-
PDF
- Split View
-
Views
-
Cite
Cite
Laura McKinnon, H. Grant Gilchrist, Kim T. Scribner, Genetic evidence for kin-based female social structure in common eiders (Somateria mollissima), Behavioral Ecology, Volume 17, Issue 4, July/August 2006, Pages 614–621, https://doi.org/10.1093/beheco/ark002
Close - Share Icon Share
Abstract
Kin-based social groups are commonly studied among cooperatively breeding species but have been less studied in “nontraditional” group breeding systems. We investigated the presence of kin-based sociality among females in the common eider (Somateria mollisima), a colonial nesting sea duck that exhibits high levels of natal philopatry in females. Previous studies of female sociality in common eiders have been restricted to observations during brood rearing. However, aggregations of female common eiders are also observed during other periods of the life cycle such as colony arrival and nesting. Here we apply a novel, empirical framework using molecular markers and field sampling to genetically characterize female social groups at several stages of the common eider life cycle. When compared with mean estimates of interindividual relatedness for the entire colony, significantly higher levels of relatedness were found between females within groups arriving to the colony in flight, between females and nearest neighbors at the time of nest site selection, and between groups of females departing the colony with ducklings. Both full-sibling and half-sibling equivalent relationships were also found within these groups. Therefore, throughout each of several stages including in-flight colony arrival, nesting, and brood rearing, we provide the first genetically confirmed evidence of female kin-based social groups in common eiders and anseriformes in general.
Kin-based social groups have been described in many species based on both duration of association (ephemeral to permanent) and composition (sex, age, etc.) (reviewed in Queller and Strassmann 1998; Griffin and West 2003). In taxa where kin-based social groups are comprised exclusively of females, group association may serve to lower the predation risk of uniparental care while increasing access to resources for adults (Sterk et al. 1997). For example, female sperm whales form “babysitting” groups so that some females can dive to optimal feeding areas without leaving their young unattended and vulnerable to predation (Whitehead 1996). Female primates also form social relationships with female kin, which decrease predation risk (Sterk et al. 1997) and increase reproductive success (Pope 2000).
Among birds, social groups have been most extensively studied in cooperatively breeding species where individuals forego their own reproduction in order to help other related members reproduce (Schoech et al. 1996). However, among some noncooperatively breeding species, female social groups occur during the brood-rearing period, apparently to decrease predation risk and increase access to resources for adults (Cooper and Miller 1992; Cezilly et al. 1994; Moreno et al. 1997). These social groups are often composed of two or more adult females caring for young from several different broods (Munro and Bedard 1977a; Eadie et al. 1988; Öst et al. 2003). This behavior, referred to as brood amalgamation, is most prevalent in the Anatidae, tribe Mergini (Eadie et al. 1988; Boos et al. 1989; Eadie and Lyon 1998; Bustnes et al. 2002). These female social groups typically form during brood amalgamation and have been most extensively studied in the common eider (Öst 1999; Öst et al. 2002, 2003, 2005).
Common eiders (Somateria mollisima) are a long-lived, iteroparous species with high adult survivorship, delayed sexual maturity (Coulson 1984), and strong female philopatry to breeding areas (Cooch 1965; Reed 1975; Wakeley and Mendall 1976; Anderson et al. 1992). Female sociality among common eiders has been described during brood amalgamation in common eiders (Öst et al. 2003, 2005). However, common eider females may form kin-based social groups throughout several stages of their life cycle, including migration and nesting. Benefits of group cohesion in female common eiders during colony arrival may include energetic savings during migration (Alerstam 1990) and the gathering of information regarding prime nesting areas. Female eiders may also benefit by nesting in related groups through increased vigilance against nest predation and intraspecific usurpation (personal observation). Finally, females with young may benefit by departing with other females if this increased access to resources and if group vigilance lowered predation.
Kin associations in other Anatidae have been inferred via behavioral observations or observations of banded individuals (Elder WH and Elder NL 1949; Regehr et al. 2001; van der Jeugd et al. 2002), though have rarely been confirmed using genetic markers (Andersson and Åhlund 2000). The objective of this study was to identify genetically whether female kin-based social groups exist in common eiders throughout several stages of their life cycle. Given that common eiders are philopatric to nesting areas, colonial, and exhibit uniparental care, we predicted that members of aggregations of adult females during colony arrival, nesting, and colony departure would be related. Specifically, we predict that estimates of mean coefficients of relatedness (a surrogate measure of pedigree relationship) between females within groups at all 3 stages would be greater than background levels of relatedness for females in the colony.
METHODS
Field methods
Study location
Research was conducted at a northern common eider colony (Somateria mollissima borealis) within the East Bay Migratory Bird Sanctuary (East Bay) on Southampton Island, Nunavut, Canada (64°02′N, 81°47′W) from June to August 2003. The common eider colony is located on a 26-ha island (Mitivik Island) within East Bay and consists of up to 4000 breeding pairs annually. The island is characterized by tundra, low-lying rocky outcrops, and several ephemeral freshwater ponds. One larger pond (0.32 ha) exists at the center of the colony and is a source of freshwater throughout the breeding season. Wooden study blinds are situated throughout the colony and are accessed by aboveground canvas tunnels to minimize disturbance to nesting common eiders.
Background levels of female relatedness in the eider colony
To detect female-based kin groups in common eiders in this study, we tested mean estimates of coefficients of relatedness among pairs of females within designated groups (arriving groups, nesting groups, and females departing together in groups) against the background levels of female relatedness for the entire colony.
Background levels of female relatedness for this study colony were determined by sampling females on arrival to the colony. In panmictic populations, the distribution of relatedness values across all pairwise comparisons will be normal with a mean of zero. In philopatric colonial nesting species, such as the common eider, higher mean background levels of relatedness between individuals may occur due to natal philopatry (Coltman et al. 2003). It was therefore necessary to select a random sample of individuals representative of the population and calculate background levels of female relatedness for the colony prior to testing for female-based social groups.
