-
PDF
- Split View
-
Views
-
Cite
Cite
Petra Wester, Regine Claßen-Bockhoff, Floral Diversity and Pollen Transfer Mechanisms in Bird-pollinated Salvia Species, Annals of Botany, Volume 100, Issue 2, August 2007, Pages 401–421, https://doi.org/10.1093/aob/mcm036
Close - Share Icon Share
Abstract
Bird-pollinated (ornithophilous) Salvia species (sages) transfer pollen either by means of a staminal lever mechanism or by immovable stamens. As the distribution of the two modes within the genus is not known, we present a survey of all ornithophilous sages. The main focus is given to floral diversity especially with respect to functional lever morphology. Thereby the hypothesis is tested that, due to a pollinator shift from bees to birds, the lever mechanism became unnecessary.
To get a general idea about the diversity of pollen transfer mechanisms, 186 ornithophilous Salvia species were classified according to the functional morphology of the stamen and the need for a lever movement. To test the functionality of the staminal levers and the fitting between flowers and birds the process of pollen transfer was examined by pollinator observations and tested by inserting museum skins and metal rods into fresh flowers.
The diversity of pollen transfer mechanisms is represented by eight case studies illustrating three main groups. In group I (approx. 50 %) the staminal lever mechanism is necessary to open access to nectar and to enable the transfer of pollen that is hidden in the upper lip. In group II (approx. 34 %) pollen is freely accessible and the lever mechanism is reduced in different ways and to different degrees. In group III (approx. 4 %) the lever works as in group I, but pollen is freely accessible as in II. The remaining approx. 13 % are not clearly classified.
It is considered that the driving force behind the diverse modes of reduction is the necessity to increase the distance between nectar and pollen, thereby ensuring pollen deposition on the bird's feathered head. This is achieved several times in parallel by corolla elongation and/or exposure of the pollen-sacs. As soon as pollen is freely accessible, the lever movement loses its significance for pollination.
INTRODUCTION
Large genera are model systems that help to reconstruct phenotypic diversification during the course of evolution. This is particularly true for taxa with specific adaptive structures assumed to be ‘key innovations’ which might have driven speciation (Hodges, 1997; Hunter, 1998). To reconstruct the genesis of adaptive radiation a phylogenetic framework is needed. This provides us with a hypothesis on the relationship among recent species based on molecular markers. As a complement, developmental, functional morphological and experimental studies as well as field investigations are needed to understand the diversity of characters as a result of developmental and functional constraints. Morphological series which are basically descriptive get their evolutionary direction by combining them with developmental pathways and with assumed selection pressures. The underlying theses are, first, that the later a specific structure is formed during ontogeny the more adaptive it is, and second, that a stable change of the developmental pathway should be caused by an internal or external selection pressure. Summarizing, there needs to be at least three sources of knowledge to reconstruct the history of phenotypic diversification: phylogeny, morphology including morphogenesis, and fitness.
In the present paper, we are interested in the phenotypic diversification of bird-pollinated sages. Salvia (‘sage’, Lamiaceae) is an adequate model system as it is a large genus with approx. 1000 species distributed worldwide (Alziar, 1988–1993). It is characterized by the well-known staminal lever mechanism of the flower (see Claßen-Bockhoff et al., 2003). The two monothecic stamens are modified to levers. They have a thin ligament between the connective and the filament, forming a joint, which enables a reversible movement causing pollen transfer. As the latter contributes to sexual reproduction, a high selection pressure on pollen transfer mechanisms is expected.
For most of the bee-pollinated species, the lever mechanism is discussed as a key innovation. It may contribute to reproductive isolation due to (precise) pollen deposition, to an increase of fitness by pollen portioning or to the decrease of possible autogamy by herkogamy (Webb and Lloyd, 1986; Grant, 1994; Claßen-Bockhoff et al., 2004b; Wester and Claßen-Bockhoff, 2006a).
Within the genus Salvia at least 186 species mainly from the New World are bird pollinated (Wester and Claßen-Bockhoff, 2007). They are supposed to have derived from melittophilous species several times in parallel (Grant and Grant, 1965; Reisfield, 1987; see also Walker and Sytsma, 2007). However, little is known about the functional constraints involved in a shift from bees to birds in Salvia. To reconstruct the phenotypic changes, the floral diversity in ornithophilous sages is analysed. Based on the general syndrome (see Wester and Claßen-Bockhoff, 2007), the focus here is on the process of pollen transfer, considering both the morphological construction and the flower–bird interaction.
The process of pollination was addressed in detail by Buzato et al. (2000), Arizmendi (2001) and Lara and Ornelas (2001). They examined several ornithophilous Salvia species and illustrated pollen deposition at the bird's body (Buzato et al., 2000; Arizmendi, 2001) and recorded seed set after bird pollination (Lara and Ornelas, 2001). Recent field studies dealt with the functional significance of the stamens during the process of pollen transfer (Wester and Claßen-Bockhoff, 2006,a, b). At least two different modes of pollen transfer were illustrated, one by means of a staminal lever mechanism as it is known from the bee-pollinated species and one without a lever mechanism.
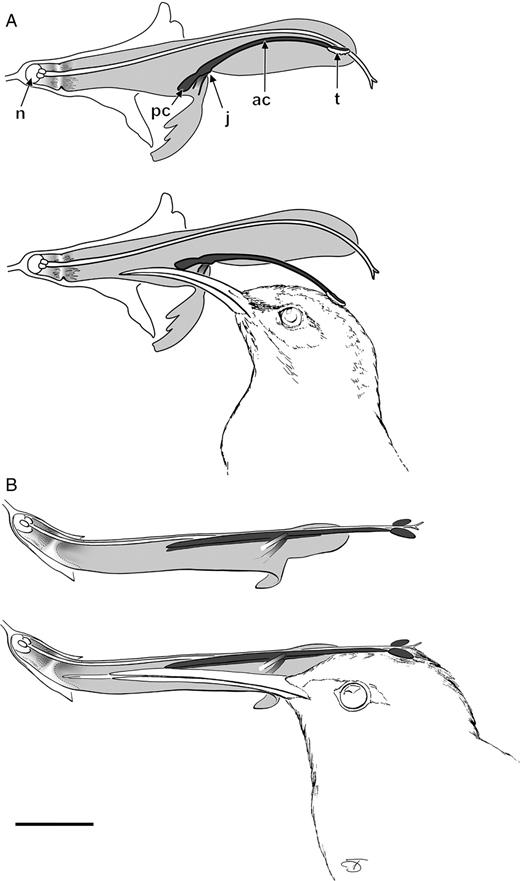
In the South African S. lanceolata pollen is transferred by the staminal lever mechanism. The lesser Double-collared Sunbird Nectarinia chalybea and the Cape White-eye Zosterops pallidus were observed to be pollinators (Figs 1A and 2A; Wester and Claßen-Bockhoff, 2006,b) (see Video 1 in Supplementary information available online). They inserted their bills into the bilabiate flowers while perching on the branches of the shrub. In this way, they pushed back the posterior connective arms of the stamens (Fig. 1A, pc) which block access to nectar (Fig. 3E). Releasing the staminal lever mechanism, the thecae (Fig. 1A, t) at the end of the anterior connective arms (Fig. 1A, ac) came out of the upper lip and were pressed onto the pollinator's head. Visiting a second flower of the same species permitted pollen transfer to the stigma.
Salvia haenkei, in contrast, represents a bird-pollinated species without a working lever mechanism (Figs 1B and 2B; Wester and Claßen-Bockhoff, 2006a) (see Video 2 in Supplementary information available online). Instead, the exserted (projecting) pollen-sacs contact a bird searching for nectar at the base of the narrow tubular corolla. Although the staminal levers are functional they cannot be moved because of the spatial arrangement. The connectives are so closely attached to the upper face of the corolla that they leave no space for a movement.
Pollen transfer with and without the staminal lever mechanism. (A) Salvia lanceolata. In order to access nectar produced by the nectary (n) the bill of Nectarinia chalybea pushes back the posterior connective arms (pc). Thereby thecae (t) at the anterior connective arms (ac) move down onto the head of the bird. The movement is enabled by the joint (j) between the filament and the connective. (B) S. haenkei. The lever mechanism is inactive with the posterior connective arms closely attached to the upper side of the tubular corolla. Pollen is deposited on the head of Sappho sparganura, touching the thecae while entering the flower. Scale bar = 1 cm. (after Wester and Claßen-Bockhoff, 2006,a, b)
Flower–bird interactions: (A) S. lanceolata visited by a perching Nectarinia chalybea; (B) S. haenkei visited by a hovering Sappho sparganura. Note in both examples the thecae on the bird's heads. Scale bars = 1 cm. Photograph A: R. Groneberg (Mainz).
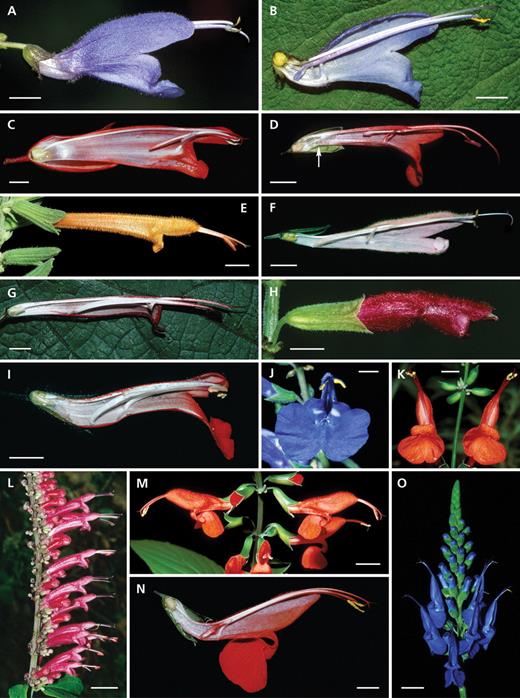
Diversity of Salvia flowers with active staminal levers and concealed pollen (group 1: Lanceolata-type). (A–C) Salvia fulgens: (A) inflorescence, (B) flower and (C) longitudinal section – note the papilla at the flower base (arrow); (D) S. atrocyanea: front view – note the fusion by hairs of the connective arms; (E) S. lanceolata: front view; (F) S. sessei: flower with large red calyx; (G) S. africana-lutea: longitudinal section with hairs at the flower base and reflexed lower lip; (H) S. oxyphora: lateral, basal constriction and papilla from the bottom; (I) S. patens: basal constriction from the bottom; (J) S. dombeyi: longitudinal section (collapsed tube due to dissection); (K) S. gravida: pendulous inflorescence with resupinated flowers; (L, M) S. confertiflora: (L) longitudinal section and (M) node with flowers in front view – note the cup-shaped lower lip and the small flower entrance; (N) S. guaranitica visited by a hovering Calypte costae with thecae on the bird's head; (O) S. penstemonoides: longitudinal section with hairs at the flower base; (P) S. divinorum with white corollas; (Q) S. madrensis with yellow corollas; (R) S. disjuncta: longitudinal section with lateral ridges at the flower base (arrow); (S) S. holwayi: longitudinal section with long nectary and a nectar chamber that is closed by two long and curved papillae. Scale bars: A–C, F–K, N = 1 cm; M, O–S = 0·5 cm; D, E, L = 0·25 cm.
