 1992; Danforth and Visscher 1993; Stone 1994; Goodell 2003). Thus, one way to limit the risk of losing the investment in a brood cell that is being provisioned is to keep provisioning time as short as possible.
The time constraints for provisioning a brood cell can become a limiting factor for the maternal investment if extrinsic or intrinsic factors reduce a bee's ability to collect food and prolong the provisioning time. A major intrinsic factor is senescence. Both the wear of the exoskeleton, especially wings, and pollen-collecting apparatus (Torchio and Tepedino 1980; Sugiura and Maeta 1989; Seidelmann 1997; Tomkins et al. 2001) and physiological aging of the flight muscles (Baker 1976; Sohal 1976; Collatz and Wilps 1986) reduce flight capacity. Old bees need more provisioning trips to collect a given amount of brood provisions (Sugiura and Maeta 1989). Consequently, cell-provisioning time and therefore the risk of parasitism are likely to increase across the lifetime of a bee. One way for an aging female to reduce the increasing risk of open-cell parasitism is to seal brood cells before they contain sufficient provisions for an offspring of optimal size. Bees are able to determine the sex of each offspring by fertilizing an egg (yielding a daughter) or not (yielding a son). The distinct sexual dimorphism in offspring size that is found in many solitary bee species (Stubblefield and Seger 1994) may give females the possibility to optimize their investment strategy in accordance with the risks of open-cell parasitism. Females could produce the larger sex that requires more provisions early in the season when they are most efficient at foraging and the smaller sex later on.
The response of female bees to an increasing risk of open-cell parasitism due to aging was studied in the Red Mason bee Osmia rufa (O. bicornis). Osmia rufa is a univoltine (1 generation per year), polylectic (visits a broad spectrum of flowers for resources), stem- or hole-nesting megachilid bee with a pronounced sexual dimorphism in body size. Females are about 1.6 times heavier than males (Raw 1972; Seidelmann 1995). This species can be easily reared in artificial trap nests. Females construct sequentially several nests of the line type (cell after cell in linear order) within their lifetime. They do not seal the nest entrance during their absence. The wet mud used as building material for cell partitions and nest plugs has to be collected freshly at mining places away from the nest.
There are 3 main parasites attacking open cells of O. rufa. The migratory hypopi of the mite Chaetodactylus osmiae are transported to a cell by the bees via phoresy (Fain 1966; Seidelmann 1990; Chmielewski 1993). Mite parasitism cannot be reduced by shortening the time away from the nest and was not examined in this study. But 2 fly species, the small drosophilid fly Cacoxenus indagator Loew (Diptera: Drosophilidae) and the bombylid fly Anthrax anthrax Schrank (Diptera: Bombyliidae), lay their eggs in open cells during the provisioning phase (Julliard 1947, 1948; Coutin and Chenon 1983; Westrich 1989; Seidelmann 1990, 1995). Females of both species inspect host nests randomly and do not guard certain nests or mark nest entrances (K Seidelmann, personal observation). Moreover, because parasitism occurs through the nest entrance, cells near the entrance should suffer a higher risk of becoming parasitized. If a higher risk of parasitism leads to a decreased maternal investment, females can be expected to store only small amounts of provisions in these cells.
The system of O. rufa and its fly parasites was used to test the hypothesis that open-cell parasites shape maternal investment patterns of their host. I build my arguments around a field study at a large artificial nest aggregation of this bee species. Field data were used to measure the provisioning efficiency of females in relation to their age. Changes in maternal investment were monitored by means of progeny body size and sex ratio in nests constructed at different times of the nesting season. The field data were also used to correlate open-cell parasitism with provisioning time and the position of a cell within a nest. The distribution of parasitism was compared with the dependence of the offspring's body size and sex on the cell position within nests. To analyze the consequences of decreasing work capacity in the presence of open-cell parasites on the optimal maternal investment, a model using a sigmoid function corollary to Smith and Fretwell (1974) was developed. The model was used to generate predictions on optimal offspring body size for different provisioning efficiencies and risks of parasitism. Moreover, the model allowed me to test the fitness benefits/costs of alternative strategies in cell provisioning. These strategies were 1) females first construct the cells of one sex and then the cells of the opposite sex (sex-assorted investment) or 2) females could allocate the cell under construction to the sex that is at that time underrepresented in her investment so far (mixed-sex investment).
MATERIALS AND METHODS
Field study
Nests of the Red Mason bee O. rufa (L.) from the Botanical Garden of the University of Halle (Germany) were analyzed to study maternal investment. One thousand females of this species were mass released from loose cocoons within 6 days in 1993. The mean emergence day was the 24 April (±1.7 days) that was assumed to be day 1 for all bees. Females were offered wooden nest blocks that contained 100 nesting holes each (8-mm diameter × 150-mm length, arranged in 10 rows and 10 columns). Approximately 600 females nested in the bee shelter, thus forming a large nesting aggregation. In order to ensure I did not disturb the nesting bees, females were not marked.
Osmia rufa females spend the night inside the nest they are constructing, facing the entrance. Every night the nest blocks were inspected visually for occupation of nest holes. The first record of a female bee in a nest hole was regarded as the start of a nest only if this nest hole was occupied during the following nights until sealing of the nest and nest-constructing activities were detected by stored provisions or partitions under construction. The days needed to fill the nest hole with cells were recorded as nest-constructing time for all nests.