Sampling of females was conducted in such a manner as to fulfill 2 fundamental requirements. First, by selecting a large number of females (n = 167) coming onto the colony over the entire arrival period, we captured the genetic diversity of the entire breeding population. Secondly, we systematically sampled across the entire prelaying and early incubation period in order to accurately represent the background levels of relatedness for the colony by including early, mid, and late nesters in the sample. A large and temporally representative sample size helped to ensure that estimates of allele frequencies and estimates of coefficients of relatedness were unbiased. In addition, the background estimate was independent of the estimates for the 3 sampling groups as females sampled for the background estimate were not resampled for the other 3 group estimates (colony arrival, nest selection, or colony departure).
Female common eiders were caught in large nylon salmon gill nets (6 m tall by 100 m long) erected on the colony during the prelaying and early laying period (14 June through 4 July 2003). Nets were set in the flight paths of common eiders flying around the seaward edge of the colony. A maximum of 2 nets were set simultaneously. At this site, eiders generally arrive in mixed sex flocks varying in size from 2 to 30 birds. Approximately 0.25 ml of blood was taken from the tarsal vein of target female samples using a 25-gauge needle and capillary tubes. Blood samples were immediately transferred to a prelabeled centrifuge tube with 1 ml of saline buffer (100 mM Tris, pH 8.0, 100 mM ethylenediaminetetraacetic acid, 10 mM NaCl, 0.5% sodium dodecyl sulfate) and stored at ambient temperature (2–5 °C) until transfer to a freezer at the end of the field season.
Colony arrival
To test the hypothesis that aggregations of female common eiders were composed of related individuals during colony arrival, mean coefficients of relatedness between females within arrival groups were compared with the background levels of relatedness for the colony. Aggregations of females arriving together were caught in the large mist nets during the prelaying and early laying period (14 June through 4 July 2003). Females were considered to be arriving in groups if they entered the net together. Only individuals that were seen flying together in a cohesive group and were subsequently captured together were considered arrival groups. This methodology is unique in that it permitted us to capture the natural composition of a distinct group of birds in flight. Blood was collected from individual females within groups (n = 16 groups) as described above. Blood samples from females captured within groups were used exclusively to estimate mean relatedness of females arriving together in groups and were not included in the background levels of relatedness for the colony discussed above.
Nest site selection
To test the hypothesis that aggregations of female common eiders were composed of related individuals during nesting, mean coefficients of relatedness were estimated between focal females (defined below) and nearest neighbors during nesting, and these were compared with the background levels of relatedness for the colony. We monitored the location and timing of nest initiation of female eiders in 2 0.175-ha study plots. The 2 study plots were located in low-and high-density nesting areas and were approximately 100 m apart from each other. Detailed maps were constructed for each monitored plot, and nest locations were plotted for each female as nests were initiated over time. Plots were monitored twice daily for the presence of nesting females from 19 June until 8 August 2003. Nest initiation date was recorded only after a female had been present on the same nest for at least 3 consecutive observation periods (approximately 1.5 days) indicating the initiation of incubation. It is possible that different females may visit the same nest earlier in the laying period (after the first or second egg) due to brood parasitism or nest takeover (Robertson 1998). By recording the initiation date only on commencement of incubation, when nest takeover is rare (Robertson 1998), and by sampling feathers at the end of incubation, it is most likely that the sample reflects the female who selected to nest in the area and not just a visiting brood parasite. Initiation dates, presence of attending males and/or females, hatch dates, and number of ducklings departing the nest were recorded for each monitored nest. Nesting females were categorized as early, mid, or late nesters based on the distribution of nest initiation dates for each plot.
Focal females were randomly selected from each of these categories using a random number generator. Plot maps were then used to select the 3 nearest neighbors that were present prior to nest initiation of each focal female. A nearest neighbor female was defined, based on mapped nest sites, as a female that was nesting closest to the focal female and was present in the study plot when the focal female initiated nesting. When approximately 80% of females in the study plots had departed the nest, nests were visited for collection of feathers for use in genetic analysis. In cases where nest material was not available for one or more of the nearest neighbors, additional females were chosen based on the same criterion until 3 nearest neighbors were sampled. A total of 13 focal females, each with 3 nearest neighbors, met this conservative criterion.
Colony departure
To test the hypothesis that aggregations of adult female common eiders departing the colony with young were composed of related individuals, mean coefficients of relatedness between females within groups departing together were compared with the background levels of relatedness for the colony. Females departing the island together in groups were captured from 24 July through 2 August 2003. Two 8 × 8 × 4–m wire walk-in traps were constructed and placed on the shore of the island. As groups departed, they were passively funneled into the walk-in traps via 50 m lengths of 0.5-m-high chicken wire fencing that extended at 45° angles from the trap entrances. Traps were checked at 2-h intervals. Both females and ducklings captured were banded and bled as above. Groups (n = 11) ranged in size from 2 to 5 females with 3 to 20 ducklings. Parent–offspring relationships were also estimated for females and ducklings within each group to identify accompanying failed breeders and/or nonbreeders with no ducklings of their own in the group.