Immovable stamens and exserted pollen-sacs were also described for S. heerii, S. tubiflora and S. coccinea by Trelease (1882) and Hildebrand (1865). In the latter species, Grases and Ramírez (1998) observed successful pollen deposition at a hummingbird's head, while McGregor (1899) carried out simulation experiments at a Salvia species wrongly determined as S. coccinea. He inserted a hummingbird skin into a flower, demonstrating the lever movement and the pollen transfer onto the skin and stigma of the flower.
Examination of the diversity of staminal levers and discussion of evolutionary tendencies, based on comparative studies, has already been undertaken by Hildebrand (1865), Correns (1891) and Hrubý (1934), Zalewska (1928; >200 species) and Himmelbaur and Stibal (1932–1934; >400 species). Zalewska (1928) stated that in the Old World, stamens evolved in adaptation to insect pollination and that most of the American ones evolved with hummingbird pollination. Although we generally confirm this statement, we disagree with her in many details (Wester and Claßen-Bockhoff, 2007). Himmelbaur and Stibal (1932–1934) examined corolla and stamen features. They stated that parallel evolutionary lineages to bilabiate corollas evolved in the New World and in the Old World. Both lineages evolved in adaptation to bees and only few species to birds. Additionally, in a separate lineage in the New World, extremely tubular ornithophilous species evolved. Although the authors suggested parallel evolution in both hemispheres concerning different morphological changes and reductions of the stamens, they never discussed a reduction of the lever mechanism and its correlation to ornithophily.
Parallel evolution of staminal modifications during the shift from bees to birds was also discussed by Reisfield (1987) for the American subgenus Calosphace, in which most of the ornithophilous sages occur (Wester and Claßen-Bockhoff, 2007). The author mentioned a shift from bee-pollinated species with hidden pollen-sacs and active lever mechanism to bird-pollinated species with exserted thecae and an inoperative lever mechanism. Species which do not fit in those categories, for instance large-flowered species with a lever mechanism, were regarded as possibly being in a transition phase and pollinated by both groups.
Stamen structures were traditionally used for classification, for instance in the revision of the subgenus Calosphace (Epling, 1939; also supplementary notes in Epling, 1940, 1941, 1944, 1947, 1951, 1960; Epling and Mathias, 1957; Epling and Játiva, 1963, 1966, 1968). In addition to other morphological characters and phytogeographical aspects they formed the base for the sectional arrangement. Walker et al. (2004) and Walker and Sytsma (2007) tested the phylogenetic significance of stamen morphology by mapping selected characters on a molecular tree. Based on about 80 species (<10 % genus coverage) they concluded that Salvia is polyphyletic. They supported the hypothesis of parallel evolution in the Old World and the New World (Himmelbaur and Stibal, 1932–1934) and their findings correlate with our finding that ornithophilous species evolved several times in parallel (Wester and Claßen-Bockhoff, 2007). Considering connective widening, theca reduction, different modes of connective fusion and lever functionality they illustrated that the lever mechanism has developed at least three times in parallel.
Ontogenetic studies revealed that the lever-like stamen is the result of specific developmental processes, the most important being inhibition and unilateral growth. Compared with the basic stamen type in Lamiaceae with a bithecate anther and a long filament, the anther in Salvia is asymmetric from the early beginning (Troll, 1929). The monothecic anther results from the inhibition of the adaxial theca; the short filament from the inhibited elongation; and the lever arms, from extension of the connective (Claßen-Bockhoff et al., 2004a). From a developmental point of view, a stamen is more derived the earlier the second theca is reduced, the shorter the filament and the more elaborate the joint and lever arms. However, it is necessary to test, in which way these developmental pathways in ornithophilous species interact with the need to adapt to the new pollinator guild.
Based on the pollination syndromes of the New World sages (Wester and Claßen-Bockhoff, 2007), the present paper deals with floral diversity and pollen transfer mechanisms in 186 Salvia species. Considering that ornithophilous species might have derived from melittophilous ones (Grant and Grant, 1965) and that inactive levers might have derived from active ones (Werth, 1956; Himmelbaur and Stibal, 1932–1934; Reisfield, 1987; Claßen-Bockhoff et al., 2004a), we test the hypothesis that, due to a pollinator shift, phenotypic changes occur and can even involve the reduction of the ‘lever mechanism’, an ancestral key innovation.
MATERIALS AND METHODS
Salvia species
Based on the classification of floral syndromes in New World sages (Wester and Claßen-Bockhoff, 2007), 186 ornithophilous Salvia species were included in the present study. Thirty-six species were observed in their natural habitats during field studies in Bolivia (February to April 2002), Mexico and Guatemala (October to December 2003), USA (April to June 2004) and South Africa (January 2004 and October and November 2005). Plant material was collected from the field and from several botanic gardens and private gardens (Wester and Claßen-Bockhoff, 2007). Fresh flowers were fixed in 70 % ethanol for further investigations. Vouchers of all the species investigated are deposited at MJG and some in B, BIGU, JEPS, K, LPB, MEXU and TEX.
The data were complemented by investigating herbarium specimens (only type material and clearly determined specimens) of 132 species and the literature (see Wester and Claßen-Bockhoff, 2006,b, 2007). For the Madagascan species, data were used from the literature (Hedge, 1972, 1974, 1998), from herbarium specimens (E: Clement et al. 2001; K: Jongkind 929, Hodgkin & Stansfield 120; MO: Phillipson 2669) and from a photograph provided by P. Phillipson (Missouri Botanical Garden and Muséum National d'Histoire Naturelle, Paris).
Fresh plants and herbarium specimens were determined from the literature (see Wester and Claßen-Bockhoff, 2007) and with the help of J. Wood (OXF), A. Vázquez (IBUG), A. Espejo (UAMIZ), H. Vibrans (CHAPA), M. Véliz (BIGU) and C. Froissart (Olivet, France). Details of the appropriate nomenclature and systematics are being compiled separately (Wester and Claßen-Bockhoff, 2007).
Methods
Bird observations of 15 Salvia species were made at their natural habitat, in the Botanic Gardens of Berkeley, Rancho Santa Ana, Riverside (California, USA) and in a private garden in Cochabamba (F. Berndt, Bolivia). The birds were identified from Fjeldså and Krabbe (1990), Hilty and Brown (1986) and Schuchmann (1999) and by J. A. Balderrama, J. C. Crespo and V. García (all from Universidad Mayor de San Simón, Cochabamba, Bolivia), J. F. Ornelas and C. González (both from Instituto de Ecología, Xalapa, Mexico), M. Ordano (Universidad Nacional Autónoma de México, México City, Mexico), O. Reyna (Universidad de Guadalajara, Mexico), G. Stiles (Universidad Nacional de Colombia, Bogotá, Colombia), N. Newfield (Metairie, USA) and A. Weller (University of Bonn, Germany).
To test the fit between flowers and birds and the functionality and movement of the staminal levers, the bird's bill was simulated by inserting either a museum skin or a metal rod into fresh flowers. Simultaneously, the area of pollen deposition on the bird's body was determined. The skins were borrowed from the ZFMK (Alexander Koenig Research Institute and Museum of Zoology, Bonn, Germany), the CBF (Colección Boliviana de Fauna, La Paz, Bolivia) and the SAM (South African Museum, Cape Town, South Africa).
Floral structures were morphologically investigated and measured morphometrically by the following: shape and length of the corolla, corolla tube (flower entrance to basal end) and corolla lips; position and stability of the lower lip; structure for nectar retention; and corolla colour. Stamen morphology and functionality was detected by establishing (a) positions of the filament, connective and thecae within the flower; (b) if thecae were enclosed by either open or closed upper lips and whether positioned either beneath or exserted from the upper lips; (c) the functionality of the joint and mobility of the lever arms; (d) shape, size, stability and flexibility of the filament, connective and joint; (e) the extent of fusion of the connective and thecae; and (f) the number of thecae.
To document the diversity of the joint structures, stamens of representative species were dehydrated through an ascending alcohol–acetone series, critical point dried (BAL-TEC CPD030, Balzar, Switzerland), mounted on SEM stubs, coated with gold (BAL-TEC SCD005) and examined with an scanning electron microscope (ESEM XL-30, Philips). Colour values were based on the CMYK colour space (Küppers, 1999). Floral nectar was measured from cultivated plants in the Botanic Garden of the University of Mainz usually in the morning of the first day of anthesis. Sugar concentration was determined using hand held refractometers (Atago, Honcho/Japan: N1: 0–32 % and N2: 28–62 % sucrose w/w and Bellingham and Stanley, Kent/UK: Eclipse 45–81: 0–50 % sucrose w/w). Volume of nectar was measured with a 25-μL microsyringe (ILS, Stützerbach, Germany).
RESULTS
Bird-pollinated Salvia species can be roughly classified into three groups: group I includes those with active levers; group II includes those with inactive levers; and group III is a heterogeneous group including those with different stamen structures. To survey their floral diversity, functionality and pollen transfer mechanisms, eight case studies were selected as representatives of all 186 ornithophilous species.
Salvia fulgens
Salvia fulgens (Calosphace) represents the species with active staminal levers. The plants, studied in two large populations in Mexico, have large inflorescences bearing several conspicuous flowers each (Fig. 3A). The latter were frequently visited by hovering White-eared Hummingbirds (Hylocharis leucotis), Blue-throated Hummingbirds (Lampornis clemenciae) and Green Violet-ears (Colibri thalassinus). Usually the hummingbirds visited the flowers from the front, but sometimes they approached from the side, pulling the flowers to themselves, which is enabled by long (4–6·5 mm), flexible pedicels. As in S. lanceolata (Figs 1A, 2A and 3E), the birds inserted their bills into the flowers, pushed the posterior connective arms back and causing the anterior connective arms to lower. Thereby, the pollen-sacs, which are oriented parallel to one anther, came out of the upper lip, depositing pollen onto the bird's head and sometimes on its bill. Pollen is deposited at the tip of the lower stigmatic branches (Fig. 3B). Nectar robbing was observed by the short-billed Cinnamon-bellied Flowerpiercers (Diglossa baritula, Emberizidae), perforating the corolla tube near the base. Bees were never observed at the flowers.
The flowers are typically ornithophilous (Fig. 3A, B), being large (about 5–6 cm) and brilliant red (M99 Y70 S10). The corolla has a bilabiate shape with large lips. The lower lips vary in their orientation from antrorse (bent downward <90°) to deflexed (bent downward about 90°) and slightly reflexed (bent downward >90°) (Fig. 3A). In each case, they leave enough space for the birds to enter the flower and touch the stigma. Perching insects would not contact the stigma, except perhaps very large bees. However, bees would never reach the nectar at the base of the 3- to 4-cm-long corolla tubes. Nectar is produced by the nectary at the base of the flower (Fig. 3C). Its large volume (12·7 ± 3·1 µL, 9–16·5, n = 7) and low sugar concentration (24·4 ± 2·2 %, 20–27, n = 15) again point to bird pollination. Two papillae near the flower base (Fig. 3C, arrow) retain the nectar, preventing the latter from overflowing.