Weather conditions strongly affect the activity of bees (Sihag 1984; Imperatriz-Fonseca et al. 1985; Stone 1994; Richards 1996). Nesting activity of O. rufa requires temperatures above 18 °C (K Seidelmann, unpublished data). Above these threshold, females provision continuously without resting in the nest. Therefore, the air temperature was recorded continuously. From the total daily temperature trajectory, the daylight hours with temperatures above 18 °C were summed for the constructing time of every nest as effective nest-constructing hours.
During the following winter, the nest blocks were opened. A random sample of 718 complete nests out of the whole flight season was analyzed. A nest was regarded as complete if it was sealed by a nest plug and there was no interruption in nest construction detected during the nightly inspections. For these nests, the length occupied by brood cells and the number of cells were counted to obtain a rough measure of the mean cell length. Also the existence and length of a vestibulum (an empty cell between the nest plug and the first brood cell) and the thickness of the nesting plug (the outermost thick wall blocking and sealing the nest entrance) were measured. The contents of every cell were recorded, and the body mass of every cocoon with a bee inside was determined to the nearest 0.1 mg. Parasitized cells were detected as the Anthrax larvae inside the host cocoon or the typical feces of Cacoxenus larvae (at the time of nest analysis, Cacoxenus larvae were found in the original infected cell and had not penetrated the cell partitions yet). If a cell was parasitized, the sex of the killed larvae was inferred from the cell size and the sex of the bees in the neighboring cells (Raw 1972; Holm 1973; Maddocks and Paulus 1987; Seidelmann 1995). The mean mass of the remaining cocoons of the appropriate sex from the same nest was used as that of the parasitized cell in further calculations. The sex ratio of cells within a nest or for a group of nests was computed as the proportion of females.
For each nest, the mean provisioning efficiency of the mother bee was computed as cocoon mass equivalents per hour (mg/h) from the total cocoon masses and the effective nest construction hours. Using the same time basis, the mean provisioning time per cell (h per cell) was calculated from the number of provisioned cells in a nest. This latter value is a rough estimation of the mean time the cells of the nest have been open and exposed to parasitism regardless of the sex and size of a particular offspring. The provisioning time per cell included also the time spent in constructing a partition for sealing a cell. The parasitism rate was determined as the number of cells parasitized per total number of provisioned cells in the nest. Only fly parasites attacking open cells during provisioning were considered. Other species parasitized only 0.51% of the cells due to the careful management of the artificial aggregation.
Open-cell parasites enter bee nests through the nest entrance. Therefore, the risk of a cell becoming parasitized should depend on its distance from the nest entrance. I developed a Turbo Pascal program to calculate the spatial distribution of the risk of parasitism within nests based on the data of the nest analysis. The mean risk of parasitism was calculated as the proportion of parasitized cells at each distance over all nests (Seidelmann 1995).
A reduction of offspring body size within nests toward the entrance was analyzed by computing the pairwise differences between the body mass of bees in neighboring cells subtracting the mass of the offspring in the cell nearer to the entrance from that in the cell farer away. Due to the size dimorphism in O. rufa, this had to be done for son and daughter cells separately. A general reduction of offspring body mass toward the nest entrance would be indicated by a positive value of the mean of the mass differences. This method does not depend on the location of cells within nests, the provisioning efficiency of the mother bee, or the progeny sex ratio within the nest. This analysis utilized 263 nests containing at least 5 brood cells (median = 8 cells), each forming a single uninterrupted series of daughter cells and/or son cells.
Collecting and storing brood provisions are the main maternal investments of solitary bees. However, to allow undisturbed nesting and development of larvae, cocoons with their diapausing imagos were instead weighed as a measure of the provision mass (cocoon mass equivalent). To verify the underlying assumption of a sex-independent proportional conversion of provision mass into body mass, a sample of freshly sealed nests was collected and the provision mass in every cell was weighed. To allow normal larval development, the eggs on their provisions were stored separately in small tubes at 25 °C up to the imago stage. In late September, the tubes were transferred to outdoor temperatures for overwintering. Diapausing bees inside their cocoons were sexed and weighed in January. The relationship between provision mass and cocoon mass was tested by means of a separate regression analysis for each sex.
Statistics
Correlations between 2 factors were investigated by linear regression. To control for seasonal effects in the observed investment pattern, a partial correlation analysis with the season date (24 April as day 1) as control variable was run before the regression analysis. Values are given as mean ± standard deviation if not stated otherwise. All statistical analyses were performed with the Statistica package (Statsoft, V. 5.1). The significance level was set at 0.05.
