-
PDF
- Split View
-
Views
-
Cite
Cite
Troy G. Murphy, Predator-elicited visual signal: why the turquoise-browed motmot wag-displays its racketed tail, Behavioral Ecology, Volume 17, Issue 4, July/August 2006, Pages 547–553, https://doi.org/10.1093/beheco/arj064
Close - Share Icon Share
Abstract
Both sexes of the turquoise-browed motmot (Eumomota superciliosa) perform a wag-display in the presence of predators, whereby their long racketed tail is repeatedly rocked side-to-side in a pendulous fashion. I tested 3 hypotheses for the function of the predator-elicited wag-display: 1) pursuit-deterrent signal, 2) warning alarm signal, and 3) self-preservation alarm signal. These hypotheses were evaluated by testing whether the presence of potential receivers (kin, conspecifics, mate) modified the way in which the wag-display was performed. Data on wag-display were collected when I experimentally presented predators to motmots and when naturally occurring predators were observed at nesting colonies. The wag-display was performed by male and female motmots who were 1) alone and not within signaling distance of conspecifics, 2) unpaired and therefore not signaling to a mate, and 3) paired but away from their mate. Motmots in these contexts performed the wag-display with similar probability and in a similar manner as individuals that were within signaling distance of conspecifics, paired birds, and paired birds who were near their mate. These results support the hypothesis that the predator-elicited wag-display is directed to the predator and functions as a pursuit-deterrent signal.
Many species perform behavioral displays when they detect predators (Cott 1940), yet the function of predator-elicited signals at first seems paradoxical. Why would an individual risk drawing attention to itself in the presence of a predator? Broadcasting one's location is especially dangerous if the signaler does not have complete information on the location of all nearby predators, as unknown predators could take advantage of the signal information and catch the signaler unaware (Bergstrom and Lachmann 2001).
Predator-elicited display can be directed to the predator and function as a pursuit-deterrent signal. Pursuit-deterrent signals represent a form of interspecific communication, whereby the prey indicates to a predator that pursuit would be unprofitable because the signaler is prepared to escape (Woodland et al. 1980). Pursuit-deterrent signals provide a benefit to both the signaler and receiver; they prevent the sender from wasting time and energy fleeing, and they prevent the receiver from investing in a costly pursuit that is unlikely to result in capture. Such signals can advertise prey's ability to escape, and reflect phenotypic condition (quality advertisement, sensu Zahavi 1977; also see Hasson 1991), or can advertise that the prey has detected the predator (perception advertisement, sensu Woodland et al. 1980). Pursuit-deterrent signals have been reported for a wide variety of taxa, including fish (Godin and Davis 1995), lizards (Cooper et al. 2004), ungulates (Caro 1995), rabbits (Holley 1993), primates (Zuberbühler et al. 1997), rodents (Shelley and Blumstein 2005), and birds (Alvarez 1993).
Predator-elicited display can also be directed to conspecifics and communicate alarm. Alarm signals can warn conspecifics of danger (warning alarm signal) and confer benefits to the signaler if receivers are related (Hamilton 1964), if they reciprocate (Trivers 1971), or if the receiver is a mate (Morton and Shalter 1977). Due to costs associated with drawing attention to oneself, warning alarm signals are typically performed only in the presence of intended receivers (Caro 1986). Such receiver discrimination occurs in many social species (Hoogland 1983, 1996; Sullivan 1985; Blumstein et al. 1997; Griesser and Ekman 2004), and in some cases, warning alarm signals are modulated depending on the degree of relatedness between the sender and particular receivers (Sherman 1977, 1985). Alternatively, alarm signals can reduce the signaler's predation risk (self-preservation alarm signal) if conspecifics group around the signaler (Hamilton 1971; Cresswell 1994a), mob the predator (Curio 1978), or are manipulated into fleeing toward the predator (Charnov and Krebs 1975).
The turquoise-browed motmot (Eumomota superciliosa), a colonially breeding neotropical bird, displays its tail in an exaggerated pendulum-like fashion (wag-display) (Snow 2001). The signal value of the motmot's wag-display has been the subject of speculation but has not yet been systematically investigated. Wagner (1950) noted that motmots invariably kept their tails still when unaware of his presence and then began to wag-display as soon as he attracted their attention, suggesting an antipredator function for the display. Likewise, Snow (2001) speculated that the wag-display serves some communicative function, while others have suggested cognitive mechanisms underlying the display, including “excitement” (Skutch 1964), “alarm” (Smith 1983), “uneasiness” (Fjeldså and Krabbe 1990), and “disturbance” (Ridgely and Greenfield 2001).