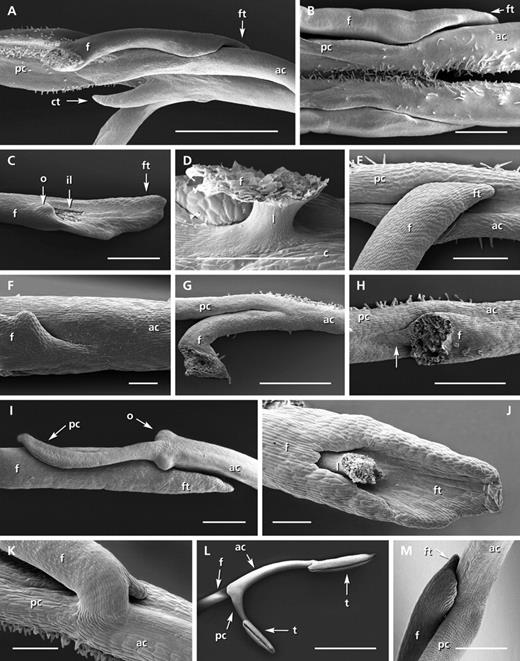
Concerning the staminal lever, the two posterior connective arms are stiff, broadened and fused to each other by hairs (Fig. 4B). They reach the lower side of the corolla (Fig. 3C), thus blocking the entrance and forming an abutment against the birds' bills. The staminal lever moves easily due to the thin and flexible ligament (Fig. 4D: l). Connective and filament outgrowths around the joint (Fig. 4B, C, o and ft; compare with A) stabilize the movement. During the release of the lever, the ligament becomes twisted. The movement is reversible. Due to the ligament's tension, the pollen-sacs swing-back into the upper lip when the birds leave the flower.
Diversity of staminal joints in ornithophilous Salvia species. (A) Salavia guaranitica: part of the stamens showing the joint area with well-developed filament tip (ft) and tooth-like structures at the connectives (ct, function unknown). (B–D) Salvia fulgens: (B) joint area of the two stamens showing the precise fit between filaments (f) and anterior and posterior connective arms (ac/pc) – note the hairs causing the fusion of the two connective arms, (C) filament showing the point of insertion of the ligament (il) and long filament tip, and (D) connective (c) with ligament (l) and point of insertion of the filament. (E) Salvia cacaliifolia: joint area with connective and filament. (F) Salvia longistyla: joint area with broad connection between filament and connective. (G, H) Salvia elegans: (G) part of the lever with a broad connection between connective and filament and (H) detail of the ‘joint’ area showing the tissue joining the filament and the connective (arrow). (I and J) Salvia spathacea: (I) joint area with connective outgrowths (o) and the short posterior connective arm and (J) filament with ligament and filament tip. (K) Salvia exserta: broad connection between connective and filament. (L, M) Salvia roemeriana: (L) whole lever with two thecae (t) and joint area and (M) joint area showing the precise fit between filament and connective. Scale bars: L = 2 mm; A–C, G, I = 1 mm; J, K = 200 µm; D–F, H, M = 500 µm. Photograph J: Sina Barth; photograph M: Norbert Holstein (both University of Mainz, Germany).
Within the ornithophilous sages, a total of 92 species correspond to S. fulgens in having an active lever movement, a well-functioning joint, hidden pollen-sacs and a blocked flower entrance (Fig. 3D, E; see Table 1). Most of the species belong to the subgenus Calosphace (88 species in 33 sections) and occur in the USA, Cuba, Mexico, Central America and South America. The North American S. penstemonoides is placed in the section Eusphace (subg. Salvia), whereas S. africana-lutea and S. lanceolata (South Africa) belong to the section Hymenosphace (subg. Salvia), respectively ‘Species group G’. Salvia thermarum from South Africa is not yet classified to a systematic group.
Functional flower types in ornithophilous Salvia species
| GROUP I (92 spp., 49·5 %) |
| Lanceolata-type |
| S. adenophora Fernald, S. africana-lutea L., S. altissima Pohl, S. articulata Epling, S. atrocaulis Fernald, S. atrocyanea Epling, S. balaustina Pohl*, S. benthamiana Gardner, S. betulifolia Epling, S. blepharophylla Brandegee ex Epling, S. buchananii Hedge, S. camarifolia Benth., S. chapadensis E.P.Santos & Harley*, S. confertiflora Pohl, S. curviflora Benth., S. darcyi J.Compton, S. diamantina E.P.Santos & Harley*, S. disjuncta Fernald, S. divinorum Epling & Játiva, S. dombeyi Epling, S. dorisiana Standl., S. dugesiana Epling*, S. ernesti-vargasii C.Nelson*, S. erythrostephana Epling*, S. espirito-santensis Brade & Barb.Per., S. falcata J.R.I.Wood & Harley*, S. formosa L'Hér., S. fulgens Cav., S. gesneriiflora Lindl. & Paxton, S. graciliramulosa Epling & Játiva, S. grandis Epling, S. gravida Epling, S. greggii A.Gray, S. grewiifolia S.Moore, S. guaranitica A.St.-Hil. ex Benth., S. harleyana E.P.Santos*, S. hatschbachii E.P.Santos*, S. herrerae Epling*, S. hidalgensis Miranda, S. hilarii Benth., S. holwayi S.F.Blake, S. involucrata Cav., S. iuliana Epling*, S. karwinskii Benth., S. lachnostoma Epling, S. lanceolata Lam., S. leucantha Cav., S. leucocephala Kunth, S. libanensis Rusby, S. lineata Benth., S. littae Vis., S. longibracteolata E.P.Santos*, S. macrocalyx Gardner, S. madrensis Seem., S. marci Epling, S. mattogrossensis Pilg.*, S. microphylla Kunth, S. miniata Fernald, S. oaxacana Fernald, S. ombrophila Dusén, S. orbignaei Benth., S. oxyphora Briq., S. patens Cav., S. pavonii Benth., S. peninsularis Brandegee, S. penstemonoides Kunth & C.D.Bouché, S. persicifolia A.St.-Hil.*, S. pringlei B.L.Rob. & Greenm., S. pubescens Benth., S. pulchella DC., S. regla Cav., S. rivularis Gardner ex H.B.Fielding, S. rufula Kunth, S. salicifolia Pohl, S. scabrata Britton & P.Wilson, S. scabrida Pohl, S. scandens Epling, S. sciaphila (J.R.I.Wood & Harley) Fern.Alonso, S. secunda Benth., S. sellowiana Benth., S. sessei Benth., S. stolonifera Benth., S. subhastata Epling, S. thermarum van Jaarsv., S. tolimensis Kunth, S. tomentella Pohl, S. tortuosa Kunth, S. tubulosa Epling*, S. venulosa Epling, S. verapazana B.L.Turner, S. wagneriana Pol., S. weberbaueri Epling* |
| GROUP II (63 spp., 33·9 %) |
| Haenkei-type (59): |
| S. alborosea Epling & Játiva, S. arbuscula Fernald†, S. arduinervis Urb. & Ekman, S. ayavacensis Kunth†, S. bahorucona Urb. & Ekman, S. cacaliifolia Benth., S. coccinea Etl., S. cocuyana Fern.Alonso†, S. curtiflora Epling, S. cyanocephala Epling, S. florida Benth., S. foveolata Urb. & Ekman, S. fruticetorum Benth.†, S. funckii Briq.†, S. gachantivana Fern.Alonso, S. haenkei Benth., S. hapalophylla Epling†, S. heerii Regel, S. hirta Kunth†, S. hirtella Vahl, S. integrifolia Ruiz & Pav.†, S. iodantha Fernald, S. iodophylla Epling†, S. jorgehintoniana Ramamoorthy, S. lachnaiclada Briq., S. lanicaulis Epling & Játiva†, S. lavendula Alain†, S. lobbii Epling, S. longistyla Benth., S. macrophylla Benth., S. medusa Epling & Játiva†, S. melaleuca Epling, S. neovidensis Benth.†, S. nervata M.Martens & Galeotti, S. oppositiflora Ruiz & Pav., S. orthostachys Epling, S. palealis Epling†, S. paryskii Skean & Judd, S. pauciserrata Benth., S. pichinchensis Benth., S. quitensis Benth.†, S. rhodostephana Epling†, S. rubescens Kunth, S. rubriflora Epling†, S. sagittata Ruiz & Pav., S. sigchosica Fern.Alonso†, S. speirematoides C.Wright, S. splendens Sellow ex Roem. & Schult., S. sprucei Briq., S. squalens Kunth, S. striata Benth., S. subrotunda A.St.-Hil., S. thormannii Urb., S. townsendii Fernald†, S. trachyphylla Epling†, S. tubiflora Sm., S. tuerckheimii Urb.†, S. uncinata Urb., S. xeropapillosa Fern.Alonso |
| Elegans-type (2): |
| S. cinnabarina M.Martens & Galeotti, S. elegans Vahl |
| Tubifera-type (1): |
| S. tubifera Cav. |
| Spathacea-type (1): |
| S. spathacea Greene |
| GROUP III (7 spp., 3·7 %) |
| Roemeriana-type (3): |
| S. henryi A.Gray, S. roemeriana Scheele, S. summa A.Nelson |
| Exserta-type (1): |
| S. exserta Griseb. |
| Lasiantha-type (3): |
| S. altimitrata Epling, S. lasiantha Benth., S. raveniana Ramamoorthy‡ |
| GROUP I + III (Lasiantha-type) (3 spp., 1·6 %) |
| S. booleana B.L.Turner*, S. rusbyi Britton§, S. sessilifolia Baker |
| Not classifiable because of lacking information (21 spp., 11·3 %) |
| S. acuminata Ruiz & Pav., S. apparicii Brade & Barb.Per., S. cubensis Britton & P.Wilson, S. cylindriflora Epling, S. itaguassuensis Brade & Barb.Per., S. melissiflora Benth., S. mentiens Pohl, S. nigrescens Alain, S. paramicola Fern.Alonso, S. phaenostemma Donn.Sm., S. plumosa Ruiz & Pav., S. psilantha Epling, S. regnelliana Briq., S. rosei Fernald, S. rubrifaux Epling, S. speciosa C.Presl ex Benth., S. subrubens Epling, S. tenuiflora Epling, S. unguella Epling, S. vestita Benth., S. xanthotricha Harley ex E.P.Santos |
| GROUP I (92 spp., 49·5 %) |
| Lanceolata-type |