Model development
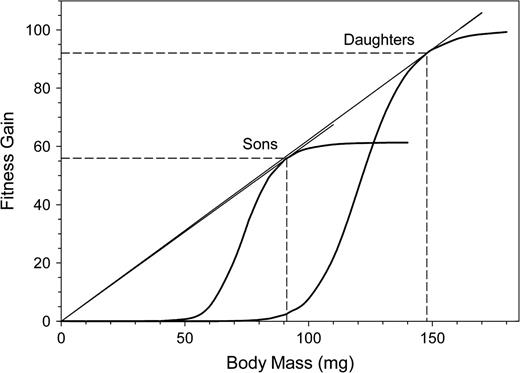
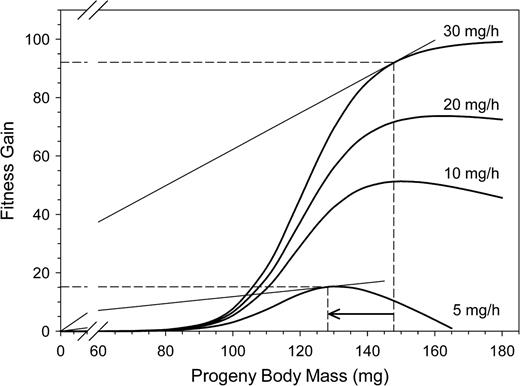
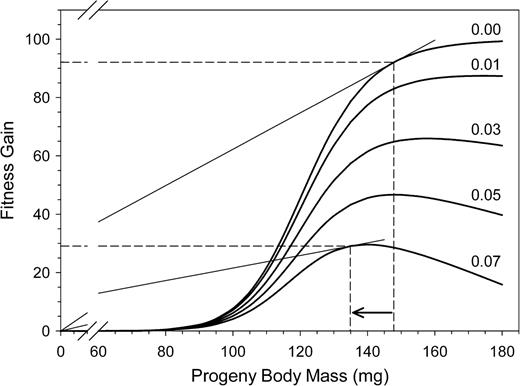
FG in relation to maternal investment expressed as offspring body mass. The FG of sons was corrected for the observed sex ratio. Dashed lines indicate optimal expenditure values and the corresponding FGs. The points of optimal investment are graphically represented by tangents through the origin to the FG curves.
The optimal investment per individual offspring is expected to maximize the benefit-to-cost ratio for a single offspring. This can be represented by the intersection point of the FG curve with the tangent through the origin (Smith and Fretwell 1974; Lloyd 1987). The slope of the tangent is the optimal rate of investment return (Figure 1).
Female bees could adopt different simple maternal investment strategies by producing one or the other sex first or by alternating the sexes. A hypothetical model bee was used to calculate the total FG of each strategy. The same amount of maternal investment was devoted in equal proportions to sons and daughters according to Fisher's principle (Bosch and Vicens 2005). For the sake of simplicity, it was assumed that all bees of a population are of the same size and age and follow the same strategy. Therefore, the simulation ignores possible conditional investment strategies and compensations for shifted sex ratios in the surviving progeny by players of other strategies. Moreover, O. rufa males invest only in matings and not in the offspring themselves. Consequently, the total FGs from sons and from daughters are equal for the whole population. The expected total FG of a strategy can be calculated as double the fitness return from the daughters alone for the scenario described above. The provisioning efficiency was assumed to decline with a female's age according to a regression function observed in the field data (Figure 4). For this calculation, the daily provisioning time was set to 6.5 h and the reproductive life span to 35 days. This results in a total investment of 2640 mg progeny body mass equivalents. Model bees reduce the body size of their offspring continuously with declining provisioning efficiency, as observed in the field study (see Results). Finally, the total FG of the strategies was compared for various rates of open-cell parasitism.
RESULTS
Correlation of provision to body mass
The sexual dimorphism in body size was found to be based on differences in the provision masses the mother bees stored in cells for daughters (306.5 ± 56.2 mg, n = 68) versus sons (183.6 ± 36.5 mg, n = 57) (degrees of freedom [df] = 123, T = 14.179, P < 0.0001). Within each sex, the provision mass was converted proportionally into cocoon mass (intercept was set to 0; males: n = 57, b = 0.278, R2 = 0.986, F1,56 = 4163.503, P < 0.0001; females: n = 68, b = 0.308, R2 = 0.993, F1,67 = 9389.757, P < 0.0001). The regression coefficients do not differ significantly (df = 121, T = 0.958, P = 0.340), pointing to equal metabolic conversion of food in both sexes.
Rate of open-cell parasitism during the nesting period
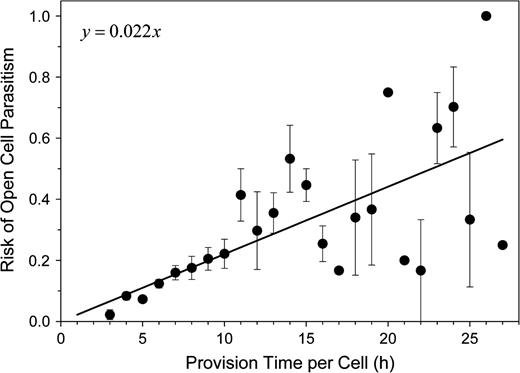
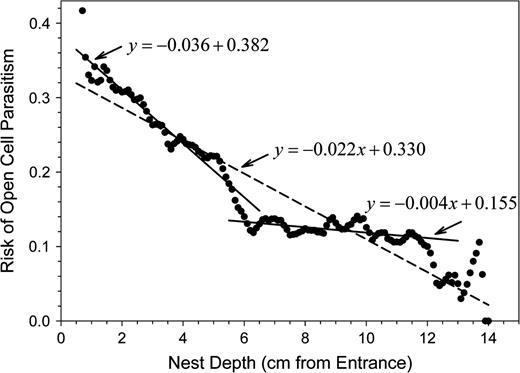
Fly parasites destroyed 15.1 ± 21.7% of the provisioned cells of an O. rufa nest. The proportion of parasitized cells in a nest was correlated to the mean provisioning time per cell (partial correlation: R = 0.211, P < 0.0001, df = 694; regression: intercept was set to 0, n = 697, b = 0.022, P < 0.0001, R2 = 0.457; Figure 2). However, the rate of parasitism of a cell depended also on its position inside the nest. The risk of cell parasitism decreased from more than 0.33 near the nest entrance to about 0.02 at the bottom of the nest (nest depth in cm; b = −0.022, R2 = 0.874, F1,275 = 1907.652, P < 0.0001; Figure 3). However, the decrease in the rate of parasitism was not uniform over the whole nest. There was a steep decrease from the nest entrance toward the middle part (depth 0.7–6 cm; b = −0.036, R2 = 0.898, F1,105 = 932.627, P < 0.0001). At the inner part of the nest, the rate of parasitism declined more slowly (depth 6–12 cm; b = −0.004, R2 = 0.360, F1,119 = 68.621, P < 0.0001; Figure 3).