During the wag-display, the motmot's tail is rocked side-to-side, similar to the regular motion of a pendulum: the tail is first cocked to approximately 50 degrees to one side of the body, where it pauses briefly before being quickly swung to the other side, in total describing an arc of approximately 100 degrees. The side-to-side motion is repeated many times during a display, and due to its recurring nature, the tail movement commonly draws attention to an otherwise hidden bird. Indeed, nearly 100 years ago, Beebe (1910) noted, “It (the motmot) would be thoroughly protected on its perch among green foliage were it not for the constant and violent jerking of the closed tail from side to side … This movement, accentuated by the large isolated rackets, calls instant attention to the bird as one looks in its direction.”
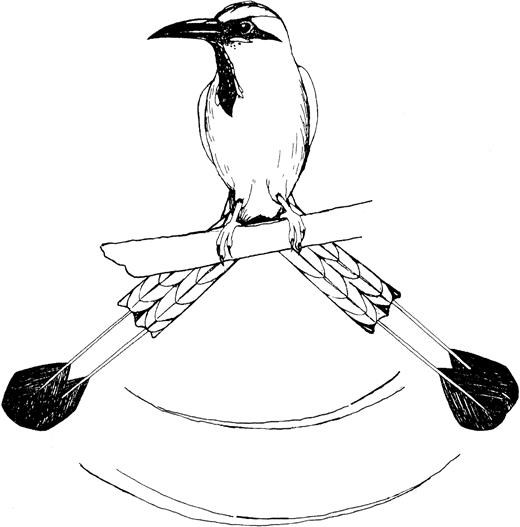
The central 2 tail feathers of the turquoise-browed motmot are long in both sexes, comprising approximately 60 % of the overall length of the bird, and they are strikingly patterned blue and black (Murphy 2005). There are 2 large rackets at the tip of the tail, which appear to hang, unattached, below the rest of the tail (Figure 1). The apparent detachment is caused by the wearing off of weakly attached vanes along the medial rachis of the 2 elongate central rectrices (Beebe 1910). The vanes of the tip of the racket are substantially wider than the other vanes on the same feather, which, in combination with the denuded feather shaft and striking coloration, augments the optical effects of the tail movements (Sick 1985).
Wag-display of the racket-tipped tail of the turquoise-browed motmot.
Based on previous reports and on my own observations that the wag-display is performed in the presence of predators, I propose 3 nonmutually exclusive hypotheses to address the function of the wag-display. These hypotheses fall into 2 categories based on the potential receivers of the signal: predators or conspecifics.
Hypotheses and predictions
Hypothesis 1: pursuit-deterrent signal. If the motmot's wag-display is directed toward the predator, it is predicted that on detecting a predator 1) the wag-display will be performed in the presence and the absence of conspecifics and 2) that the wag-display will not vary in the way it is performed when conspecifics are present or absent. Hypothesis 2: warning alarm signal. If the motmot's wag-display is directed to conspecifics, it is predicted that 1) on detecting a predator the wag-display will be performed only when appropriate conspecific receivers are present (kin, conspecifics, mate) and will not be performed in the absence of conspecifics and that 2) if the mate is the intended receiver (i.e., if other potential receivers are excluded as possibilities), unpaired birds should not perform the wag-display. Hypothesis 3: self-preservation alarm signal. This hypothesis predicts that on detecting a predator and performing a wag-display, conspecifics will 1) move closer together (group) or 2) move closer to the predator (mob or flee toward predator).
I tested these 3 hypotheses by recording the responses of turquoise-browed motmots when they encountered natural predators and when they were experimentally presented with a feral cat and a human. Specifically, I tested the prediction that the presence or absence of potential receivers would affect the probability of performing the predator-elicited wag-display or the manner in which the display was performed.
MATERIALS AND METHODS
Study organism
The turquoise-browed motmot is a socially monogamous insectivore that nests in tunnels built in earthen banks (0.4–2.2 m in depth, mean = 1.3 m). The species breeds colonially in the Yucatan Peninsula, and colonies are located in the walls of sinkholes, freshwater wells, limestone quarries, and ancient man-made structures (e.g., Maya ruins; Scott and Martin 1983). Colony size ranges from 2 to 60 pairs, with colonies of 10–20 pairs being most common (Orejuela 1977; Murphy 2005). The species is migratory, and pairs arrive at breeding colonies in March approximately 3 months before clutch initiation. During the prelaying period, the birds spend mornings at the colony renovating and defending tunnel nests. After the rainy season begins (May–June), activity levels increase at the colony, and motmots defend nest sites throughout the day. Both males and females incubate, brood, and provision nestlings. Pairs also defend off-colony foraging and roosting territories, located up to 2 km from the colony. Pairs forage and roost on off-colony territories throughout the breeding season, except during incubation and early-stage brooding, when the female alone incubates or broods at night.