| S. adenophora Fernald, S. africana-lutea L., S. altissima Pohl, S. articulata Epling, S. atrocaulis Fernald, S. atrocyanea Epling, S. balaustina Pohl*, S. benthamiana Gardner, S. betulifolia Epling, S. blepharophylla Brandegee ex Epling, S. buchananii Hedge, S. camarifolia Benth., S. chapadensis E.P.Santos & Harley*, S. confertiflora Pohl, S. curviflora Benth., S. darcyi J.Compton, S. diamantina E.P.Santos & Harley*, S. disjuncta Fernald, S. divinorum Epling & Játiva, S. dombeyi Epling, S. dorisiana Standl., S. dugesiana Epling*, S. ernesti-vargasii C.Nelson*, S. erythrostephana Epling*, S. espirito-santensis Brade & Barb.Per., S. falcata J.R.I.Wood & Harley*, S. formosa L'Hér., S. fulgens Cav., S. gesneriiflora Lindl. & Paxton, S. graciliramulosa Epling & Játiva, S. grandis Epling, S. gravida Epling, S. greggii A.Gray, S. grewiifolia S.Moore, S. guaranitica A.St.-Hil. ex Benth., S. harleyana E.P.Santos*, S. hatschbachii E.P.Santos*, S. herrerae Epling*, S. hidalgensis Miranda, S. hilarii Benth., S. holwayi S.F.Blake, S. involucrata Cav., S. iuliana Epling*, S. karwinskii Benth., S. lachnostoma Epling, S. lanceolata Lam., S. leucantha Cav., S. leucocephala Kunth, S. libanensis Rusby, S. lineata Benth., S. littae Vis., S. longibracteolata E.P.Santos*, S. macrocalyx Gardner, S. madrensis Seem., S. marci Epling, S. mattogrossensis Pilg.*, S. microphylla Kunth, S. miniata Fernald, S. oaxacana Fernald, S. ombrophila Dusén, S. orbignaei Benth., S. oxyphora Briq., S. patens Cav., S. pavonii Benth., S. peninsularis Brandegee, S. penstemonoides Kunth & C.D.Bouché, S. persicifolia A.St.-Hil.*, S. pringlei B.L.Rob. & Greenm., S. pubescens Benth., S. pulchella DC., S. regla Cav., S. rivularis Gardner ex H.B.Fielding, S. rufula Kunth, S. salicifolia Pohl, S. scabrata Britton & P.Wilson, S. scabrida Pohl, S. scandens Epling, S. sciaphila (J.R.I.Wood & Harley) Fern.Alonso, S. secunda Benth., S. sellowiana Benth., S. sessei Benth., S. stolonifera Benth., S. subhastata Epling, S. thermarum van Jaarsv., S. tolimensis Kunth, S. tomentella Pohl, S. tortuosa Kunth, S. tubulosa Epling*, S. venulosa Epling, S. verapazana B.L.Turner, S. wagneriana Pol., S. weberbaueri Epling* |
| GROUP II (63 spp., 33·9 %) |
| Haenkei-type (59): |
| S. alborosea Epling & Játiva, S. arbuscula Fernald†, S. arduinervis Urb. & Ekman, S. ayavacensis Kunth†, S. bahorucona Urb. & Ekman, S. cacaliifolia Benth., S. coccinea Etl., S. cocuyana Fern.Alonso†, S. curtiflora Epling, S. cyanocephala Epling, S. florida Benth., S. foveolata Urb. & Ekman, S. fruticetorum Benth.†, S. funckii Briq.†, S. gachantivana Fern.Alonso, S. haenkei Benth., S. hapalophylla Epling†, S. heerii Regel, S. hirta Kunth†, S. hirtella Vahl, S. integrifolia Ruiz & Pav.†, S. iodantha Fernald, S. iodophylla Epling†, S. jorgehintoniana Ramamoorthy, S. lachnaiclada Briq., S. lanicaulis Epling & Játiva†, S. lavendula Alain†, S. lobbii Epling, S. longistyla Benth., S. macrophylla Benth., S. medusa Epling & Játiva†, S. melaleuca Epling, S. neovidensis Benth.†, S. nervata M.Martens & Galeotti, S. oppositiflora Ruiz & Pav., S. orthostachys Epling, S. palealis Epling†, S. paryskii Skean & Judd, S. pauciserrata Benth., S. pichinchensis Benth., S. quitensis Benth.†, S. rhodostephana Epling†, S. rubescens Kunth, S. rubriflora Epling†, S. sagittata Ruiz & Pav., S. sigchosica Fern.Alonso†, S. speirematoides C.Wright, S. splendens Sellow ex Roem. & Schult., S. sprucei Briq., S. squalens Kunth, S. striata Benth., S. subrotunda A.St.-Hil., S. thormannii Urb., S. townsendii Fernald†, S. trachyphylla Epling†, S. tubiflora Sm., S. tuerckheimii Urb.†, S. uncinata Urb., S. xeropapillosa Fern.Alonso |
| Elegans-type (2): |
| S. cinnabarina M.Martens & Galeotti, S. elegans Vahl |
| Tubifera-type (1): |
| S. tubifera Cav. |
| Spathacea-type (1): |
| S. spathacea Greene |
| GROUP III (7 spp., 3·7 %) |
| Roemeriana-type (3): |
| S. henryi A.Gray, S. roemeriana Scheele, S. summa A.Nelson |
| Exserta-type (1): |
| S. exserta Griseb. |
| Lasiantha-type (3): |
| S. altimitrata Epling, S. lasiantha Benth., S. raveniana Ramamoorthy‡ |
| GROUP I + III (Lasiantha-type) (3 spp., 1·6 %) |
| S. booleana B.L.Turner*, S. rusbyi Britton§, S. sessilifolia Baker |
| Not classifiable because of lacking information (21 spp., 11·3 %) |
| S. acuminata Ruiz & Pav., S. apparicii Brade & Barb.Per., S. cubensis Britton & P.Wilson, S. cylindriflora Epling, S. itaguassuensis Brade & Barb.Per., S. melissiflora Benth., S. mentiens Pohl, S. nigrescens Alain, S. paramicola Fern.Alonso, S. phaenostemma Donn.Sm., S. plumosa Ruiz & Pav., S. psilantha Epling, S. regnelliana Briq., S. rosei Fernald, S. rubrifaux Epling, S. speciosa C.Presl ex Benth., S. subrubens Epling, S. tenuiflora Epling, S. unguella Epling, S. vestita Benth., S. xanthotricha Harley ex E.P.Santos |
* Classification has to be checked.
† Joint has to be checked to exclude the Elegans-type.
‡ Possibly affinities to group I.
§ Possibly also Haenkei-type.
Functional flower types in ornithophilous Salvia species
| GROUP I (92 spp., 49·5 %) |
| Lanceolata-type |
| S. adenophora Fernald, S. africana-lutea L., S. altissima Pohl, S. articulata Epling, S. atrocaulis Fernald, S. atrocyanea Epling, S. balaustina Pohl*, S. benthamiana Gardner, S. betulifolia Epling, S. blepharophylla Brandegee ex Epling, S. buchananii Hedge, S. camarifolia Benth., S. chapadensis E.P.Santos & Harley*, S. confertiflora Pohl, S. curviflora Benth., S. darcyi J.Compton, S. diamantina E.P.Santos & Harley*, S. disjuncta Fernald, S. divinorum Epling & Játiva, S. dombeyi Epling, S. dorisiana Standl., S. dugesiana Epling*, S. ernesti-vargasii C.Nelson*, S. erythrostephana Epling*, S. espirito-santensis Brade & Barb.Per., S. falcata J.R.I.Wood & Harley*, S. formosa L'Hér., S. fulgens Cav., S. gesneriiflora Lindl. & Paxton, S. graciliramulosa Epling & Játiva, S. grandis Epling, S. gravida Epling, S. greggii A.Gray, S. grewiifolia S.Moore, S. guaranitica A.St.-Hil. ex Benth., S. harleyana E.P.Santos*, S. hatschbachii E.P.Santos*, S. herrerae Epling*, S. hidalgensis Miranda, S. hilarii Benth., S. holwayi S.F.Blake, S. involucrata Cav., S. iuliana Epling*, S. karwinskii Benth., S. lachnostoma Epling, S. lanceolata Lam., S. leucantha Cav., S. leucocephala Kunth, S. libanensis Rusby, S. lineata Benth., S. littae Vis., S. longibracteolata E.P.Santos*, S. macrocalyx Gardner, S. madrensis Seem., S. marci Epling, S. mattogrossensis Pilg.*, S. microphylla Kunth, S. miniata Fernald, S. oaxacana Fernald, S. ombrophila Dusén, S. orbignaei Benth., S. oxyphora Briq., S. patens Cav., S. pavonii Benth., S. peninsularis Brandegee, S. penstemonoides Kunth & C.D.Bouché, S. persicifolia A.St.-Hil.*, S. pringlei B.L.Rob. & Greenm., S. pubescens Benth., S. pulchella DC., S. regla Cav., S. rivularis Gardner ex H.B.Fielding, S. rufula Kunth, S. salicifolia Pohl, S. scabrata Britton & P.Wilson, S. scabrida Pohl, S. scandens Epling, S. sciaphila (J.R.I.Wood & Harley) Fern.Alonso, S. secunda Benth., S. sellowiana Benth., S. sessei Benth., S. stolonifera Benth., S. subhastata Epling, S. thermarum van Jaarsv., S. tolimensis Kunth, S. tomentella Pohl, S. tortuosa Kunth, S. tubulosa Epling*, S. venulosa Epling, S. verapazana B.L.Turner, S. wagneriana Pol., S. weberbaueri Epling* |
| GROUP II (63 spp., 33·9 %) |
| Haenkei-type (59): |
| S. alborosea Epling & Játiva, S. arbuscula Fernald†, S. arduinervis Urb. & Ekman, S. ayavacensis Kunth†, S. bahorucona Urb. & Ekman, S. cacaliifolia Benth., S. coccinea Etl., S. cocuyana Fern.Alonso†, S. curtiflora Epling, S. cyanocephala Epling, S. florida Benth., S. foveolata Urb. & Ekman, S. fruticetorum Benth.†, S. funckii Briq.†, S. gachantivana Fern.Alonso, S. haenkei Benth., S. hapalophylla Epling†, S. heerii Regel, S. hirta Kunth†, S. hirtella Vahl, S. integrifolia Ruiz & Pav.†, S. iodantha Fernald, S. iodophylla Epling†, S. jorgehintoniana Ramamoorthy, S. lachnaiclada Briq., S. lanicaulis Epling & Játiva†, S. lavendula Alain†, S. lobbii Epling, S. longistyla Benth., S. macrophylla Benth., S. medusa Epling & Játiva†, S. melaleuca Epling, S. neovidensis Benth.†, S. nervata M.Martens & Galeotti, S. oppositiflora Ruiz & Pav., S. orthostachys Epling, S. palealis Epling†, S. paryskii Skean & Judd, S. pauciserrata Benth., S. pichinchensis Benth., S. quitensis Benth.†, S. rhodostephana Epling†, S. rubescens Kunth, S. rubriflora Epling†, S. sagittata Ruiz & Pav., S. sigchosica Fern.Alonso†, S. speirematoides C.Wright, S. splendens Sellow ex Roem. & Schult., S. sprucei Briq., S. squalens Kunth, S. striata Benth., S. subrotunda A.St.-Hil., S. thormannii Urb., S. townsendii Fernald†, S. trachyphylla Epling†, S. tubiflora Sm., S. tuerckheimii Urb.†, S. uncinata Urb., S. xeropapillosa Fern.Alonso |
| Elegans-type (2): |
| S. cinnabarina M.Martens & Galeotti, S. elegans Vahl |
| Tubifera-type (1): |
| S. tubifera Cav. |
| Spathacea-type (1): |
| S. spathacea Greene |
| GROUP III (7 spp., 3·7 %) |
| Roemeriana-type (3): |
| S. henryi A.Gray, S. roemeriana Scheele, S. summa A.Nelson |
| Exserta-type (1): |
| S. exserta Griseb. |
| Lasiantha-type (3): |
| S. altimitrata Epling, S. lasiantha Benth., S. raveniana Ramamoorthy‡ |
| GROUP I + III (Lasiantha-type) (3 spp., 1·6 %) |
| S. booleana B.L.Turner*, S. rusbyi Britton§, S. sessilifolia Baker |
| Not classifiable because of lacking information (21 spp., 11·3 %) |
| S. acuminata Ruiz & Pav., S. apparicii Brade & Barb.Per., S. cubensis Britton & P.Wilson, S. cylindriflora Epling, S. itaguassuensis Brade & Barb.Per., S. melissiflora Benth., S. mentiens Pohl, S. nigrescens Alain, S. paramicola Fern.Alonso, S. phaenostemma Donn.Sm., S. plumosa Ruiz & Pav., S. psilantha Epling, S. regnelliana Briq., S. rosei Fernald, S. rubrifaux Epling, S. speciosa C.Presl ex Benth., S. subrubens Epling, S. tenuiflora Epling, S. unguella Epling, S. vestita Benth., S. xanthotricha Harley ex E.P.Santos |