Genetic analysis
DNA was extracted from all blood samples using Qiagen DNEasy extraction kits (Qiagen, Bothel, WA). DNA was quantified using a spectrophotometer and diluted to 20 ng/μl for amplification. DNA was extracted from all feather samples in the same manner. Only 5 contour feathers were used per nest due to the potential for genetic contamination (i.e., feathers from different individuals in the same nest). Reducing the number of feathers selected can reduce the potential for contamination (Pearce et al. 1997). Extracted feather samples were amplified undiluted due to low concentration of DNA. DNA was amplified using polymerase chain reaction (PCR) for 10 microsatellite loci: Sfiμ5, Alaμ1(Fields and Scribner 1997), Sfiμ9, Sfiμ10, Sfiμ11 (Libants S, Oswald K, Olle E, Scribner K, unpublished data), Hhiμ5, Bcaμ11 (Buchholz et al. 1998), Smoμ4, Smoμ10 (Paulus and Tiedemann 2003), and Aphμ23 (Maak et al. 2003). All loci were run under published PCR conditions with slight modification (McKinnon 2005). Due to the low concentration of DNA extracted from the feather samples, feather samples could not be reliably amplified at 2 of these loci (Sfiμ11 and Sfiμ5), so analyses for the nest site selection study were conducted using only 8 loci. PCR products were run on 6% denaturing acrylamide gels along with a molecular base pair ladder and standards of known size. Gels were scanned with a Hitachi FMBIO II scanner. Alleles were hand scored for each individual. Initial allele scores were double checked by a second experienced lab member. Approximately 8–10% of samples, randomly selected from across all groups, were reamplified for each locus to test for genotyping error due to allelic dropout, contamination, scoring etc. The genotyping error was reported as the proportion of alleles scored incorrectly, averaged across all loci.
To obtain background allele frequencies for the populations, a total of 167 individuals were selected systematically from all females caught in the net (not including those within arrival groups), representing the entire range of arrival times for all females. Estimates of allelic frequencies were generated in the program Microsatellite analyzer, version 3.15 (Dieringer and Schlotterer 2002). Allelic diversity and observed and expected heterozygosity were calculated using the program Genepop, version 3.3 (Raymond and Rousset 1995). Tests for deviation from Hardy Weinberg equilibrium and tests for linkage disequilibrium between loci (Bonferroni corrected [Rice 1989] to adjust nominal alpha levels for multiple testing) were also conducted using the program Genepop, version 3.3 (Raymond and Rousset 1995), with exact tests using the Markov chain method. Pairwise coefficients of relatedness were calculated using the program Kinship, version 1.3.1 (Goodnight and Queller 1999). Pairwise coefficients of relatedness provide a measure of relatedness between 2 individuals and are estimated based on the proportion of alleles shared between individuals adjusted based on population allele frequencies. Standard parametric statistics cannot be used to detect differences in mean estimates of relatedness between groups due to the interdependence of the pairwise estimates (Danforth and Freeman-Gallant 1996). Instead, differences in mean pairwise coefficients of relatedness between groups were determined via a one-tailed distribution free permutation test with 1000 repetitions. A one-tailed test was chosen due to the testing of directional hypotheses (i.e., the mean of the selected group is greater than the background). The permutation test was performed using a SAS-based program. This program calculates the difference between the means of the 2 groups, then pools the data from both groups, and randomly subsamples 2 new groups from the pooled data. The difference between the means of the 2 subsampled groups is calculated for each iteration. The number of times that the difference in means of the subsampled groups is greater than the observed difference in means provides a measure of significance, reported as a P value. The permutation test provides a more robust test than alternative nonparametric statistical tests (Danforth and Freeman-Gallant 1996).
To provide a description of group composition in terms of relatedness between individuals within groups, we further estimated the likelihood that alleles between individuals were identical by descent using the program Kinship, version 1.3.1. (Goodnight and Queller 1999). We tested whether coefficients of relatedness were significantly more likely under the hypotheses of full-sibling equivalent relationships (rxy = 0.50) or half-sibling equivalent relationships (rxy = 0.25) than under the null hypothesis of 2 individuals being unrelated (rxy = 0). Program Kinship calculates the probability of detecting observed pairs of individuals with multilocus genotypes consistent with these pedigrees conditional on estimated population allele frequencies. Estimates of statistical significance and power were calculated by simulating genotypes given the allele frequencies of the population. P values (the probability of falsely rejecting the null hypothesis) under 0.05 were considered significant. Estimates of statistical power (the probability of failing to reject a false null hypothesis) were reported as β (power = 1 − β). The calculation of β was based on the number of loci and estimated heterozygosity for each locus (Goodnight and Queller 1999).
RESULTS
Genetic analysis
Allelic frequencies, allelic diversity, and observed and expected heterozygosity for each locus were high (Table 1). The mean number of alleles per locus was 12.4 (standard deviation [SD] = 3.16, range 4–33) (Table 1). Genotypic frequencies of 8 loci were consistent with Hardy Weinberg expectations (P > 0.005, Bonferroni corrected), whereas genotypic frequencies at 2 loci were not (Smoμ4, P = 0.0016, and Hhiμ5, P = 0.0033). Smoμ4 was highly polymorphic (33 alleles) (Table 1). The background sample size (n = 167) was not large enough to represent the full range of possible genotypes (531) for such a polymorphic locus, a common problem when estimating population gene frequencies from samples (Nei 1987). However, Smoμ4 and Hhiμ5 were included in both estimates of coefficients of relatedness and likelihood tests of pedigrees as the deviation from Hardy Weinberg expectations was either minimal (Hhiμ5) and/or due to small sample size (Smoμ4) and consequently would not likely bias estimates. Tests for linkage disequilibrium confirmed that all loci were independently inherited (P > 0.005, Bonferroni corrected). The genotyping error rate was estimated to be 0.0302 averaged across all loci, well within the lower range of error rates (0.001–0.127) reported in previous studies (Hoffman and Amos 2005). Genotype scores were corrected, and all gels were reviewed again. As an additional precautionary measure, all homozygotes for several loci were reamplified to reduce errors due to allelic dropout.