Study area and general methods
I studied turquoise-browed motmots during the 1999–2002 breeding seasons (March–August) in the thorn-scrub forest near the Ria Lagartos Biosphere Reserve in northern Yucatan, Mexico (21°33′N, 88°05′W). I studied 4 colonies located in abandoned limestone quarries (range 7–39 pairs), and 3 colonies located in freshwater wells (approximate range 20–30 pairs). To facilitate individual identification, individuals were marked with color bands. Approximately 98% of all breeders and approximately 85% of nonbreeding floaters were banded. In the final year of study, I observed 488 banded motmots at the 7 colonies.
During approximately 9100 observation hours at 7 colonies, my research team collected data on wag-displays when motmots encountered natural predators. In 2002, I experimentally presented a feral cat or a human to motmots at colonies located in limestone quarries. Observations were conducted with spotting scopes from within permanent blinds located 45–55 m from the colony. Predator-presentation trials were video taped for later analysis, and monitoring of multiple focal individuals was facilitated by simultaneous recording of behavior by 2 observers with spotting scopes. To minimize human disturbance, observers entered blinds before sunrise while motmots were away from the colony (likely on their off-colony territories).
Encounters with natural predators
When a potential predator or other large animal was observed at a colony, I recorded the species and whether any motmots at the colony performed a wag-display. To further establish if the wag-display was tied to the presence of a potential predator, I recorded the time between the departure of the animal from the colony and the termination of wag-displays by one focal individual under observation (n = 18 individuals, each on a separate day).
Predator-presentation experiment
Predator-presentation experiments were originally conducted by presenting a feral cat to motmots. Before sunrise, I placed a cat, enclosed within a cage, 10 m in front of the colony face. The cage was divided into 2 parts: a small compartment was covered with an opaque cloth that prevented the motmots from seeing inside, and this opened into an uncovered larger compartment via a remote-controlled door. After motmots arrived at the colony in the morning, I collected 10 min of baseline data. The baseline survey was divided into 1-min intervals, and for each interval, I noted if any bird at the colony performed the wag-display. I then opened the remote-controlled door so that the cat emerged, and continued to collect data for 10 min. Data were collected in the same way by scoring each minute interval for the presence or absence of wag-display across the entire colony. I performed the experiment with the cat once, and the reactions of 11 motmots were collected.
Because the feral cat proved difficult to work with, as an alternative, I used a human as a simulated predator. The human emerged from a blind located approximately 80 m from the colony and slowly walked toward the colony face. Before the human emerged, I collected 10 min of baseline data by visually scanning the area around the colony, including all trees and perches within 50 m of the colony face. The baseline survey was divided into 1-min intervals, and for each interval, I noted if any motmot at the colony performed the wag-display. In the second half of the experiment, after the human emerged, I continued to collect data in the same way by scoring each minute interval for the presence or absence of wag-display by any individual across the entire colony. I collected data until all birds were flushed away from the colony or until 10 min had elapsed after human emergence (trial length after emergence of human: mean = 7.7 min, range = 4.0–10.0 min). A human was presented 14 times on separate days, and experiments were divided among 3 colonies.
To establish if the wag-display conveyed information about immediacy or level of threat, I tested whether the intensity of wagging changed as the human approached the colony. I quantified the intensity of wagging (number of side-to-side wags of the tail per minute) performed by one individual per trial, over 10 trials, and correlated the average number of wags with the distance to the approaching human.
Test between hypotheses 1 and 2: pursuit-deterrent signal verses warning alarm signal (receivers: kin or conspecifics)
To test the prediction that the wag-display would be performed in the absence of conspecifics, I monitored whether individuals performed the wag-display when a human appeared in 3 locations where conspecifics (other than the mate) were unlikely to be present: 1) at off-colony territories, where only one pair foraged and roosted; 2) at noncolonial nest sites in Yucatan, Mexico, where single nests were separated by at least 100 m; and 3) away from the breeding colonies during the nonbreeding season (November). In each of these circumstances, I recorded whether the focal bird performed the wag-display when I approached it and whether potential conspecific receivers were observed. Note that by testing this prediction, I concurrently addressed the prediction of “hypothesis 2” that the wag-display would only be performed in the presence of kin or conspecifics.