Risk of cell parasitism by the drosophilid fly Cacoxenus indagator or the bombylid fly Anthrax anthrax in relation to the time a cell was open. For clarity of presentation, values were grouped into 1-h classes (mean ± standard error). The regression was calculated from the raw data.
Risk that a cell would become parasitized by Cacoxenus indagator or Anthrax anthrax in relation to its position inside the nest. The outermost centimeter of the nest is occupied by the nest plug and, usually, a vestibulum (an empty cell). The innermost centimeter at the bottom of the nest hole is rarely used for cell construction. Dashed line: linear regression line for the whole nest depth, solid lines: linear regression lines for the first 6 cm of nest depth and for the inner part of the nest (6–12 cm).
Provisioning efficiency and cell-provisioning time
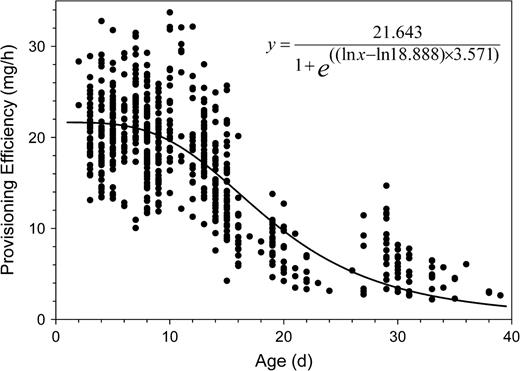
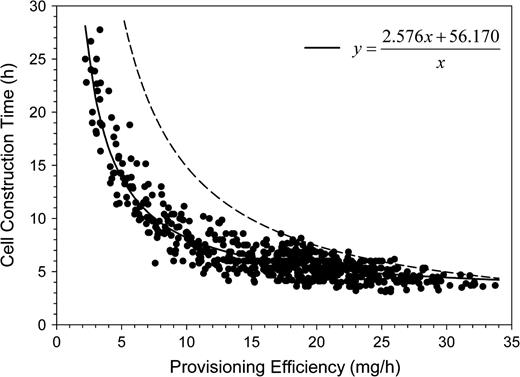
Osmia rufa females constructed nests from 24 April until the end of May. The main nesting activity was in April and the first 10 days of May. During the nesting season, the provisioning efficiency decreased from about 20 to 5 mg/h at the end of May (Figure 4). This decrease was correlated with estimated female age (provisioning efficiency log transformed; n = 635, b = −0.056, R2 = 0.662, F1,633 = 1236.871, P < 0.0001). Although cell provisioning time should depend on provisioning efficiency, it did not increase in proportion to decreasing provision efficiency (Figure 5). The median of the mean cell provision times of the 635 nests analyzed was 6.0 h (quartiles: 5.0, 7.1 h).
Decline in provisioning efficiency as female Osmia rufa grew older during the flight season of 1993. Points indicate the mean provisioning efficiency (measured as mean progeny body mass equivalent per effective nest-constructing hour) during the construction of a nest by an anonymous bee. Data are plotted by the assumed bee's age (see text) at the start of a nest.
Mean time needed by Osmia rufa females to construct and provision a cell in relation to the mother bee's provisioning efficiency. Each point refers to the mean of all cells per individual nest regardless of the occupant's gender and the mean provisioning efficiency during the construction of the entire nest. Provisioning efficiency is measured as progeny body mass equivalents per effective nest-constructing hour. The dashed line indicates the cell construction time that had to be expected at a constant provision mass.