Allelic diversity and observed and expected heterozygosity for background samples at 10 polymorphic loci
| Locus . | Het. obs.a . | Het. exp.b . | Fc . | Allelic diversity . |
|---|---|---|---|---|
| Sfiμ10 | 0.813 | 0.855 | 0.049 | 23 |
| Sfiμ9 | 0.714 | 0.744 | 0.040 | 8 |
| Alaμ1 | 0.652 | 0.631 | −0.034 | 6 |
| Hhiμ5 | 0.103 | 0.122 | 0.153 | 4 |
| Sfiμ5 | 0.730 | 0.769 | 0.052 | 16 |
| Sfiμ11 | 0.462 | 0.460 | −0.012 | 6 |
| Smoμ10 | 0.813 | 0.801 | −0.014 | 18 |
| Aphμ23 | 0.613 | 0.586 | −0.046 | 6 |
| Smoμ4 | 0.850 | 0.943 | 0.099 | 33 |
| Bcaμ11 | 0.130 | 0.130 | −0.007 | 4 |
| Mean | 0.588 | 0.603 | 0.026 | 12.4 |
| Locus . | Het. obs.a . | Het. exp.b . | Fc . | Allelic diversity . |
|---|---|---|---|---|
| Sfiμ10 | 0.813 | 0.855 | 0.049 | 23 |
| Sfiμ9 | 0.714 | 0.744 | 0.040 | 8 |
| Alaμ1 | 0.652 | 0.631 | −0.034 | 6 |
| Hhiμ5 | 0.103 | 0.122 | 0.153 | 4 |
| Sfiμ5 | 0.730 | 0.769 | 0.052 | 16 |
| Sfiμ11 | 0.462 | 0.460 | −0.012 | 6 |
| Smoμ10 | 0.813 | 0.801 | −0.014 | 18 |
| Aphμ23 | 0.613 | 0.586 | −0.046 | 6 |
| Smoμ4 | 0.850 | 0.943 | 0.099 | 33 |
| Bcaμ11 | 0.130 | 0.130 | −0.007 | 4 |
| Mean | 0.588 | 0.603 | 0.026 | 12.4 |
Observed heterozygosity.
Expected heterozygosity.
Deviation from Hardy Weinberg equilibrium; positive values indicate heterozygote deficiency and negative values indicate heterozygote excess.
Allelic diversity and observed and expected heterozygosity for background samples at 10 polymorphic loci
| Locus . | Het. obs.a . | Het. exp.b . | Fc . | Allelic diversity . |
|---|---|---|---|---|
| Sfiμ10 | 0.813 | 0.855 | 0.049 | 23 |
| Sfiμ9 | 0.714 | 0.744 | 0.040 | 8 |
| Alaμ1 | 0.652 | 0.631 | −0.034 | 6 |
| Hhiμ5 | 0.103 | 0.122 | 0.153 | 4 |
| Sfiμ5 | 0.730 | 0.769 | 0.052 | 16 |
| Sfiμ11 | 0.462 | 0.460 | −0.012 | 6 |
| Smoμ10 | 0.813 | 0.801 | −0.014 | 18 |
| Aphμ23 | 0.613 | 0.586 | −0.046 | 6 |
| Smoμ4 | 0.850 | 0.943 | 0.099 | 33 |
| Bcaμ11 | 0.130 | 0.130 | −0.007 | 4 |
| Mean | 0.588 | 0.603 | 0.026 | 12.4 |
| Locus . | Het. obs.a . | Het. exp.b . | Fc . | Allelic diversity . |
|---|---|---|---|---|
| Sfiμ10 | 0.813 | 0.855 | 0.049 | 23 |
| Sfiμ9 | 0.714 | 0.744 | 0.040 | 8 |
| Alaμ1 | 0.652 | 0.631 | −0.034 | 6 |
| Hhiμ5 | 0.103 | 0.122 | 0.153 | 4 |
| Sfiμ5 | 0.730 | 0.769 | 0.052 | 16 |
| Sfiμ11 | 0.462 | 0.460 | −0.012 | 6 |
| Smoμ10 | 0.813 | 0.801 | −0.014 | 18 |
| Aphμ23 | 0.613 | 0.586 | −0.046 | 6 |
| Smoμ4 | 0.850 | 0.943 | 0.099 | 33 |
| Bcaμ11 | 0.130 | 0.130 | −0.007 | 4 |
| Mean | 0.588 | 0.603 | 0.026 | 12.4 |
Observed heterozygosity.
Expected heterozygosity.
Deviation from Hardy Weinberg equilibrium; positive values indicate heterozygote deficiency and negative values indicate heterozygote excess.
Background levels of female relatedness in the colony
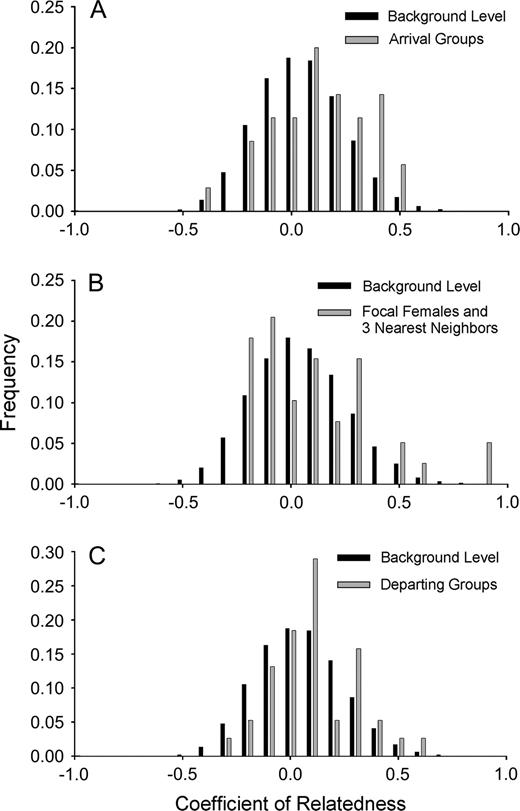
Background levels of female relatedness (rxy) in this colony were normally distributed with a mean close to zero (10 loci: rxy = −0.004, standard error [SE] = 0.002; 8 loci: rxy = −0.006, SE = 0.002) (Figure 1 and Table 2). A mean rxy of zero for background random female samples indicates that, on average, females in the colony that were not sampled as part of identified groups were not related to one another.