Test of hypothesis 2: warning alarm signal (receiver: mate)
To test if the mate is the likely receiver of the signal, I observed wag-behavior of 3 categories of birds at the colonies during 14 human-presentation trials. First, I determined if unpaired floaters without a mate performed the wag-display. Second, I compared behavior of paired and unpaired birds. Third, I compared behavior of paired birds whose mates were either present or away from the colony. For the latter 2 comparisons, I compared the probability of performing the wag-display and the intensity of wagging for each category of bird. Probability of performing the display was computed as the number of individuals that performed a wag-display divided by the total number of individuals observed of each type (paired or unpaired; mate present or away). The intensity of wagging was computed as the average number of side-to-side wags of the tail over a 1-min period (standardized for the amount of time each individual was under observation). Data were collected by simultaneously following 1–5 individuals (with a video camera), and birds were followed for as long as they remained on the colony or for a maximum of 10 min.
Test of hypothesis 3: self-preservation alarm signal
To determine if motmots react to the wag-display by grouping, mobbing, or fleeing toward a predator, I monitored the reaction of conspecifics to wag-displays during the 14 human-presentation trials. To test if motmots group, I chose 2 focal birds within a 10 m2 area and monitored the distance between them just before the human emerged and then again 2.5 min after the human emerged. By waiting 2.5 min, this ensured that the birds observed the human and any conspecific wag-display, yet was not long enough that the focal birds left the colony. In 7 of the trials, both focal birds remained at the colony 2.5 min after the trial begun.
To test if motmots mob or flee toward the predator, I monitored whether individuals flew toward the human. I randomly chose one individual and monitored it for 10 min after the emergence of the predator, noting whether the individual moved, even slightly, toward the human.
Statistical analysis
Nonparametric statistics (Kruskal–Wallis, Fisher's Exact, Spearman's rho) were used to analyze data. Values are reported as mean ± standard error, unless otherwise noted as standard deviation (SD). All probabilities are two tailed.
RESULTS
General description
The tail was generally moved side-to-side multiple times within a bout of wag-display, and bouts were generally repeated, after short pauses (4.7 s ± 3.5 (SD), n = 20 individuals), for the entire period a predator (human or cat) was present. The mean number of side-to-side wags within each bout did not differ between the sexes (during human-presentation trials—male: 4.2 ± 1.9 (SD), n = 21; female: 4.5 ± 2.4 (SD), n = 12; Kruskal–Wallis: χ2 = 0.01, P = 0.91, n = 33), and there was not a significant sexual difference in probability of performing the wag-display (during human-presentation trials—male: 71% (15/21); female: 71% (10/14); Fisher's Exact: P = 0.99, n = 35). At the beginning of most bouts the tail was raised above the head as it simultaneously swung side-to-side, causing the tail to trace a pattern resembling the letter “Z” on both its upward and downward trajectory.
Most predator-elicited wag-displays (71% [15/21]) were accompanied by a clucking vocalization. The call is easily localizable because 1) of its high amplitude, 2) it is repeated on and off for long periods (up to many minutes), and 3) the call structure has signal design characteristics of a localizable signal, with a full spectrum up to 10 kHz and a short pulse duration (Klump and Shalter 1984; Bradbury and Vehrencamp 1998).
There was not a significant difference in the mean number of side-to-side wags within each bout during different parts of the breeding season (prenestling stage compared with postnestling stage; Kruskal–Wallis: χ2 = 0.04, P = 0.84, n = 32) or between the breeding and nonbreeding seasons (Kruskal–Wallis: χ2 = 0.28, P = 0.60, n = 45).
Encounters with natural predators
Motmots generally performed the wag-display when potential predators approached the colony but did not wag-display in the presence of every type of animal. Six types of potential predators elicited the wag-display at the colony, and all were potential predators on adult motmots and were close enough to see the wag-display being performed (Table 1). Three other types of potential predators and 2 types of nonthreatening animals were observed at the colony that never elicited the wag-display (Table 1).