Sex ratio and body mass of progeny
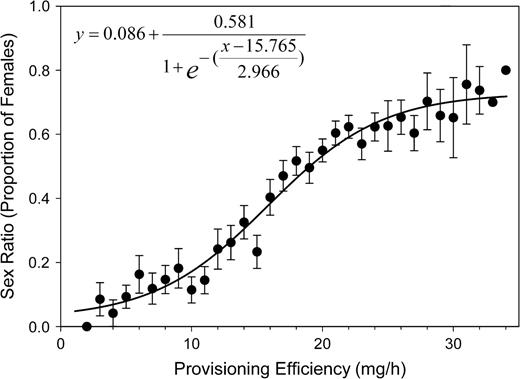
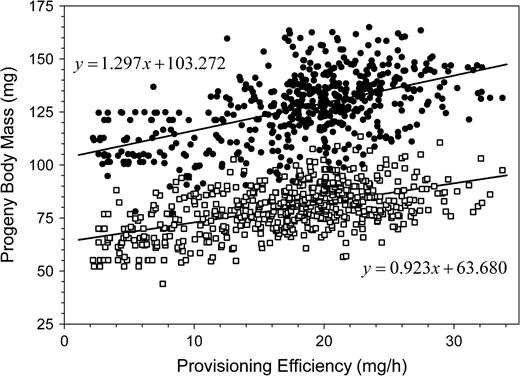
During the nesting period, the sex ratio of progeny shifted from a female bias toward a male bias (Figure 6). This shift was correlated with the declining provisioning efficiency of female bees (partial correlation: R = 0.317, df = 632; regression: n = 635, b = 0.028, R2 = 0.372, F1,633 = 374.185, P < 0.0001). The mean body mass (measured as cocoon mass) of offspring decreased by 27% during the nesting period for both sexes and was correlated to provisioning efficiency (sons—partial correlation: R = 0.194, P < 0.0001, df = 587; regression: n = 590, b = 0.923, R2 = 0.298, F1,588 = 249.104, P < 0.0001; daughters—partial correlation: R = 0.282, P < 0.0001, df = 513; regression: n = 516, b = 1.297, R2 = 0.281, F1,514 = 202.648, P < 0.0001; Figure 7).
Change in the sex ratio (proportion of daughters per nest) in relation to the mother bee's provisioning efficiency measured as progeny body mass equivalents. Provisioning efficiency was classified to the nearest 1 mg/h. For clarity of the graphic presentation, values were grouped into 1-h classes (mean ± standard error). The regression was calculated from the raw data.
Increase in offspring body mass with increasing provisioning efficiency by Osmia rufa females. Solid circles: daughters, open squares: males.
From the 656 O. rufa nests analyzed, only 183 nests (27.9%) contained cells of one sex (sons only: 157 nests [23.9%], daughters only: 26 nests [4.0%]). The mixed nests consisted of a series of cells containing daughters (large cells) at the inner side and a series of son cells (smaller cells) toward the nest entrance. Within nests, body size was successively reduced only in the cell series of sons from the innermost cells toward the nest entrance. The cocoon mass differences of neighboring cells differed from zero for sons (3.06 ± 13.58 mg, n = 511, T(510) = 5.090, P < 0.0001) but not for daughters (0.34 ± 18.97 mg, n = 792, T(791) = 0.500, P = 0.6173). Cocoon masses have not been changed systematically in the daughter cell series. As expected by the sexual body size dimorphism, the cocoon mass difference was significant at the transition from the daughter cell series to the son cell series (41.78 ± 17.84 mg, n = 231, T(230) = 35.583, P < 0.0001).
Exploitation of the available space in the nest hole
The average size of cells remained constant over the nesting period and was not correlated with provisioning efficiency (partial correlation: R = −0.002, P = 0.9691, df = 632; regression: n = 635, b = −0.011, R2 = 0.001, F1,633 = 1.644, P = 0.2002). This is remarkable because smaller offspring (reduced body size and higher proportion of males) produced at the end of the nesting period would need less space (shorter cells) and mothers could thereby increase the number of cells per nest. In contrast, the number of cells per nest decreased with decreasing provisioning efficiency (partial correlation: R = 0.367, P < 0.0001, df = 632; regression: n = 635, b = 0.148, R2 = 0.304, F1,633 = 278.414, P < 0.0001). However, the vestibulum (an empty cell between the nest plug and the first brood cell) was lengthened slightly with estimated age of the bees (regression: n = 409, b = 0.102, R2 = 0.018, F1,407 = 8.586, P = 0.0036) but was statistically not correlated with provisioning efficiency (partial correlation: R = −0.082, P = 0.1341, df = 332). The nesting plug (the outermost thick wall blocking and sealing the nest entrance) became thinner with estimated age (regression: n = 373, b = −0.032, R2 = 0.036, F1,371 = 14.909, P = 0.0001; partial correlation with provisioning efficiency: R = −0.074, P = 0.1933, df = 305).
Fitness reward and optimization of offspring body mass
The model reproduces the expected decline of FG from a given offspring body mass with decreasing provisioning efficiency for a given risk of parasitism due to a higher loss of cells to parasites (Figure 8). As the FG curve flattens with decreasing provisioning efficiency, the optimal body mass of an offspring also drops. That is, females should reduce the body mass of their offspring (sons as well as daughters) when their age increases and their provisioning efficiency declines. A similar effect results from an increase in the rate of open-cell parasitism for a constant level of provisioning efficiency (Figure 9). The FG curve is flatter with increasing rate of parasitism because of the higher loss of cells to parasites. Also, the optimal body size of offspring declines, as shown for declining provisioning efficiency at a constant rate of parasitism.
Decline in the expected FG from daughters with decreasing provisioning efficiency of the mother bee. The risk of open-cell parasitism was set at 0.03. Dashed lines indicate the optimal investment and the corresponding expected FG for high (30 mg/h) and low (5 mg/h) provisioning efficiencies. The point of optimal investment is graphically represented by tangents through the origin to the FG curves.