| GROUP I (92 spp., 49·5 %) |
| Lanceolata-type |
| S. adenophora Fernald, S. africana-lutea L., S. altissima Pohl, S. articulata Epling, S. atrocaulis Fernald, S. atrocyanea Epling, S. balaustina Pohl*, S. benthamiana Gardner, S. betulifolia Epling, S. blepharophylla Brandegee ex Epling, S. buchananii Hedge, S. camarifolia Benth., S. chapadensis E.P.Santos & Harley*, S. confertiflora Pohl, S. curviflora Benth., S. darcyi J.Compton, S. diamantina E.P.Santos & Harley*, S. disjuncta Fernald, S. divinorum Epling & Játiva, S. dombeyi Epling, S. dorisiana Standl., S. dugesiana Epling*, S. ernesti-vargasii C.Nelson*, S. erythrostephana Epling*, S. espirito-santensis Brade & Barb.Per., S. falcata J.R.I.Wood & Harley*, S. formosa L'Hér., S. fulgens Cav., S. gesneriiflora Lindl. & Paxton, S. graciliramulosa Epling & Játiva, S. grandis Epling, S. gravida Epling, S. greggii A.Gray, S. grewiifolia S.Moore, S. guaranitica A.St.-Hil. ex Benth., S. harleyana E.P.Santos*, S. hatschbachii E.P.Santos*, S. herrerae Epling*, S. hidalgensis Miranda, S. hilarii Benth., S. holwayi S.F.Blake, S. involucrata Cav., S. iuliana Epling*, S. karwinskii Benth., S. lachnostoma Epling, S. lanceolata Lam., S. leucantha Cav., S. leucocephala Kunth, S. libanensis Rusby, S. lineata Benth., S. littae Vis., S. longibracteolata E.P.Santos*, S. macrocalyx Gardner, S. madrensis Seem., S. marci Epling, S. mattogrossensis Pilg.*, S. microphylla Kunth, S. miniata Fernald, S. oaxacana Fernald, S. ombrophila Dusén, S. orbignaei Benth., S. oxyphora Briq., S. patens Cav., S. pavonii Benth., S. peninsularis Brandegee, S. penstemonoides Kunth & C.D.Bouché, S. persicifolia A.St.-Hil.*, S. pringlei B.L.Rob. & Greenm., S. pubescens Benth., S. pulchella DC., S. regla Cav., S. rivularis Gardner ex H.B.Fielding, S. rufula Kunth, S. salicifolia Pohl, S. scabrata Britton & P.Wilson, S. scabrida Pohl, S. scandens Epling, S. sciaphila (J.R.I.Wood & Harley) Fern.Alonso, S. secunda Benth., S. sellowiana Benth., S. sessei Benth., S. stolonifera Benth., S. subhastata Epling, S. thermarum van Jaarsv., S. tolimensis Kunth, S. tomentella Pohl, S. tortuosa Kunth, S. tubulosa Epling*, S. venulosa Epling, S. verapazana B.L.Turner, S. wagneriana Pol., S. weberbaueri Epling* |
| GROUP II (63 spp., 33·9 %) |
| Haenkei-type (59): |
| S. alborosea Epling & Játiva, S. arbuscula Fernald†, S. arduinervis Urb. & Ekman, S. ayavacensis Kunth†, S. bahorucona Urb. & Ekman, S. cacaliifolia Benth., S. coccinea Etl., S. cocuyana Fern.Alonso†, S. curtiflora Epling, S. cyanocephala Epling, S. florida Benth., S. foveolata Urb. & Ekman, S. fruticetorum Benth.†, S. funckii Briq.†, S. gachantivana Fern.Alonso, S. haenkei Benth., S. hapalophylla Epling†, S. heerii Regel, S. hirta Kunth†, S. hirtella Vahl, S. integrifolia Ruiz & Pav.†, S. iodantha Fernald, S. iodophylla Epling†, S. jorgehintoniana Ramamoorthy, S. lachnaiclada Briq., S. lanicaulis Epling & Játiva†, S. lavendula Alain†, S. lobbii Epling, S. longistyla Benth., S. macrophylla Benth., S. medusa Epling & Játiva†, S. melaleuca Epling, S. neovidensis Benth.†, S. nervata M.Martens & Galeotti, S. oppositiflora Ruiz & Pav., S. orthostachys Epling, S. palealis Epling†, S. paryskii Skean & Judd, S. pauciserrata Benth., S. pichinchensis Benth., S. quitensis Benth.†, S. rhodostephana Epling†, S. rubescens Kunth, S. rubriflora Epling†, S. sagittata Ruiz & Pav., S. sigchosica Fern.Alonso†, S. speirematoides C.Wright, S. splendens Sellow ex Roem. & Schult., S. sprucei Briq., S. squalens Kunth, S. striata Benth., S. subrotunda A.St.-Hil., S. thormannii Urb., S. townsendii Fernald†, S. trachyphylla Epling†, S. tubiflora Sm., S. tuerckheimii Urb.†, S. uncinata Urb., S. xeropapillosa Fern.Alonso |
| Elegans-type (2): |
| S. cinnabarina M.Martens & Galeotti, S. elegans Vahl |
| Tubifera-type (1): |
| S. tubifera Cav. |
| Spathacea-type (1): |
| S. spathacea Greene |
| GROUP III (7 spp., 3·7 %) |
| Roemeriana-type (3): |
| S. henryi A.Gray, S. roemeriana Scheele, S. summa A.Nelson |
| Exserta-type (1): |
| S. exserta Griseb. |
| Lasiantha-type (3): |
| S. altimitrata Epling, S. lasiantha Benth., S. raveniana Ramamoorthy‡ |
| GROUP I + III (Lasiantha-type) (3 spp., 1·6 %) |
| S. booleana B.L.Turner*, S. rusbyi Britton§, S. sessilifolia Baker |
| Not classifiable because of lacking information (21 spp., 11·3 %) |
| S. acuminata Ruiz & Pav., S. apparicii Brade & Barb.Per., S. cubensis Britton & P.Wilson, S. cylindriflora Epling, S. itaguassuensis Brade & Barb.Per., S. melissiflora Benth., S. mentiens Pohl, S. nigrescens Alain, S. paramicola Fern.Alonso, S. phaenostemma Donn.Sm., S. plumosa Ruiz & Pav., S. psilantha Epling, S. regnelliana Briq., S. rosei Fernald, S. rubrifaux Epling, S. speciosa C.Presl ex Benth., S. subrubens Epling, S. tenuiflora Epling, S. unguella Epling, S. vestita Benth., S. xanthotricha Harley ex E.P.Santos |
* Classification has to be checked.
† Joint has to be checked to exclude the Elegans-type.
‡ Possibly affinities to group I.
§ Possibly also Haenkei-type.
Besides S. fulgens, hummingbirds were observed at nine further species: in Mexico an unidentified species at S. gravida (Fig. 3K) and three unidentified hummingbirds at S. sessei (Fig. 3F); in Bolivia the Glittering-bellied Emerald Chlorostilbon aureoventris at S. orbignaei, a Blue-capped Puffleg Eriocnemis glaucopoides at S. atrocyanea (Fig. 3D) and an unidentified hummingbird at S. grewiifolia. Costa's Hummingbirds Calypte costae visited cultivated plants of S. guaranitica in California (Fig. 3N) (see Video 3 in Supplementary information available online), the Glittering-bellied Emerald Chlorostilbon aureoventris visited cultivated plants of S. leucantha in Bolivia and an unidentified hummingbird in California also visited cultivated plants of S. leucantha. Calypte costae was observed at S. microphylla var. wislizenii and at white cultivars of S. greggii, both cultivated in California.
The birds were observed to hover, except in S. guaranitica where they also perched on branches or hover-clasped, even on flowers or leaves. Body size and bill length of the hummingbirds observed fit well to the particular flowers, so that successful pollination is to be expected. The birds were observed releasing the lever mechanism in S. sessei and being dusted with pollen on the forehead. At S. guaranitica, the birds glided along the thecae and got pollen either smeared on their head or precisely deposited at one spot (Fig. 3N). When approaching from the side, the birds pulled the flowers to themselves. When flying from below into hanging flowers the birds lifted them up. In general, though entering the flowers from different directions, the birds touched the reproductive organs, either the stigma or the thecae first. Salvia gravida differs from all other Salvia species in having large obligatory pendulous inflorescences (Fig. 3K). The flowers resupinate during anthesis. Thereby, they compensate for the hanging position, being pollinated in the usual nototribic way. It was observed that the visiting hummingbird was dusted with pollen on the dorsal side of its head. Nectar robbers were observed at S. sessei (butterflies), S. leucantha (a large bee and a honeybee), S. guaranitica and S. grewiifolia (bees) and S. orbignaei (bees including Xylocopa sp.). At S. orbignaei, honeybees also stole pollen.
Although each of the 92 species possesses the specific features of the lever mechanism, they clearly differ in many other floral characters. These aspects are outlined below.
• The posterior lever arms always block the flower entrance (Fig. 3D and E, front view); however, they can be short (Fig. 3G) or long (Fig. 3H), oriented in a diagonal manner (Fig. 3H) or rarely in a more or less upright position (Fig. 3G). In any case, the posterior lever arms act as an abutment against the bird's bill, a function which is optimized by their fusion in most of the species. Epidermal hairs usually cause a fusion, which can be strong (e.g. S. fulgens, S. oxyphora) or weak (e.g. S. patens). The connective arms are free in S. penstemonoides, S. africana-lutea, S. lanceolata and S. thermarum and maybe in S. sessilifolia. The posterior connective arms are always sterile in the New World (Fig. 3C, H, L), but may have a small fertile theca in the Old World species (e.g. S. africana-lutea; Fig. 3G).
• Simulation experiments showed that in most species movement proceeds smoothly (especially in S. patens). In several species the release is somewhat hindered by the closely attached or overlapping lobes of the upper lip, which slow down the movement (e.g. S. oxyphora, S. involucrata, S. wagneriana and S. grewiifolia). Sometimes the connective arms return slowly into the upper lip. In all species, the birds are easily able to release the levers.