Wag-display was performed in response to most potential predators but not to all types of animals
. | Occasions wag-display was performed . | Occasions wag-display was not performed . |
|---|---|---|
| Animals eliciting wag-display | ||
| Feral cats | 7 | 0 |
| Feral dogs | 7 | 8 |
| Gray foxes (Urocyon cinereoargenteus) | 4 | 0 |
| Coatimundis (Nasua narica) | 2 | 3 |
| Perched birds of prey | 17 | 5 |
| Humans | >100 | 0 |
| Animals not eliciting wag-display | ||
| Snakes | 0 | 12 |
| Black iguanas (Ctenosaura similis) | 0 | >100 |
| Flying birds of prey | 0 | >50 |
| Domestic cattle | 0 | 3 |
| Eastern cottontails (Sylvilagus floridanus) | 0 | 7 |
. | Occasions wag-display was performed . | Occasions wag-display was not performed . |
|---|---|---|
| Animals eliciting wag-display | ||
| Feral cats | 7 | 0 |
| Feral dogs | 7 | 8 |
| Gray foxes (Urocyon cinereoargenteus) | 4 | 0 |
| Coatimundis (Nasua narica) | 2 | 3 |
| Perched birds of prey | 17 | 5 |
| Humans | >100 | 0 |
| Animals not eliciting wag-display | ||
| Snakes | 0 | 12 |
| Black iguanas (Ctenosaura similis) | 0 | >100 |
| Flying birds of prey | 0 | >50 |
| Domestic cattle | 0 | 3 |
| Eastern cottontails (Sylvilagus floridanus) | 0 | 7 |
Wag-display was performed in response to most potential predators but not to all types of animals
. | Occasions wag-display was performed . | Occasions wag-display was not performed . |
|---|---|---|
| Animals eliciting wag-display | ||
| Feral cats | 7 | 0 |
| Feral dogs | 7 | 8 |
| Gray foxes (Urocyon cinereoargenteus) | 4 | 0 |
| Coatimundis (Nasua narica) | 2 | 3 |
| Perched birds of prey | 17 | 5 |
| Humans | >100 | 0 |
| Animals not eliciting wag-display | ||
| Snakes | 0 | 12 |
| Black iguanas (Ctenosaura similis) | 0 | >100 |
| Flying birds of prey | 0 | >50 |
| Domestic cattle | 0 | 3 |
| Eastern cottontails (Sylvilagus floridanus) | 0 | 7 |
. | Occasions wag-display was performed . | Occasions wag-display was not performed . |
|---|---|---|
| Animals eliciting wag-display | ||
| Feral cats | 7 | 0 |
| Feral dogs | 7 | 8 |
| Gray foxes (Urocyon cinereoargenteus) | 4 | 0 |
| Coatimundis (Nasua narica) | 2 | 3 |
| Perched birds of prey | 17 | 5 |
| Humans | >100 | 0 |
| Animals not eliciting wag-display | ||
| Snakes | 0 | 12 |
| Black iguanas (Ctenosaura similis) | 0 | >100 |
| Flying birds of prey | 0 | >50 |
| Domestic cattle | 0 | 3 |
| Eastern cottontails (Sylvilagus floridanus) | 0 | 7 |
When a potential predator that had elicited the wag-display departed the colony (was out of view from the observer), 72% (13/18) of the focal motmots stopped performing the wag-display within 1 min, and the remaining 28% stopped within 3 min.
Predator-presentation experiment
There was not a significant difference in the probability of performing the wag-display when presented with a feral cat, 73% (8/11 birds) (one experiment), or a human, 71% (32/45 birds) (14 experiments) (Fisher's Exact: P = 0.99, n = 56). There was not a significant difference in the intensity of wagging performed when a feral cat or a human was presented (wags per minute—cat: 9.9 ± 2.2, n = 8; human: 10.1 ± 1.6, n = 32; Kruskal–Wallis: χ2 = 0.15, P = 0.70, n = 40).
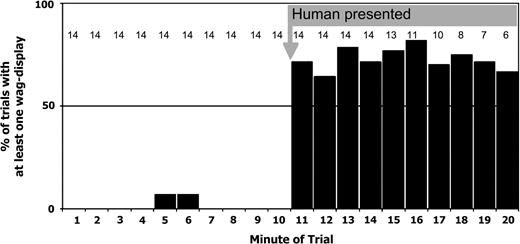
During the 14 human-presentation experiments, motmots rarely performed the wag-display during the 10-min period before the human emerged from the blind; the wag-display was performed during 2 of 140 (<2%) observation minutes. The occurrence of wag-display increased dramatically when a human emerged from hiding. Thereafter, at least one motmot performed the wag-display during 73.0% (81/111) of observation minutes over 14 trials (baseline vs. when human visible—Fisher's Exact: P ≤ 0.0001, n = 251; Figure 2).
The occurrence of wag-display increased when a human was experimentally presented to a colony of motmots. Ten minutes of baseline data were collected before a human emerged from hiding. The human emerged after minute 10, and data were collected for 10 min or until all motmots had left the colony. Fourteen human-presentation trials were conducted. The gray numbers along the top of the graph denote the sample size (number of trials where ≥1 bird was at colony) for each 1-min interval.
The intensity of wagging (wags per minute) did not significantly change with distance between the human and the focal individual performing the wag-display (Spearman's rho: 0.49, P = 0.15, n = 10).