Decline in the expected FGs from daughters with increasing risk of open-cell parasitism. The provisioning efficiency was set to 15 mg/h. Dashed lines indicate the optimal investment and the corresponding expected FGs when the risk of parasitism ranged from none (0.00) to high (0.07). The point of optimal investment is graphically represented by tangents through the origin to the FG curves.
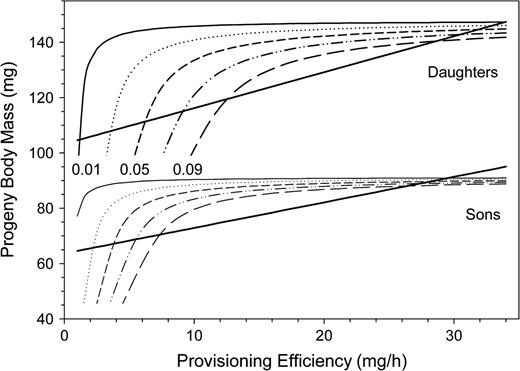
Plotting the optimal body size of offspring for various rates of parasitism against provisioning efficiency generated a group of hyperbolic curves for both sexes (Figure 10). However, the observed body size reduction in progeny can be described best by a straight line (see Figures 7 and 10). That is, females do not optimize offspring size according to the actual rate of parasitism (0.02 in the study site).
Optimal offspring body mass in relation to the mother bee's provisioning efficiency plotted for various risks of open-cell parasitism: solid line: 0.01, dotted line: 0.03, short-dashed line: 0.05, dashed-dotted line: 0.07, long-dashed line: 0.09. Solid straight lines indicate the observed offspring body mass reduction with declining provisioning efficiency in Osmia rufa.
Optimal nesting strategy
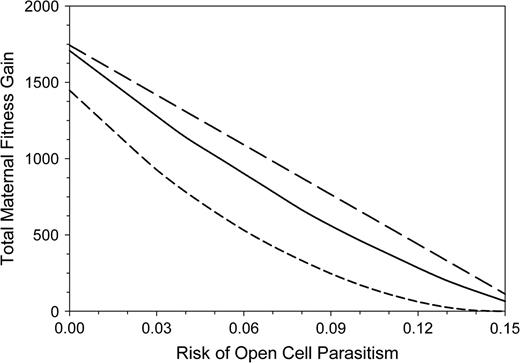
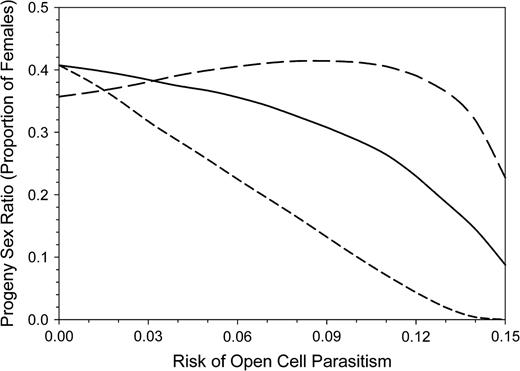
The total FGs of the 3 alternative strategies tested were different despite identical total investment (Figure 11). The daughters-first strategy gave the highest profit, followed by the alternating strategy. The advantage of the daughters-first strategy is due to the higher proportion of surviving daughters. Moreover, the smaller body size of late daughters is responsible for an additional handicap of the alternating and son-first strategies, even without open-cell parasitism. However, the numerical sex ratio remained male biased for all strategies and risks of open-cell parasitism (Figure 12). The male-biased sex ratio in the daughter-first strategy without open-cell parasitism results from the production of large daughters when mothers are still young and efficient, whereas the older, less efficient bees produce small sons later on. As the same amount of about 1320 mg body mass equivalents are devoted each to daughters and to sons, fewer daughters and more sons are produced compared with the other strategies.
Outcomes of the total maternal FG in arbitrary units for 3 strategies of maternal investment in relation to the risk of open-cell parasitism. Long-dashed line: daughters-first strategy, solid line: alternating strategy, and short-dashed line: sons-first strategy. All mother bees invested equal total amounts to sons and daughters.
Secondary sex ratio resulting from the survival probability of son and daughter larvae in different simple maternal investment strategies. Long-dashed line: daughters-first strategy, solid line: alternating strategy, and short-dashed line: sons-first strategy. In all 3 strategies, mother bees invested equally in sons and daughters.
DISCUSSION
Estimation of maternal investment in O. rufa
Cocoon mass is generally used as a measure of maternal investment and for calculations of the Fisherian sex ratio in solitary bees (e.g., Torchio and Tepedino 1980; Strickler 1982; Frohlich and Tepedino 1986; Tepedino and Parker 1988; Sugiura and Maeta 1989; Stark 1992). This practice is based on a high correlation between provision mass and cocoon mass in solitary bees with a moderate sexual dimorphism in body size (Phillips and Klostermeyer 1978; Maddocks and Paulus 1987; Johnson 1988; Danforth 1990; Bosch and Vicens 2002). A high correlation between provision mass and cocoon mass exists also for O. rufa. This correlation was found to be independent of the sex of the offspring. Possible differences in the metabolic conversion of food between the sexes (Boomsma 1989; Visscher and Danforth 1993; Bosch and Vicens 2002) do not affect body mass in O. rufa (see also Seidelmann 1995). Cocoon mass can therefore be used as a good estimate both of maternal investment in provisioning as well as body size of progeny.