Distribution of relatedness values (A) between females within arriving groups (n = 16 groups), (B) between females and 3 nearest neighbors (n = 13 groups), and (C) between females in departing groups (n = 11 groups) compared with background levels of relatedness for the colony (n = 167 individuals).
Mean pairwise relatedness values as calculated in Kinship 1.3.1 (Goodnight and Queller 1999) for all groups and results of one-tailed permutation tests testing for differences between mean relatedness of females in groups versus background levels
| Group . | Sample size . | Mean pairwise relatedness values . | Permutation test (P value) . |
|---|---|---|---|
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Arrival groups | 16 groups | 0.069 (0.036) | 0.012 |
| Background (8 loci) | 167 individuals | −0.006 (0.002) | |
| Focal females and 3 nearest neighbors | 13 groups | 0.060 (0.046) | 0.039 |
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Departing groups | 11 groups | 0.055 (0.033) | 0.047 |
| Group . | Sample size . | Mean pairwise relatedness values . | Permutation test (P value) . |
|---|---|---|---|
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Arrival groups | 16 groups | 0.069 (0.036) | 0.012 |
| Background (8 loci) | 167 individuals | −0.006 (0.002) | |
| Focal females and 3 nearest neighbors | 13 groups | 0.060 (0.046) | 0.039 |
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Departing groups | 11 groups | 0.055 (0.033) | 0.047 |
SEs of the means are reported in parentheses.
Mean pairwise relatedness values as calculated in Kinship 1.3.1 (Goodnight and Queller 1999) for all groups and results of one-tailed permutation tests testing for differences between mean relatedness of females in groups versus background levels
| Group . | Sample size . | Mean pairwise relatedness values . | Permutation test (P value) . |
|---|---|---|---|
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Arrival groups | 16 groups | 0.069 (0.036) | 0.012 |
| Background (8 loci) | 167 individuals | −0.006 (0.002) | |
| Focal females and 3 nearest neighbors | 13 groups | 0.060 (0.046) | 0.039 |
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Departing groups | 11 groups | 0.055 (0.033) | 0.047 |
| Group . | Sample size . | Mean pairwise relatedness values . | Permutation test (P value) . |
|---|---|---|---|
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Arrival groups | 16 groups | 0.069 (0.036) | 0.012 |
| Background (8 loci) | 167 individuals | −0.006 (0.002) | |
| Focal females and 3 nearest neighbors | 13 groups | 0.060 (0.046) | 0.039 |
| Background (10 loci) | 167 individuals | −0.004 (0.002) | |
| Departing groups | 11 groups | 0.055 (0.033) | 0.047 |
SEs of the means are reported in parentheses.
Colony arrival
We tested the hypothesis that aggregations of female common eiders were composed of related individuals during colony arrival in flight. The mean estimate of pairwise relatedness for females within arriving groups (rxy = 0.069, SE = 0.036) was significantly greater than was estimated for the mean background level of relatedness for the colony (rxy = −0.004, SE = 0.002; permutation test, P = 0.034; Table 2 and Figure 1A), indicating that aggregations of females during colony arrival were composed of some related individuals. Estimates of relatedness consistent with full-sibling equivalent relationships (rxy = 0.50) were present in 4 of 16 arrival groups (P < 0.05, β = 0.044). Included in these groups was one group of 4 females with 3 full-sibling equivalent relationships. Thus, the null hypothesis that female associations present at the time of colony arrival are random was rejected.
Nest site selection
We tested the hypothesis that aggregations of female common eiders were composed of related individuals during nesting. When focal females initiated nests, there was an average of 32 (SD = 4.8, range 16–83) females nesting in the study plots.
Nearest neighbors initiated nests on average 2.6 (SE = 0.44, range = 0–13) days before focal females initiated their own nests. The mean estimate of pairwise relatedness for focal females and their 3 nearest neighbors (rxy = 0.060, SE = 0.046) was significantly greater than the mean background level of relatedness for the colony (rxy = −0.006, SE = 0.002; permutation test, P = 0.012; Table 2 and Figure 1B), indicating that females that were spatially aggregated during nesting were related to each other. Two focal females had 2 full-sibling equivalent relationships with their nearest 3 neighbors (P < 0.05, β = 0.101). Another 3 focal females had one full-sibling equivalent relationship within the nearest 3 neighbors (P < 0.05, β = 0.101). An additional 2 focal females had half-sibling equivalent relationships (rxy = 0.25) within the nearest 3 neighbors (P < 0.05, β = 0.481). Thus, a total of 7 of 13 focal females had at least half-sibling equivalent relationships within the 3 nearest neighbors. The null hypothesis that female associations during nest site selection are random was rejected.