Test between hypotheses 1 and 2: pursuit-deterrent signal verses warning alarm signal (receivers: kin or conspecifics)
When I approached motmots at each of the 3 locations where they were unlikely to be associating with conspecifics (except possibly the mate), they generally responded by performing the wag-display and were generally outside of signaling distance of observed conspecifics. At off-colony territories, 87% (27/31) of the individuals who performed the wag-display were not near other observed motmots. At isolated noncolonial nest sites, 100% (10/10) of individuals performed the wag-display when approached, and in each case, no other motmots were observed in the vicinity. During the nonbreeding season, 75% (12/16) of individuals performed the wag-display when approached, and no other motmots were observed in the vicinity. Thus, motmots wag-display in the absence of apparent conspecific receivers. The probability of performing the wag-display in these 3 solitary circumstances did not differ significantly from the probability of performing the wag-display during human-presentation trials at the colony (71%, 32/45) (Fisher's Exact: P > 0.05 in all comparisons).
Test of hypothesis 2: warning alarm signal (receiver: mate)
Unpaired birds
Unpaired birds were observed performing the wag-display during human-presentation trials at the colony; in total 7 unpaired individuals performed the wag-display.
Paired versus unpaired birds
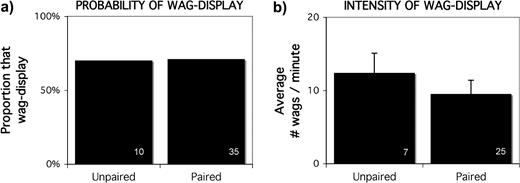
There was not a significant difference in the probability of performing the wag-display by unpaired and paired individuals. During 14 human-presentation trials, 70% (7/10) of unpaired birds performed the wag-display and 71% (25/35) of paired birds performed the wag-display (Fisher's Exact: P = 0.99, n = 45; Figure 3a). There was not a significant difference in the intensity of wagging performed by unpaired and paired individuals (wags per minute—unpaired: 12.4 ± 2.7, n = 7; paired: 9.5 ± 1.9, n = 25; Kruskal–Wallis: χ2 = 2.3319, P = 0.1267, n = 32; Figure 3b).
When a human was experimentally presented to a colony of motmots, paired status was not related to (a) the probability of performing the wag-display or (b) the intensity of wagging. Sample size is shown in the lower right of each bar.
Paired and away from mate versus paired and near mate
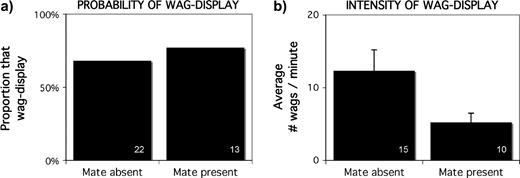
There was not a significant difference in the probability of performing the wag-display by paired birds that were either away from or near their mate: the wag-display was performed by 68% (15/22) of individuals that were away from their mate and 77% (10/13) of individuals that were near their mate (Fisher's Exact: P = 0.71, n = 35) (Figure 4a). Contrary to the prediction, there was a tendency for paired individuals to wag-display with greater intensity when they were away from their mate (wags per minute—away from mate: 12.3 ± 2.9, n = 15; near their mate: 5.2 ± 1.3, n = 10; Kruskal–Wallis: χ2 = 2.96, P = 0.09, n = 25; Figure 4b).
When a human was experimentally presented to a colony of motmots, the presence or absence of an individual's mate was not related to (a) the probability of performing the wag-display or (b) the intensity of wagging. Sample size is shown in the lower right of each bar.
Test of hypothesis 3: self-preservation alarm signal
Motmots did not move significantly closer to one another after the appearance of the human (Intermotmot distance 1 min before predator emergence: 3.2 ± 0.91 m; 2.5 min after predator emergence: 3.4 ± 0.80 m; Wilcoxon signed-rank: P = 0.99, n = 7). They also did not mob or flee toward the predator during the human-presentation trials: during the 10 min after the human emerged, the focal motmot either stayed where it was or moved away from the predator in 93% (13/14) of the trials, and only one individual was observed to move, even slightly, toward the predator, 7% (1/14) (Fisher's Exact: P = 0.99, n = 14).
DISCUSSION
When the turquoise-browed motmot encounters a predator, it reacts in a predictable and stereotypical manner by performing the wag-display. There are 4 lines of evidence that link the presence of a predator to the wag-display: 1) when no predators are present, the wag-display is rarely performed; 2) when a predator is experimentally presented, motmots immediately begin to display; 3) while a predator is present, the wag-display is repeatedly performed; and 4) when a predator departs (as observed with natural predators), motmots stop performing the wag-display.