An additional component of investment is the time and labor the mother bee devotes to the construction of cell partitions and a nest plug (Seidelmann 1995; Bosch and Vicens 2005). This effort is equal for all cells of a nest and adds a constant small amount of investment to the expenditure involved in cell provisioning. Calculations of parental investment based only on offspring size will therefore slightly underestimate maternal investment. Due to the sexual dimorphism in body size, the relative error in estimation becomes higher in small sons than in larger daughters, resulting in an overestimation of expenditure for sons. However, O. rufa females spend at least 80% (up to 95%) of the total time for cell preparation in collecting food (Raw 1972; Tasei 1973; Maddocks and Paulus 1987; Strohm et al. 2002). The error in estimation of the Fisherian sex ratio using cocoon masses is therefore small and can be neglected for most investigations (for a detailed study on Osmia cornuta, see Bosch and Vicens 2005). It shifts the expected sex ratio of O. rufa by a maximum of 7% toward males (Seidelmann 1995). In terms of modeling the fitness reward of an offspring with Equation 3, the time needed for sealing the cell would add a little extra time in the calculation of the risk of parasitism. That is, the sealing time leads to an additional small decrease in the fitness reward value. This effect strengthens the discovered effect of body mass reduction with decreasing provisioning efficiency or increasing rate of parasitism.
Adjustment of optimal body size
Smith and Fretwell (1974) postulated that parents should attempt to adjust offspring number rather than size to maximize fitness per unit invested. That is, parents should invest no more than that required to obtain the highest fitness reward per unit investment even if a given offspring does not secure the maximal fitness possible. Therefore, the point of optimal investment depends on the shape of the FG curve and might shift as the fitness return per unit invested changes.
The decreasing probability of survival due to open-cell parasitism changes the FG curve in O. rufa (Figures 8 and 9). This effect is independent of the model used to describe the FG function. In rapid gain models (Lloyd 1987) or domed models (Rosenheim et al. 1996), a decrease in survival probability would generate a flatter FG curve and reduced optimal offspring size too. Because open-cell parasitism depends on maternal behavior, it has to be taken into account by female bees (Lloyd 1987; Visscher and Danforth 1993) in contrast to the mortality due to parasites and predators that occurs after termination of the expenditure (Trivers 1985; Clutton-Brock and Godfray 1991).
In O. rufa, open-cell parasitism links the FG per unit investment to provisioning efficiency, which depends on the flight capacity of the female bee. Therefore, females have to tune their investment depending on their physiological and physical state. Female bees should be able to assess their constitution easily by the time or energy they need to provision a cell. The constancy in cell size demonstrates that mothers do not reduce the projected offspring size with age but terminate provisioning before supplying sufficient provisions to provide for an offspring of optimal fitness. As predicted by the model, females of O. rufa limit provisioning time and optimize the FG per unit investment. However, to tune their expenditure exactly, bees have to respond to the actual risk of open-cell parasitism. The estimation of this factor seems to be quite difficult as females meet their enemies rarely and the risk of parasitism varies widely from year to year and from location to location (Seidelmann 1995). In Osmia pumila, females also do not respond to the absence or presence of brood enemies (Goodell 2003). The habit of O. rufa females of kneading the provision mass surface with their mandibles before depositing freshly collected pollen (Raw 1972) could be interpreted as a protective behavior designed to destroy the eggs or hatched larvae of open-cell parasites. Instead of adapting offspring body mass to the actual risk of parasitism, O. rufa females were found to use a simpler strategy that permits them to deal with a broad range of parasites. The bees reduced the size of their offspring in proportion to their declining provisioning efficiency.
Adjustment of the sex allocation
In O. rufa and many other aculeate species, the shape of the sigmoid curve of size on fitness differs for the 2 sexes (Stubblefield and Seger 1994). The curve for the larger sex (females) reaches an asymptote at the upper fitness limit at higher investment values than the curve for the smaller sex (males). Fitness depends on body mass in females of nest-constructing species. Large females tend to produce more eggs, live longer, and forage more efficiently than smaller females (Alcock 1979; Freeman 1981a, 1981b; Larsson 1990; Stubblefield and Seger 1994; Kim 1997; Molumby 1997; Strohm and Linsenmair 1997; Kim and Thorp 2001; Tomkins et al. 2001). However, large sons gain no comparable advantage in species with scramble competition mating systems (Alcock 1980; Thornhill and Alcock 1983; Wcislo et al. 1992; Seidelmann 1999b) as in O. rufa. Therefore, according to the Smith–Fretwell model, mother bees should supply more food to daughters than to sons.
The different FG curves of sons and daughters enable mothers to cope with the constraints of parasitism by reducing progeny body mass over their lifetime. Because females are able to determine the sex of an offspring without any cost, they could respond to their declining provisioning efficiency by producing daughter cells at the beginning of the flight season and son cells at the end. Indeed, the calculations of the total FG of 3 different expenditure strategies demonstrate that the top strategy is based on this kind of sex-assorted investment (Figure 11). These calculations do not consider possible effects of a higher sex-specific reproductive value of daughters due to haplodiploidy (e.g., Crozier and Pamilo 1993; Yanega 1996). A switch in the progeny sex ratio would not cause any negative secondary effects on such things as mating opportunities or resource allocation. After a wintering diapause, all bees eclose within a short period depending on the microclimatic conditions in next spring, regardless of whether their brood cells were built early or late in the season.