Colony departure
We tested the hypothesis that aggregations of adult female common eiders departing the colony with young were composed of related individuals. Mean departing group size was 11.33 (SD = 5.80, range 5–23). The average number of adult females per departing group was 2.40 (SD = 1.24, range 1–5), the average number of ducklings was 8.93 (SD = 1.28, range 4–20), and the average ratio of ducklings to adult females in captured groups was 3.72:1. The mean estimate of pairwise relatedness for adult females within departing groups (rxy = 0.055, SE = 0.033) was significantly greater than the mean background level of relatedness for the colony (rxy = −0.004, SE = 0.002; permutation test, P = 0.047; Table 2 and Figure 1C), indicating that aggregations of females during colony departure were composed of related individuals. Each departing group had one female that did not have parent–offspring equivalent relationships with any of the ducklings in the brood. Full-sibling equivalent relationships were found between females in 2 departing groups. One departing group included 2 full-sibling equivalent relationships (P < 0.05, β = 0.052) and a second departing group had 1 full-sibling equivalent relationship (P < 0.01, β = 0.163) between adult females. Thus, the null hypothesis that female associations present at the time of colony departure are random was rejected.
DISCUSSION
Based on genetic evidence, we found that kin groups occurred during colony arrival, nest site selection, and colony departure and, therefore, we reject the null hypothesis that social groups in common eider females are random with respect to relatedness. When compared with the mean estimates of interindividual relatedness for the entire colony, significantly higher levels of relatedness were found between females within groups arriving to the colony in flight, between females and nearest neighbors at the time of nest site selection, and between groups of females departing the colony with ducklings. Collectively, results provide evidence for kin-mediated sociality in common eider females.
Colony arrival
Confirmation of female kin groups arriving in flight together at the breeding colony provides the first direct evidence for female–female bonds documented in sea ducks during flight. The presence of full-sibling equivalent relationships within these groups suggests that offspring may follow the females and/or siblings from hatch, beyond the brood-rearing period, throughout the fall molt and migration to wintering areas, and during their return to the colony. This has not been previously observed in tribe Mergini. Evidence of extended kin groups (young with adults) has been suggested for another sea duck, the harlequin duck (Histrionicus histrionicus; Cooke et al. 2000; Regehr et al. 2001), and geese (Elder WH and Elder 1949) based on sightings of juveniles arriving with adults on the wintering areas. These suggested relationships, however, have not previously been confirmed genetically.
Detection of half-sibling equivalent relationships may also indicate that family groups can persist across several years or across extended family members. An rxy value of 0.25 can be explained through a variety of mechanisms including, but not limited to, an offspring following other female relatives, young from multiple years following a mother, or half-siblings from a multiply sired clutch (e.g., Hario et al. 2002). Prolonged (>2 years) mother–offspring and sibling relationships have not been commonly observed in anseriformes (Black and Owen 1989; review in Raveling 1979; Owen 1980; Prevett and MacInnes 1980). Though patterns of prolonged parent–offspring bonds and extended family units have been documented in migrating geese (Ely 1993; Warren et al. 1993) and swans (Scott 1980), studies were based on behavioral observations or visual observations of banded individuals and lacked genetic confirmation of mother–offspring and sibling relationships. The presence of prolonged or extended bonds within migrating groups can increase the potential fitness benefits of group travel as younger individuals may benefit from the experience of older group members. Here, we present the first genetic evidence of mother–offspring or sibling equivalent bonds during colony arrival and possibly migration and the first genetic evidence of potential prolonged or extended family relationships for common eiders specifically and among anseriformes in general.
It is possible that the females captured in arrival groups could have already been in the colony, selected a nesting area and left to forage with females from that same area, and were captured on return to the colony. Relationships within these groups may then be explained by the clustering of kin during nesting at the colony (discussed in detail below) and not by clustering of kin prior to arrival at the colony. Thus, females in arrival groups could represent migrating individuals or simply individuals foraging together. Regardless, the elevated levels of relatedness within the arrival groups do indicate some form of in-flight kin-based group cohesion, which could be present during migration.
Nest site selection
Spatial clustering of kin during nesting has been documented in many species by identifying general spatial patterns of relatedness in breeding areas (i.e., correlation of relatedness and geographic proximity) (e.g., Friesen et al. 1996; Burland et al. 2001; Ratnayeke et al. 2002; van der Jeugd et al. 2002). Fewer studies actually examine the decision-making process during nest site selection by individual animals (Andersson and Åhlund 2000). Female common eiders investigate several nest sites, often sitting in them, before choosing the final nest cup (Cooch 1965; Schmutz et al. 1982; Bottita et al. 2003). By examining the levels of relatedness between focal females and their nearest neighbors at the time of nest site selection, we have confirmed a preference for nesting in proximity to kin. Given the abundance of females nesting in these areas when focal females selected nests and the high prevalence of full- and half-sibling equivalent relationships between focal females and their 3 nearest neighbors, these results suggest that kinship is a factor in nest site selection.
Given evidence of kin groups in arriving females, it is not surprising to find higher levels of relatedness between nesting females and their nearest neighbors. If females were arriving together, it is possible that they may be selecting nest sites at the same time, in the same locations based on other ecological factors such as proximity to water, distance to the nests of predators, and characteristics of available nest cups themselves (Kilpi and Lindström 1997). Females arriving together may nest together in the highest quality areas available at the time of arrival. However, the nearest neighbors selected in this study initiated nests, on average, 2.6 days earlier than the focal females, clearly indicating separate nest initiation times. In addition, females arriving together in groups can vary in breeding condition. For example, of females caught in the net at the same time, some laid an egg while in the holding box within an hour of capture, whereas others prospected for nesting areas for several days before finally selecting a nest site and laying. This suggests that although individuals may arrive together, nesting among arriving individuals is not necessarily synchronous, and therefore it is unlikely that these patterns are solely an artifact of similar colony arrival times. It is more likely that there is some level of kin recognition among female common eiders, similar to that suggested for goldeneye ducks during brood parasitism, where females preferentially parasitize the nests of close relatives (Andersson and Åhlund 2000).