• The thecae are usually enclosed by the upper lip but, rarely in some species, they may be slightly exserted (e.g. S. patens). The fertile thecae of the upper lever arm are mostly free of each other, except in S. africana-lutea and S. lanceolata where the fusion contributes to stabilize the lever movement.
• Regarding the corolla, the species differ in colour, with >50 % of the species being red. They also differ in flower length, being (0·7 cm)–3·8 cm–(13 cm) (n = 92) as well as in the length of the flower tube, being (0·5 cm)–2·6 cm–(9 cm) (n = 91). The latter varies in shape, being mostly tubular (Fig. 3F and J), but also funnel shaped (Fig. 3G), bellied (Fig. 3H) or rarely more or less sigmoid (Fig. 3P).
• The upper lip varies from being closed by adjacent (e.g. S. miniata) or overlapping lobes (sometimes S. oxyphora) to being completely open (e.g. S. confertiflora, Fig. 3M), but the thecae are always covered by the upper lip.
• The lower lips are long (Fig. 3I) to short (Fig. 3P), reflexed (Fig. 3G), additionally revolute (S. lanceolata, Fig. 3E) to antrorse (Fig. 3B, F, J–L, P, Q). In the latter case, they may lengthen the corolla tube (Fig. 3L). They can be additionally cup-shaped, their front margins oriented upward (Fig. 3L, M) and their lateral lobes may be oriented vertically (Fig. 3L, Q). The lower lips are weak (e.g. S. patens) to firm (e.g. S. confertiflora). They often make landing for insects difficult or even impossible.
• Nectar is retained by structures at the base of the corolla, by lateral (Fig. 3H) or abaxial constrictions (Fig. 3I), by lateral ridges (S. disjuncta; Fig. 3R, arrow), abaxial papillae (Fig. 3S) or by hairs (Fig. 3G, O).
Salvia cacaliifolia
Salvia cacaliifolia (Calosphace; Fig. 5A, B) represents the species that transfer pollen without a lever movement. Plants were observed in the Botanic Garden of the University of Riverside (California), where they were frequently visited by Costa's Hummingbird (Calypte costae). The birds were dusted with pollen on their heads while hovering at the flowers, hovering-clasping on neighbouring branches or leaves and perching.
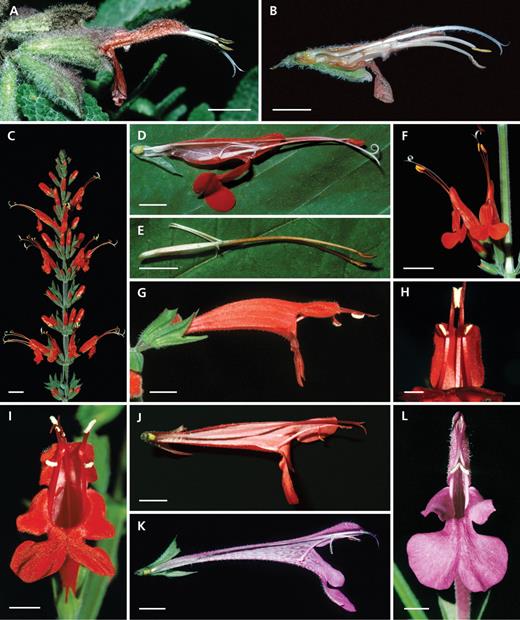
Diversity of Salvia flowers with freely accessible pollen and lacking a lever mechanism (group 2): Haenkei-type. (A, B) Salvia cacaliifolia: (A) flower with greatly exserted thecae and (B) longitudinal section with the short posterior lever arm as a nectar cover. (C) Salvia splendens: longitudinal section with the posterior lever arms not reaching the lower side of the flower tube and not blocking the entrance. (D) Salvia hirtella: longitudinal section with the posterior lever arms attached to the upper corolla wall and papillae at the base of the flower (arrow). (E) Salvia haenkei: tubular flowers with greatly exserted thecae. (F) Salvia striata: longitudinal section with posterior lever arms reaching the lower side of the flower tube, note the cup-shaped lower lip. (G) Salvia longistyla: longitudinal section with posterior lever arms reaching the lower side of the corolla tube. (H) Salvia curtiflora: slightly exserted thecae and short lower lip. (I) Salvia rubescens: longitudinal section with long nectary and a large amount of nectar. (J) Salvia sagittata: front view of flower with reflexed, large lower lip. (K, M, N) Salvia pauciserrata: (K) front view of flowers, showing greatly exserted thecae and strongly reflexed, large, lower lips, (M) node with flowers, and (N) longitudinal section with strongly curved basal part of the corolla tube and short posterior lever arms reaching the lower side of the tube. (L) Salvia iodantha: part of the inflorescence bearing many tubular flowers with greatly exserted thecae. (O) Salvia macrophylla: inflorescence showing flowers with strongly reflexed lower lips. Scale bars: A–K, N = 0·5 cm; L, M, O = 1 cm.
The blue (M75 C95 S00), funnel-shaped flowers are about 25–30 mm long and have short lower lips (Fig. 5A). The latter are often cup-shaped with their front margins oriented upward, making landing difficult for insects. It is unlikely that bees are pollinators because they would hardly touch the exposed reproductive organs. The nectar fits to the ornithophilous syndrome, being large to medium in volume (7·8 ± 2·9 µL, 2–15·5; n = 32) and having a low sugar concentration (22·8 ± 3·4 %, 16·3–30·0; n = 45).
Pollen is freely accessible as the thecae are exserted. The posterior connective arms do not block the flower entrance. They partly lean against the adaxial tube wall before they reach the abaxial tube wall at the base of the flower (Fig. 5B). Being closely attached to the adaxial tube wall, they leave no space for releasing the lever, although the joint operates freely. The ligament is thin and flexible (Fig. 4C, D, o and l). The distal part of the posterior connective arms retains nectar which rises only slightly over it.
There are 59 species corresponding to S. cacaliifolia that have freely accessible pollen and lack lever movement and have a more or less functional joint (Table 1). The posterior connective arms never block the entrance. The species occur only in the subgenus Calosphace (18 sections) and are distributed from South America, Central America and Mexico to Hispaniola. Chlorostilbon aureoventris was observed visiting flowers of the cultivated Brazilian S. splendens (Fig. 5C) in Bolivia. An unidentified hummingbird visited the flowers of S. nervata in Guatemala.
Apart from the specific characters of the lever mechanism, the 59 species are highly diverse in many floral characters, as summarized below.
• The lever arms do not block the flower entrance. They are usually attached to the upper tube wall along their whole length (Fig. 5D). Their proximal ends may reach the lower side of the corolla either near the base (Fig. 5B, F) or about mid-tube (Fig. 5G). In S. splendens, the posterior connective arms are not closely attached to the upper tube wall but only lie nearby (Fig. 5C). In some species such as S. splendens and S. iodantha the position of the posterior connective arms is variable, sometimes positioned a small distance from the upper tube wall. Simulation experiments illustrated that there is, rarely, a very weak movement depending on the position and direction of the bill insertion.
• As to their stability, the connective arms are firm throughout their length as, for instance, in S. curtiflora and S. rubescens (Fig. 5H, I), while their posterior parts at least are thin and relatively flexible, as in S. striata (Fig. 5F). In this species, a bird's bill may push the posterior lever arms upward, but would never cause lever movement.
• The posterior connective arms are usually fused, but in some cases they are only weakly attached to each other (e.g. S. sagittata) or even free (S. cacaliifolia). They are short (Fig. 5B, N) or long (Fig. 5G).
• The joints differ in their functionality. When they are well developed they are often stabilized by outgrowths, coming from both the filament and the connective (S. cacaliifolia in Fig. 4E and S. iodantha). The ligaments of less functional joints are either more or less easily breakable (e.g. S. striata) or are broad and therefore partly stiffened (e.g. sometimes in S. longistyla, Fig. 4F) often having reduced outgrowths around the joint area (e.g. S. longistyla, Fig. 4F). The joints are usually located near the entrance (Fig. 5G), but also at the middle of the tube (Fig. 5F) or even at its base (Fig. 5B).
• Pollen is always freely accessible from the thecae, which are either exserted to varying degrees (Fig. 5A, D, E, G, J, L, M, O; C, F, H) or positioned beneath the upper lip (Fig. 5I). Their position varies within some species (e.g. S. striata, S. nervata, S. splendens, S. curtiflora and S. rubescens). The thecae are oriented parallel (Fig. 5I), diagonal (Fig. 5E), across (Fig. 5O) or in a variable position (S. cacaliifolia, S. pauciserrata) relative to one another. They are always free of each other except in S. hirtella (Fig. 5D).
• In the corolla, the species differ in colour (around 50 % red), flower length [(1·2 cm)–3 cm–(5·9 cm), n = 59] and tube length [(1 cm)–2·3–(4·6 cm), n = 59]. The shape of the flower tube is mostly tubular (Fig. 5G), sometimes funnel shaped (Fig. 5A) or rarely sigmoid (e.g. in the basal part in S. pauciserrata; Fig. 5M, N).
• The lower lip is long and broad (Fig. 5J) to short (Fig. 5H), reflexed (Fig. 5M, O) to antrorse (Fig. 5A, F). In the latter case it might be cup-shaped (Fig. 5F) or the lateral lobes of the lower lip might be vertically oriented (e.g. S. striata, Fig. 5F). The lower lip differs in its stability, ranging from being weak (e.g. S. sagittata and S. hirtella) to more or less firm (e.g. S. cacaliifolia).
• Nectar is often retained by basal corolla constrictions (Fig. 5C), papillae (S. hirtella, Fig. 5D, arrow) or by posterior connective arms reaching the lower corolla side (Fig. 5B, F).
The species summarized so far represent >80 % of the ornithophilous sages. Correspondingly, each of the following case studies represents only relatively few species.
Salvia elegans
In contrast to the above-mentioned species, a joint is completely missing in S. elegans (Calosphace: Incarnatae, Fig. 6A–C) as there is no flexible thin ligament between the connective and the filament (Fig. 4G, H). On the contrary, there is even further stiffening as the filament is fixed to the posterior connective arm by thin tissue (Fig. 4H, arrow). Movement of the lever is impossible. As in S. hirtella, the connectives are attached so closely to the upper side of the tubular corolla that there is no space left for any release (Fig. 6C). They are fused, very thin and flexible, and do not block the entrance. As in S. cacaliifolia, pollen is freely accessible. The thecae, oriented diagonally or across each other, are either exserted or beneath the open upper lip. Simulation experiments confirmed that the visitor will touch the thecae on entering the flower.
Diversity of Salvia flowers with freely accessible pollen and lacking a lever mechanism (group 2, continued): (A–D) Elegans-type, (E–G) Tubifera-type and (H–K) Spathacea-type. (A–C) Salvia elegans: (A) inflorescence with red, conspicuous flowers, (B) flower with slightly exserted thecae, and (C) longitudinal section with connective closely attached to the upper side of the corolla tube. (D) Salvia cinnabarina: flower with a narrow basal part of the tube. (E–G) Salvia tubifera: (E) flower with strongly reflexed lower lip and well-developed upper lip, (F) longitudinal section, and (G) flowers from below showing the thecae enclosed by the upper lip – the upper flower shows the condition after a bird inserts its bill causing a slight opening of the upper lip and making pollen available. (H–K) Salvia spathacea: (H) plant with a richly flowered inflorescence, (I) flower with a large calyx and exserted thecae, (J) longitudinal section with constriction at the flower tube and stamen, and (K) stamen with long filament, long anterior connective arm and very short posterior connective arm – note its end indicated by the arrow. Scale bars: A = 1 cm; B–G, I–K = 0·5 cm; H = 3 cm.