Short-term adjustment of maternal investment
Osmia rufa females construct several nests in sequence in beetle holes or cracks in wood as well as empty stalks. Within the linear series of cells of a given nest, the risk of open-cell parasitism increases from the innermost part toward the nest entrance (Figure 3). Females respond to the increasing risk of parasitism by reducing their investment per offspring (Figure 9). As a consequence, females start with daughters at the bottom of the nest and switch to sons toward the entrance (Raw 1972; Holm 1973; Seidelmann 1995). (The arrangement of daughters in the inner cells and sons in the outer cells of mixed nests is also selected for by protandry.) Because the risk of parasitism increases steeply from the middle part toward the nest entrance, females reduce the body size of their sons in cells located toward the entrance. Finally, the outermost cell is usually sealed unprovisioned (Raw 1972; Maddocks and Paulus 1987), presumably because this cell is not worth provisioning due to extremely high likelihood of parasitism (Seidelmann 1999a).
Besides the avoidance of high parasitism risk, other factors also preclude a pure sex-assorted investment regardless of which sex is produced first. Both the principles of risk spreading as well as the unpredictability of life expectancy, weather conditions, and floral resources favor a mixed-sex allocation (Torchio and Tepedino 1980). The advantages of mixed nests force mother bees to shift the proportion of daughters and sons between different nests, as observed in the field (Figure 6), instead of a sex-assorted investment.
CONCLUSIONS
The phenomena of a shift in offspring sex ratio as well as declining offspring body size has been described for several other solitary bee species (e.g., Torchio and Tepedino 1980; Strickler 1982; Tepedino and Torchio 1982; Frohlich and Tepedino 1986; Sugiura and Maeta 1989; Danforth and Visscher 1993; Tomkins et al. 2001; Bosch and Vicens 2005). These changes have been attributed to gradual reductions in the quality and quantity of floral resources and/or to a reduced capacity of aging females to forage. A seasonal floral dearth can be excluded for this study because there was plenty of flowering food plants and trees in the Botanical Garden during the whole flight season. Thus, the aging of mother bees was the most probably cause for the observed age-dependent changes in maternal investment in this study of O. rufa (see comparable results in a recent study on O. cornuta by Bosch and Vicens 2005).
Both declines in food resources and the senescence of female bees result in prolonged provisioning times that would be selectively neutral without a time-constraining force. Torchio and Tepedino (1980) suggest that heightened mortality rates later in the season favor females that produce sons then because they have a better chance of completing cells for these smaller individuals. In contrast, the ovulation cycle of chorionated eggs was instead proposed by Sugiura and Maeta (1989) as the mechanism driving constant egg-laying intervals. As demonstrated in this study, the risk of open-cell parasitism acts immediately as an evolutionary force driving optimization of maternal investment behavior in O. rufa and should be considered for other nest-constructing bees and wasps as well.
I thank M. Dorn for providing helpful support. I am especially grateful to John Alcock, Robert Paxton, and 2 anonymous referees for their helpful critiques on an earlier version of the manuscript. This work was supported by a scholarship from the Studienstiftung des deutschen Volkes.
References
Alcock J.
Boomsma JJ.
Bosch J, Vicens N.
Bosch J, Vicens N.
Brechtel F.
Brockmann HJ, Grafen A.
Chmielewski W.
Clutton-Brock TH, Godfray C.
Collatz KG, Wilps H.
Coutin R, Chenon RD de.
Crozier RH, Pamilo P.
Danforth BN.
Danforth BN, Visscher PK.
Fain A.
Freeman BE.
Freeman BE.
Frohlich DR, Tepedino VJ.
Gebhardt M, Röhr G.
Goodell K.
Holm SN.
Imperatriz-Fonseca VL, Kleinert-Giovannini A, Pires JT.
Johnson MD.
Julliard C.
Julliard C.
Kim J-Y.
Kim J-Y, Thorp RW.
Krombein KV.
Larsson FK.
Lloyd DG.
Maddocks R, Paulus HF.
Molumby A.
Phillips JK, Klostermeyer EC.
Raw A.
Richards KW.
Rosenheim JA, Nonacs P, Mangel M.
Seidelmann K.
Seidelmann K.
Seidelmann K.
Seidelmann K.
Seidelmann K.
Sihag RC.
Smith CC, Fretwell SD.
Stark RE.
Stone GN.
Strickler K.
Strohm E, Daniels H, Warmers C, Stoll C.
Strohm E, Linsenmair KE.
Stubblefield JW, Seger J.
Sugiura N, Maeta Y.
Tasei J-N.
Tepedino VJ, Parker FD.
Tepedino VJ, Torchio PF.
Thornhill R, Alcock J.
Tomkins JL, Simmons LW, Alcock J.
Torchio PF, Tepedino VJ.
Visscher PK, Danforth BN.
Wcislo WT, Minckley RL, Spangler HC.