Female philopatry might also account for higher levels of relatedness between nearest neighbors. Common eiders are often philopatric to nesting areas within colonies (Cooch 1965), and fidelity to specific nest sites, though rare, has been documented (Goudie et al. 2000). Natal philopatry could explain close proximity of kin in nesting areas regardless of nest site selection processes. Studies conducted on other seabird colonies (Osorio-Beristain and Drummond 1993; Friesen et al. 1996; Schjorring 2001) have shown that individuals showed a greater preference for nesting in proximity to natal nesting areas as opposed to kin. These species, however, exhibit both male and female philopatry, and pair bonds form on the breeding grounds. Nesting in close proximity to kin could be detrimental in species where both sexes are highly philopatric and breeding colonies are small in number due to increased risk of inbreeding (Shields 1982, 1987). In northern common eiders, only females are philopatric, and pair bonds are thought to form on the wintering grounds. Consequently, in common eiders, the process of selecting nests in close proximity to kin should not lead to the negative consequences of inbreeding. Based on the data presented here, it is more likely that there is a nest site selection process that involves kin recognition. Though the mechanisms of kin recognition remain poorly understood in birds (Komdeur and Hatchwell 1999), several studies in philopatric species have documented a preference for nesting in proximity to kin even when nesting away from natal breeding sites (Andersson and Åhlund 2000; van der Jeugd et al. 2002).
Colony departure
Relatively higher levels of relatedness between females in departing groups could be a result of arrival in kin groups, nesting in kin groups, or a combination of both. If females are arriving together, they may be nesting at the same time, and consequently clutches would hatch synchronously and females would depart with young at the same time. It is likely then, if brood amalgamation is to occur, that females in departing groups may be relatively closely related to each other due to their timing of arrival and nesting associations (discussed above). One proposal, the accidental mixing hypothesis (Munro and Bedard 1977b; Warhurst and Bookhout 1983; Eadie et al. 1988), partially explains this phenomenon in breeding females, where there is a propensity for broods to amalgamate accidentally due to high brood density (Patterson et al. 1982; Warhurst and Bookhout 1983; Savard et al. 1989) and/or during the confusion of a predation attack (Munro and Bedard 1977b). Associations of adult females that amalgamate broods could be random due to accidental mixing of young, but patterns of relatedness would still exist due to higher relatedness between females that arrived together in the same breeding condition and thus were departing the island at the same time. However, not all female associations during colony departure occur as a result of the accidental mixing of broods. Females with no young of their own also accompany departing groups. Recent satellite telemetry data from our field site indicate that many nonbreeders or failed breeders remain within 10 km of the colony, feeding in the bay, throughout the breeding season (HG Gilchrist, unpublished data). These females routinely return to the colony during late incubation periods and attend incubating females (Munro and Bedard 1977a; Schmutz et al., 1982; HG Gilchrist, unpublished data). Nonbreeders or failed breeders will often attend incubating females at the nest just prior to hatch (usually 24 h prior) (Munro and Bedard 1977a; Schmutz et al. 1983; Bustnes and Erikstad 1991) and subsequently escort the brood from the nest site to the marine habitats.
The presence of failed breeders or nonbreeders in departing groups might be explained by female nest site selection, prolonged female offspring or sibling bonds, female philopatry to natal nesting areas, or combinations of these. For example, when nesting attempts fail, female eiders often depart the breeding area to recover nutritional reserves (Gorman and Milne 1972) but may return to attend broods in the same area of their failed nesting attempt as opposed to a different area in the colony. The adaptiveness of attending departing groups with relatives for a failed or nonbreeder could be explained by kin selection if duckling survival was enhanced by this attendance. Campbell (1975) presents evidence suggesting that the time period between hatching and departure from the nest (24 h), not the departure itself, is the period of greatest predation risk for ducklings (Campbell 1975). At the East Bay eider colony, attending females, on average, tend to arrive at the nests of incubating females 1.86 days before departure, precisely during this critical hatching period. Their presence at the nest may decrease predation pressure because we have observed attending females defending eider nests against herring gull attacks. However, we recognize that despite evidence of vigilant behavior by failed breeders or nonbreeders during predation attacks on ducklings (Schmutz et al. 1982), increases in duckling survival due to these attending females remain unconfirmed (Ahlen and Andersson 1970; Campbell 1975).
The size, degree of isolation, and spatial contiguity of nesting habitat characterizing breeding colonies may also affect the extent to which philopatry can influence the kin composition of departing female groups. On relatively large, isolated breeding colonies such as our site, breeding habitat is contiguous and philopatry is often strong (Baillie and Milne 1989; Bustnes and Erikstad 1993) and may be maintained via isolation. However, common eider females may also nest in colonies on archipelagos or other disjunct habitats where breeding sites are widely dispersed (Goudie et al. 2000) and where the composition (social or familial) of contiguously nesting groups that are available to form coalitions may vary. Öst et al. (2005) collected genetic data on 24 coalitions of female common eiders from 18 nesting islands in an archipelago in the Baltic sea and concluded that common eider females formed brood-rearing coalitions composed of unrelated individuals. Despite high levels of female philopatry in the archipelago, Öst et al. (2005) noted that the spatial dispersion of nesting females across many small islands may have affected the kin composition of coalitions.
Intense social interactions between common eider females begin during the first few days after hatch (Öst and Kilpi 2000). By capturing females and ducklings in broods during departure from the island itself and prior to the formation of stable creches (as studied in Ost et al. 2005), we have generated the first genetic information confirming kin relationships between females early in coalition formation. Although there was the potential for biases in composition of groups captured due to the nature of the walk-in traps and the varying cohesiveness of departing groups, the ratio of ducklings to females in captured broods (3.72:1) was consistent with those recorded for naturally occurring departing broods in other studies (3.5:1, Gorman and Milne 1972; 2.1–3.1:1, Swennen 1989; 5:1, Öst 1999).