Plants were studied at different localities in Mexico and in the Botanic Garden of Mainz. Their flowers have typical ornithophilous characters. The slender tubular corollas are long (approx. 3–4 cm, their tube 2–3 cm) and bright red (M99 Y80 S20; Fig. 6A, B). The lower lips are antrorse or deflexed. The moderate amount of nectar is of low sugar concentration (17·6 ± 4·3 %, 7·5–24; n = 18).
The only other species completely lacking a joint is S. cinnabarina (Calosphace, Incarnatae). It differs from S. elegans in having longer posterior connective arms reaching in or even behind the basal constriction of the corolla tube (Fig. 6D). Hovering hummingbirds (Selasphorus rufus in Mexico, and an unidentified hummingbird species in Guatemala) were observed visiting the flowers. Occasionally, butterflies and bees were observed stealing nectar and pollen, respectively.
Salvia tubifera
A completely different pollen transfer mechanism operates in S. tubifera (Calosphace; Fig. 6E). Pollen is totally enclosed by the upper lip (Fig. 6F, G, lower flower). As the staminal lever mechanism is lacking, the upper lip has to be opened by the pollinator. Simulation experiments illustrated that a bird has to open the upper lip by pushing the lobes aside with its bill. The movement is facilitated by a weak constriction of the lip lobes at their proximal ends. After removal, the thecae are exposed and pollen is accessible for deposition onto the bird (Fig. 6G, upper flower).
Although the joint of the staminal lever is well developed and thus potentially functional, movement is prevented by the position of the posterior connective arms. Similarly to S. cacaliifolia, the latter run parallel to the upper tube wall before reaching the lower wall in the basal part (Fig. 6F), leaving no room for any staminal movement. The posterior lever arms contribute to nectar retention.
The plants were studied in Mexico. They have conspicuous red (M99 Y60 S30) tubular flowers (approx. 3 cm) and reflexed lower lips (Fig. 6E).
Salvia spathacea
In S. spathacea (Audibertia; Fig. 6H) the lever movement is also lacking, in this case due to the extreme reduction of the posterior connective arms (Fig. 6J, K). The latter are only a few millimetres long, very thin and positioned more or less parallel to the filament (Figs 6J, K and 4I, pc). Simulation experiments confirmed that the reduced arms offer no abutment and that there is no movement of the stamens. However, from its construction, the joint is more or less functional, being composed of a thin and flexible ligament and of outgrowths (Fig. 4I, J, l, o and ft). The original lateral position of the joint is turned by a right angle, causing a more or less sideways movement (Fig. 6K). As the whole lever is exserted by its long and firm filament, pollen is freely accessible.
Hovering hummingbirds (Calypte sp.) were observed visiting the conspicuous flowers of cultivated plants in two botanic gardens in California. The plants were studied in cultivation and at two large populations at the San Bruno Mountain in California. They have large tubular flowers (approx. 3–4·5 cm long, their tube 2–3·5 cm long; Fig. 6I). The flowers show a colour change from salmon pinkish in young flowers (M80 Y40 S30–40) to dark pink (M99 C20 S10 to M99 C50 S00) or dark red (M99 Y30 S50) with age. The lower lips are either deflexed or reflexed and offer no landing platform. Nectar is of low sugar concentration (20 ± 5·34 %, 6·8–25·4, n = 23) and its large volume (32·6 ± 16·3 µL, 13–51, n = 10) rises slightly over a basal constriction.
The following three case studies include all species which have both an active lever mechanism and exposed thecae.
Salvia lasiantha
The flowers of S. lasiantha (Calosphace, Mitratae) differ from those of S. fulgens in having freely accessible pollen (Fig. 7A, B). Only in abnormally small flowers are the thecae enclosed by the upper lip. The posterior connective arms block the entrance (Fig. 7B) and have to be pushed away to allow access to nectar.
Salvia flowers with active staminal levers and freely accessible pollen (group 3): (A and B) Lasiantha-type, (C–F) Exserta-type, (G–L) Roemeriana-type. (A, B) Salvia lasiantha: (A) flower with exserted thecae and (B) longitudinal section with the posterior connective arm reaching the lower side of the corolla tube. (C–F) Salvia exserta: (C) inflorescence, (D) flower longitudinal section with the long curved filament, (E) dissected straight filament, and (F) pair of flowers showing the greatly exserted thecae and deflexed lower lip. (G–J) Salvia roemeriana: (G) flower with bithecate stamens, (H) proximal part of the flower from the bottom showing united anterior thecae, (I) front view with non-united thecae, and (J) longitudinal section showing the long filament and hairs at the flower base. (K, L) Salvia summa: (K) longitudinal section also with hairs at the flower base and (L) front view with thecae not blocking the entrance. Scale bars: A, B, D, E, G, J, K = 0·5 cm; C, F = 1 cm; H, I, L = 0·25 cm.
Although the flowers have relative short corollas (tube about 10–12 mm; flower about 20 mm), they are regarded as ornithophilous because of their usually deflexed or reflexed lower lips and exserted thecae. Insects which land or hang on the lower lip would neither become dusted with pollen by the long exserted thecae nor touch the stigma.
The corollas are variable in colour, ranging from orange (M80 Y70 S10 to M60 Y60 S10), orange-ochre (M80 Y99 S50), dark red-orange (M90 Y70 S60) to dark red (M99 Y70 S40), red-brown (M99 Y50 S70), dull pink-red (M60 Y30 S60), dark pink (M90 Y40 S50) and dull pink (M80 Y30 S60). The calyx varies from green to pink-purple.
At the plants examined in Mexico, no birds were observed, instead nectar drinking honeybees hang on the upper lip. They inserted their head into the flower entrance, thereby sometimes touching the thecae and becoming dusted with pollen at the rear part of the abdomen.
Two other species resembling S. lasiantha in their floral construction are S. altimitrata (Mitratae) and S. raveniana (Purpureae). The latter species has slightly exserted thecae.
Salvia exserta
Salvia exserta (syn.: S. praeclara; Calosphace, Mineatae; Fig. 7C) is unique within the genus as the easily triggered movement of the lever is not enabled by joints, but by extremely long (up to 9·5 mm), thin and flexible filaments (Fig. 7D). The tension of the filaments, which enables reversibility of the movement, becomes apparent when excising the filaments from the corolla tube. Normally heavily curved, the filaments become straight (Fig. 7E). Simulation experiments illustrated that a bird's bill will push back the posterior lever arms, thereby increasing the bending of the filaments and forcing the lowering of the thecae.
The joint area is partly stiffened as the connection between the filament and the connective is broad (Fig. 4K).
The annual plants, studied in several large populations in Bolivia, bear large conspicuous inflorescences with many flowers (Fig. 7C). The flowers were frequently visited and pollinated by various hovering hummingbirds: the Sparkling Violet-ear (Colibri coruscans), the White-bellied Hummingbird (Amazilia chionogaster) and the Glittering-bellied Emerald (Chlorostilbon aureoventris). The birds visited several flowers of an inflorescence before flying to another one. Usually, the hummingbirds visited the flowers from the front, but when coming from the side they pulled the flowers to themselves, which is enabled by long (about 4 mm), flexible pedicels. On entering the flowers, the birds were dusted with pollen on their heads and touched the stigma with the same side. Honeybees (Apis mellifera) and unidentified bee species landed on the exposed connectives, thecae or styles and stole pollen, only occasionally touching the stigma. Carpenter bees (Xylocopa sp.) landed on the corolla tube and robbed nectar through holes near the corolla base.
The flowers are brilliant red (M99 Y99 S10) and relatively long (approx. 15–30 mm, their tube 10–20 mm). They lack a landing platform as the lower lip is either reflexed or deflexed (Fig. 7C, D, F). The large amount of nectar is of low sugar concentration (25·4 ± 5·9 %, 12–45, n = 117). Nectar is prevented from overflowing by lateral corolla constrictions and hairs near the flower base.
Salvia roemeriana
Salvia roemeriana (sect. Heterosphace, subg. Leonia) differs from all the above-mentioned species in having bithecate anthers (Fig. 7G–J). As in S. spathacea, the filaments are long and firm, exserting the whole lever from the corolla tube (Fig. 7H–J). Thereby, at least the anterior thecae are exserted. The lower thecae do not restrict access to nectar (Fig. 7I).
Simulation experiments illustrated that touching the lower thecae causes a relatively weak movement. The joint is functional and well developed with a thin and flexible ligament and with stabilizing outgrowths (Fig. 4L, M). The thecae of the anterior connective arms may be weakly postgenitally fused (Fig. 7H), then often separating after being touched (Fig. 7I).
The plants, examined at different localities in Texas and in cultivation (BG Mainz), have brilliant red (M90-99 Y60-80 S20), tubular, long flowers (approx. 3–4 cm, their tube 25–30 mm). The weak lower lips are usually oriented downwards (Fig. 7G). The large volume of nectar is of low sugar concentration (24·4 ± 3·5 %; 18·8–32·5, n = 58). One time a butterfly was observed stealing nectar at cultivated plants (Texas).
Salvia henryi and S. summa (Fig. 7K, L; both Heterosphace) resemble S. roemeriana in having bithecate anthers. The thecae are also exposed in front of the upper lip or placed beneath the open upper lip.
Species with varying stamen characters
There are three species which are not represented by the case studies because their characters were variable. Salvia rusbyi (Calosphace, Cylindriflorae) varies in thecae exposition, ranging from greatly exserted (approx. 1 cm) to slightly exserted pollen-sacs which may even be enclosed by the upper lip. The lever mechanism is functional, moving either strongly or sometimes slightly. The Madagascan S. sessilifolia (‘Species group B’) and the Mexican S. booleana have both a functional lever mechanism and thecae, either enclosed by the upper lip or shortly exserted.
DISCUSSION
Diversity of pollen transfer in ornithophilous Salvia species
All Salvia flowers have the same organization (Bauplan) with sympetalous, more or less monosymmetric corollas and development of only the two abaxial stamens, which are modified to act as levers. However, the floral organs greatly differ in their relative proportions, their relative positions and their synorganization. With respect to the lever mechanism and its necessity for pollen transfer, the case studies have shown that two major constructions can be distinguished: one with a staminal lever mechanism (group I) and those without a staminal lever mechanism (group II). Beside these two groups, some species show unique constructions (group III).
Group I is represented only by the Lanceolata-type, illustrated in S. lanceolata (Fig. 1A; Wester and Claßen-Bockhoff, 2006,b) and here in S. fulgens. The lever movement is needed to release the thecae out of the upper lip and to unblock the flower entrance (Table 2; see also Hildebrand, 1865; Ogle, 1869; Trelease, 1882; Vogel, 1954; Wester and Claßen-Bockhoff, 2006,b). It is additionally needed to lower the thecae further, as birds also may visit the flowers from below (Fig. 2A) without necessarily touching the thecae (Wester and Claßen-Bockhoff, 2006b).