For predator-elicited communication to be maintained by selection, the benefit associated with the signal must outweigh the costs associated with drawing attention to oneself. The motmot's wag-display is likely to incur considerable costs because it is easy to detect and locate. High detectability and localizability arise because: 1) the display involves repeated and exaggerated movements, 2) the display involves flashing of conspicuous colors, and 3) it is accompanied by a high-amplitude clucking call, which bears the vocal signal design of a localizable signal (Bradbury and Vehrencamp 1998). Taken together, the visual and vocal components of the wag-display appear to be designed to draw the attention of the predator to the signaler. In fact, the ease with which one is able to detect and locate the wag-display is supported by the observation made by many naturalists that the wag-display draws attention to an otherwise hidden bird (Fjeldså and Krabbe 1990; Hilty 2003; Jones 2003).
When a predator is detected, the wag-display is performed by both sexes with similar probability and with a similar number of side-to-side wags of the tail. In addition, the wag-display is performed throughout the long breeding season, during the nonbreeding season on the wintering grounds, at both colonial and solitary nesting sites, and away from the colony on off-colony territories. In all locations and at all times of year the wag-display is performed in a similar manner. These results suggest that the signal value of the wag-display is similar for both sexes and that the signal value does not change in different locations or seasons.
Evidence is most consistent with the hypothesis that the intended recipient of the wag-display is the predator and that the display functions as a pursuit-deterrent signal. When a human approached a motmot away from the colony, the bird generally performed the wag-display regardless of the presence of potential conspecific receivers. Specifically, motmots performed the wag-display in 3 locations where it was unlikely that conspecifics (other than the mate) were nearby: 1) at off-colony territories where only mated pairs forage and roost and other conspecifics rarely pass through, 2) at noncolonial nest sites where nests were separated by at least 100 m and individuals from different nests seldom interact, and 3) away from the breeding colonies during the nonbreeding season when these birds no longer are gregarious. In further support of the hypothesis that the intended recipients of the display are not conspecifics, the probability and intensity of the wag-display performed by lone birds in these 3 locations were not different from wag-displays performed when birds were near conspecifics at the colony. In addition, Skutch's (1947) observation that the wag-display is performed by turquoise-browed motmots in the southern subspecies, which are noncolonial, supports the hypothesis that the display is not directed to kin or to nonmate conspecifics.
The possibility that the predator-elicited wag-display functions to warn mates was not supported. The wag-display was performed by unpaired birds, which do not gain a selective advantage from warning a mate. Furthermore, unpaired birds were similarly likely to perform the wag-display and displayed at the same intensity as paired birds. Also, paired birds who were away from their mate were similarly likely to perform the wag-display and displayed at the similar intensity as paired birds who were near their mate.
The self-preservation alarm signal hypothesis was also not supported. When a human approached a colony of motmots, the resulting wag-display did not cause conspecifics to move closer to one another (i.e., group) or to move closer to the predator (i.e., mob or flee toward predator). These results are further supported by behavioral observations when natural predators arrived at the colony: no mobbing, grouping, or fleeing toward the predator was observed.
The wag-display fulfils the signal design criteria of a pursuit-deterrent signal because it is easy to locate, which is in sharp contrast to the design features of many warning alarm signals, which reduce localizability (Marler 1955). Indeed, alarm signaling within a visual modality is not likely in this species because motmots are often widely distributed among thick vegetation, and it is doubtful that conspecific receivers could reliably detect visual signals (sensu Woodland et al. 1980).
It is worth noting that the wag-display is performed in a second context: during a short (4 week) period of the breeding cycle, male and female motmots occasionally perform the wag-display immediately before delivering food to nestlings. Such wag-displays are performed before approximately 20% of feedings performed without the clucking vocalization, and performed in the absence of apparent predators (Murphy 2005). In this context, the wag-display may be performed due to a lowered response threshold to threatening stimuli during the dangerous nestling period (for discussion, see Murphy 2005).
Many species perform pursuit-deterrent signals in order to deter predators from ambush (artiodactyls, Caro et al. 2004; great gerbil (Rhombomys opiums), Randall et al. 2000; kangaroo rats (Dipodomys), Randall and Boltas King 2001; sciurids, Clark 2005), and in some cases pursuit-deterrent signals are selectively given only in the presence of predators who hunt by ambush (i.e., cats and birds of prey) and are not performed in the presence of predators that do not rely on stealth and ambush (Diana monkeys Cercopithecus Diana; Zuberbühler et al. 1997). This form of pursuit deterrent has been shown to be effective, and ambush predators abandon hunting when prey are aware of their presence (timber rattlesnakes, Crotalus horridus (Clark 2005); African lions, Panthera leo (Elliot et al. 1977); and tigers, Panthera tigris (Schaller 1967).