CONCLUSIONS
Throughout each of several stages, colony arrival, nesting, and brood rearing, we have provided evidence of sociality and kin groups among common eiders. Though we have not measured direct or indirect associated fitness benefits, previous avian studies have indicated possible fitness benefits of sociality and kin groups during migration (Alerstam 1990), nesting (van der Jeugd et al. 2002), and brood amalgamation (Munro and Bedard 1977b; Öst et al. 2002). Benefits of prolonged female bonds in common eiders might include an increase in individual adult survival due to increased group size. For example, larger group size on the wintering grounds could increase survival via thermoregulatory benefits during roosting (Gilchrist and Robertson 2000) and/or by lowering predation risk by vigilance and dilution effects. However, indirect benefits may be reduced via increased competition between relatives (Griffin and West 2002).
Here we provide genetic evidence for kin-based female social structure in common eiders throughout the breeding season. Though the selective consequences of these social groups are still unknown, the genetic data presented here suggest that kin selection is a potential hypothesis to test the adaptiveness of these social behaviors in the common eider. Further research directed at measuring fitness benefits of sociality in common eiders and other anseriformes could provide important insight into the benefits and evolution of sociality among these species.
Funding for this project was provided by the Department of Zoology and the Department of Fisheries and Wildlife at Michigan State University and grants from the Dr Marvin Hensley Foundation and the George and Martha Wallace Foundation through Michigan State University. Fieldwork was funded by the Canadian Wildlife Service, Environment Canada. Additional logistical support was provided by Polar Continental Shelf Project (Natural Resources Canada) and the Nunavut Research Institute. We thank all those who participated in fieldwork especially H. Jewell, D. McCruer, C. Fournier, K. McKay, J. Bety, C. Anderson, P. Fast, M. Fast, and K. Allard. Invaluable laboratory assistance was provided by S. Libants, K. Filcek, L.Garzel, B. Williams, and J. McGuire. We also thank C. Lindell, H. Prince, and K. Holekamp for their comments on the manuscript. Special thanks to the Hunter's and Trapper's Association and community members of Coral Harbour, Nunavut, for supporting the research conducted at the East Bay Migratory Sanctuary, specifically Joe Nakoolak for the essential transportation to Coral Harbour.
References
Ahlen I, Andersson A.
Andersson M, Åhlund M.
Anderson MG, Rhymer JM, Rohwer FC.
Baille SR, Milne H.
Black JM, Owen M.
Boos JD, Nudds TD, Sjoberg K.
Bottita G, Nol E, Gilchrist HG.
Buchholz WG, Pearce JM, Pierson BJ, Scribner, KT.
Burland TM, Barratt EM, Nichols RA, Racey PA.
Bustnes JO, Erikstad KE.
Bustnes JO, Erikstad KE.
Bustnes JO, Erikstad KE, Bjorn TH.
Campbell LH.
Cezilly F, Tourenq C, Johnson A.
Coltman DW, Pilkington JG, Pemberton JM.
Cooch FG.
Cooke F, Robertson GJ, Smith C.
Cooper JM, Miller EH.
Coulson JC.
Danforth BN, Freeman-Gallant CR.
Dieringer D, Schlotterer C.
Eadie JM, Kehoe FP, Nudds T.
Eadie JC, Lyon BE.
Fields RL, Scribner KT.
Friesen VL, Montevecchi WA, Gaston AJ, Barrett RT, Davidson WS.
Gilchrist HG, Robertson GJ.
Goodnight KF, Queller DC.
Goudie KI, Robertson GJ, Reed A.
Griffin AS, West SA.
Hario M, Hollmén TE, Morelli TL, Scribner KT.
Hoffman JI, Amos W.
Kilpi M, Lindström K.
Komdeur J, Hatchwell GJ.
Maak S, Wimmers K, Weigend S, Neumanns K.
McKinnon L.
Moreno J, Barbosa A, Potti J, Merino S.
Munro J, Bedard J.
Osorio-Beristain M, Drummond H.
Öst M.
Öst M, Kilpi M.
Öst M, Mantila L, Kilpi M.
Öst M, Vitikainen E, Waldeck P, Sundrström L, Lindström K, Hollmen T, Franson CJ, Kilpi M.
Öst M, Ydenberg R, Kilpi M, Lindstrom K.
Patterson IJ, Gilboa A, Tozer DJ.
Paulus KB, Tiedemann R.
Pearce JM, Fields RL, Scribner KT.
Pope TR.
Raveling DG.
Ratnayeke S, Tuskan GA, Pelton MR.
Raymond M, Rousset F.
Reed A.
Regehr HM, Smith CM, Arquilla B, Cooke F.
Robertson GJ.
Savard JPL, Reed A, Lesage L.
Schjorring S.
Schmutz JK, Robertson RJ, Cooke F.
Schmutz JK, Robertson RJ, Cooke F.
Schoech SJ, Mumme RL, Wingfield JC.
Scott DK.
Shields WM.
Shields WM.
Sterk EHM, Watts DP, van Schaik CP.
Swennen C.
van der Jeugd JP, van der Veen IG, Larsson K.
Wakeley JS, Mendall HL.
Warhurst RA, Bookhout TA.
Warren SM, Fox AD, O'Sullivan P.
Author notes
aDepartment of Zoology, Michigan State University, 13 Natural Resources Building, East Lansing, MI 48824-1222, USA
bCanadian Wildlife Service, National Wildlife Research Centre, Carleton University, 1125 Colonel By Drive, Raven Road, Ottawa, ON K1A 0H3, Canada
cDepartment of Fisheries and Wildlife, Michigan State University, 13 Natural Resources Building, East Lansing, MI 48824-1222, USA