Although pursuit-deterrent signals have only been reported for a few avian species (Woodland et al. 1980; Alvarez 1993; Cresswell 1994b; Spitznagel 1996; Laiolo et al. 2004), they may be common in avian species like motmots, which are frequently preyed on by ambush predators such as bird hawks, foxes, and small cats. Because the turquoise-browed motmot is a rather large and slow flying bird, 2 life-history characters make this species especially susceptible to ambush predators: 1) motmots place their tunnel nest near or on the ground and 2) motmots commonly forage on the ground and restrict their foraging attempts to small areas, frequently using the same perch between repeated sallies. As a result, motmots make many repeated movements in small areas, which may make them especially susceptible to predators that lie in wait where they anticipate their prey to occur. Because ambush predators rely on being hidden or undetected while hunting, a motmot's pursuit-deterrent signal could effectively dissuade such predators from attempting ambush. It is thus likely that the motmot's wag-display functions as a perception advertisement that communicates the bird's awareness of the predator and its preparedness to escape.
If the motmot's wag-display does inform ambush predators that they have been detected, it might be more appropriate to think of the wag-display as an ambush-deterrent, rather than a pursuit-deterrent signal. Although the data presented in this paper are consistent with the pursuit/ambush-deterrent hypothesis, to fully test this hypothesis it will be necessary to experimentally present natural predators with motmots who wag-display and who do not wag-display. I predict that mammalian and avian predators who rely on ambush will be less likely to attempt an ambush on a motmot that has been observed performing the wag-display.
This research was supported by American Museum of Natural History, American Ornithologists' Union, Andrew Mellon Foundation, Animal Behavior Society, Cornell Laboratory of Ornithology, Explorer's Club, Mario Enaudi Foundation, National Science Foundation (DDIG-0206584), Sigma Xi, and the Western Bird Banding Association. Stephen T. Emlen, Paul W. Sherman, Elizabeth Adkins-Regan, Hudson K. Reeve, and David W. Winkler, the Cornell Behavioral Lunch Bunch, and the Winkler Lab provided useful comments throughout the study, and their suggestions greatly improved the manuscript. Two anonymous reviewers and Rulon W. Clark provided helpful comments on the organization of the manuscript. I am especially thankful to all the field assistants who participated in the study: Nicole Murphy, Marcel Flores, Ismael Hau, Taxo Marfil, Tim Poole-DiSalvo, Wayne Hsu, Summer Names, Lara Fondow, Kiersten Cook, Benjamin Clock, Zachary Nelson, Andrew Rassweiler, Christopher Egan, Valerie Steen, Erin Macchia, Jennifer Smith. I would also like to thank Dan Rubin for his analysis of video footage, Bruce R. Land and Samuel Walcott for designing and building of a mot-bot that was worth a try, and Benjamin M. Clock for the scientific illustration. I am also indebted to technical support while in Mexico, provided by Barbara MacKinnon de Montes, Rodrigo Migoya von Bertrab, Mauricio Quijano Farjat, and Melgar J. Tabasco Contreras, the staff of Niños y Crías, and the staff of the Ria Lagartos Biosphere Reserve. This research was conducted under Cornell University's Institutional Animal Care and Use Committee protocol 99-23-02.
References
Bergstrom CT, Lachmann M.
Blumstein DT, Steinmetz J, Armitage KB, Daniel JC.
Bradbury JW, Vehrencamp SL.
Caro TM.
Caro TM, Graham CM, Stoner CJ, Vargas JK.
Clark RW.
Cooper WE, Perez-Mellado V, Baird TA, Caldwell JP, Vitt LJ.
Cresswell W.
Cresswell W.
Curio E.
Elliot JP, McTaggart Cowan I, Holling CS.
Godin JGJ, Davis SA.
Griesser M, Ekman A.
Hasson O.
Hoogland JL.
Klump GM, Shalter MD.
Laiolo P, Tella JL, Carrete M, Serrano D, Lopez G.
Morton ES, Shalter MD.
Murphy TG.
Orejuela JE.
Randall JA, Boltas King DK.
Randall JA, Rogovin KA, Shier DM.
Scott PE, Martin RF.
Shelley EL, Blumstein DT.
Sherman PW.
Smith SM.
Snow D.
Spitznagel A.
Sullivan K.
Woodland DJ, Jaafar Z, Knight ML.
Zahavi A.