Functional flower types of 186 ornithophilous Salvia species
For species-specific data see Wester and Claßen-Bockhoff (2007).
OW, Old World; NA, North America; CB, Caribbean; MX, Mexico; CA, Central America; SA, South America.
* The widespread S. coccinea (Haenkei-type) was not considered as its native distribution is unknown.
‡ Related to sect. Salviastrum per Walker & Sytsma (2007).
Functional flower types of 186 ornithophilous Salvia species

For species-specific data see Wester and Claßen-Bockhoff (2007).
OW, Old World; NA, North America; CB, Caribbean; MX, Mexico; CA, Central America; SA, South America.
* The widespread S. coccinea (Haenkei-type) was not considered as its native distribution is unknown.
‡ Related to sect. Salviastrum per Walker & Sytsma (2007).
Group II is represented by the Haenkei-type, the Elegans-type, the Tubifera-type and the Spathacea-type. The Haenkei-type (Fig. 1B), already illustrated in S. haenkei (Wester and Claßen-Bockhoff, 2006,a), is here represented by S. cacaliifolia. All species are characterized by a lack of lever movement and freely accessible pollen, except in S. tubifera (for S. heerii see also Trelease, 1882; Table 2). The lever movement is not needed for unblocking the flower entrance or for pollen transfer (Table 2; see also Hildebrand, 1865; Meehan, 1871; Himmelbaur and Stibal, 1932–1934). The flower tube is often long and narrow, forcing the bird into a specific position whereby the thecae are touched (compare Werth, 1956; Castellanos et al., 2003; Figs 1B and 2B; Wester and Claßen-Bockhoff, 2006a). In S. tubifera pollen is hidden in the upper lip that has to be opened by the visitor (Table 2).
Group III is heterogeneous, including all species with staminal lever movement as in type I, and freely accessible pollen as in type II (except S. tubifera). The staminal lever is not necessary for pollen transfer, but is needed to unblock the flower entrance in the Lasiantha-type and in the Exserta-type (Table 2). In the Roemeriana-type where the entrance is free (but see Walker and Elisens, 2001), the lever has a different morphology with bithecate anthers and a long filament (Table 2).
The two main types and group III clearly illustrate the functional morphological range of the flowers and their stamens. It is surprising that most species fit to the grouping and only few species vary in the staminal lever construction. For instance, in S. iodantha and S. splendens (type II) stamen movement rarely occurs (see also Trelease, 1881; Ogle, 1896; Hrubý, 1934; Werth, 1956; Faegri and van der Pijl, 1971; Proctor et al., 1996). In S. patens (type I) the thecae might be slightly exposed (see also Hildebrand, 1865; Hrubý, 1934). In S. rusbyi the thecae are either exserted or enclosed. Thus, this species ranges between the Lasiantha-type and group I. The same is probably true for S. booleana, a species separated from S. fulgens by Turner (1995), which is described as having slightly exserted thecae.
Considering the geographical distribution of the different floral constructions in bird-pollinated sages, it becomes apparent that group I flowers, which comprise 50 % of all species, are underrepresented in the northern Andes (Venezuela to Bolivia; 33 %). They are overrepresented in southern South America, especially in Brazil (74 %), and in North to Central America (64 %). Only three representatives occur in the Old World. As to group II flowers, which comprise 34 % of all species, their distribution in the New World is complementary to that of group I: Northern Andes: 51 %, southern South America and Brazil (11 %), and North to Central America (21 %). Group II is completely absent from the Old World. An account of the distribution of all species is being prepared (Wester and Claßen-Bockhoff, 2007).
Phenotypic changes due to pollinator shift from bees to birds
Since ornithophilous Salvia species may have been derived from bee-pollinated ancestors (Grant and Grant, 1965; Reisfield, 1987; Wester and Claßen-Bockhoff, 2007), we have tried to elucidate the adaptational constraints involved in the shift from bee to bird pollination.
Compared with birds, bees often collect pollen for their offspring. This pollen is lost for pollination. Specialized bee flowers often conceal their pollen and reserve it for pollination. In most of the melittophilous Salvia flowers, pollen is concealed in the upper lip where it is invisible. This can be regarded as a protection against pollen collecting bees (Müller, 1871; Loew, 1886; Correns, 1891; Westerkamp, 1997). By means of the staminal lever mechanism pollen is transferred out of the upper lip and on to the back of the bee. There the bee cannot see the pollen and it is difficult or impossible to reach it with its legs (see also Westerkamp, 1996, 1997; Westerkamp and Claßen-Bockhoff, 2007).
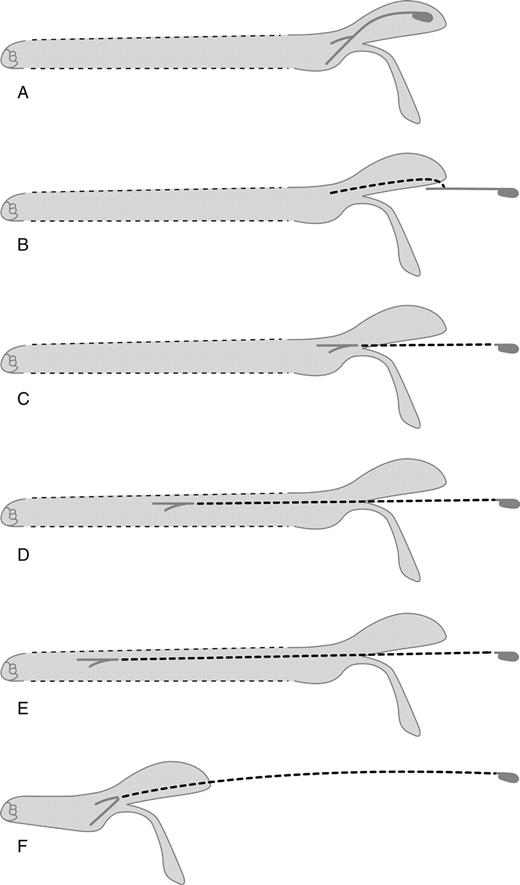
Compared with bees, birds are regarded as more reliable pollinators; they cover larger distances and are more independent of weather, particularly in highlands (Cruden, 1972; Stiles, 1978; Thomson et al., 2000). Concealment of pollen is not necessary because birds in general do not seek pollen (Wolf, 1985; Westerkamp and Claßen-Bockhoff, 2007; see also Stiles, 1981; Brice et al., 1989). Although birds occasionally groom their feathers, they do not scrape them selectively in order to obtain pollen. Consequently, pollen adheres longer to the bird and is available to be deposited on the stigmas. It is expected that bees were excluded from bird-pollinated flowers in the course of evolution. An optimization towards birds can be achieved by different modifications. First, the lower lip might be reduced, reflexed or arranged in such a way that landing for bees is impossible (e.g. S. confertiflora, Fig. 3L; S. striata, Fig. 5F). Second, the distance between the nectar and the flower entrance, i.e. the corolla tube length, might be increased to such an extent that bees are excluded (Fig. 8A; see also Reisfield, 1987; but see Himmelbaur and Stibal, 1932–1934). The same effect comes about when both the upper and lower lips functionally lengthen the flower tube. A bee's proboscis is too short to reach the nectar in comparison to a bird's bill. The increased distance between the nectar and the flower entrance also increases the distance between nectar and pollen. This might facilitate pollen deposition on the feathered head which is the better vector for pollen transport and delivery on the stigma compared with the smooth bill (Kugler, 1970; Faegri and van der Pijl, 1971; Johnsgard, 1983; Rose, 1990; Arizmendi et al., 1996).
Modes to increase the distance between nectar and pollen in Salvia flowers. (A–E) elongation of corolla tube: only corolla tube elongated (A), with additional, long filament (B), with additional, long anterior connective arm and different positions of the filament indicating an elongation of the proximal part of the corolla tube (C), of both the proximal and distal parts of the corolla tube (D) or predominantly the distal part of the corolla tube (E) compared with the usual case of an elongated proximal part (A–C); elongation of the anterior connective arm (F). Dashed lines: elements increasing distance in the individual cases.
The increase of distance between the nectar and the pollen in Salvia is caused by various developmental processes (Fig. 8). First, the corolla tube may be long, a characteristic of many ornithophilous species (Fig. 8A). Second, the filament might be long which is rarely observed in ornithophilous sages, e.g. in S. exserta, S. roemeriana or S. spathacea (Fig. 8B). Third, the connectives may elongate which is very common in bird-pollinated sages (Fig. 8C). Long filaments and connectives usually appear together with corolla tube elongation (Fig. 8B–E), and only seldom with short-tubed flowers (Fig. 8F, e.g. S. lasiantha and S. speciosa). The corolla either elongates in its proximal part (Fig. 8A–C) and/or, more rarely, in its distal part displacing the insertion of the filaments towards the flower base (Fig. 8D, E). Depending on the position of the filament insertion within the tube, the connectives need to be elongated to make pollen transfer possible or more reliable (compare Fig. 8C–E). Often, elongation of the connective and/or the long filaments is associated with exposition of the thecae (and stigma) (Fig. 8B–F).
Parallel evolution of floral constructions
Referring to our functional morphological grouping, the flowers of ornithophilous sages range from the typical bilabiate flower with hidden pollen (Fig. 1A) to tubular flowers with freely exposed pollen (Fig. 2B). The first requires the lever mechanism to lower the thecae and to unblock access to nectar. The latter releases the flower from the need of staminal movement, allowing the reduction of the lever mechanism to different degrees. The narrow (and long) corolla often forces the pollinator in a fixed position which is not necessarily the case in the bilabiate construction.
The two main floral constructions are caused by different morphogenetic processes. These occurred several times in parallel, illustrating the broad amplitude of phenotypic responses to a given selection pressure. In detail we distinguish the following analogies.
• Pollen availability is usually achieved by the lever movement, which evolved in both hemispheres. A second mode is the exposition of the anthers, which, with only one exception, is an exclusively New World novelty. The rare mechanism of upper lip movement by a pollinator is interpreted as an apomorphy for S. tubifera. The latter species may be compared with the bee-pollinated S. verticillata from the Old World, which also has a reduced lever mechanism and a movable upper lip (Hildebrand, 1865). In both species weak tissue at the base of the upper lip enables the reversible movement.
• Mobility of the lever mechanism is usually enabled by the flexible ligament of the joint. A completely different mechanism by means of a flexible filament is observed in S. exserta (Table 2), again an apomorphy.
• Even the reduction or immobilization of the lever mechanism is effected by different morphological constructions. There is either no space for the release of the lever mechanism (Haenkei-type, Elegans-type and Tubifera-type) or the posterior lever arms are too weak to cause a movement (Elegans-type and Haenkei-type), or they are almost completely reduced (S. spathacea). The latter has a parallel in the aforementioned bee-pollinated S. verticillata (Hildebrand, 1865). Furthermore, the joints are reduced (Haenkei-type p.p.), partly stiffened by a broad insertion of the ligament (Haenkei-type p.p.; S. excelsa), or the lack of a ligament (Elegans-type; see also Himmelbaur and Stibal, 1932–1934).
The diversity of phenotypes following the same functional construction indicates a high degree of parallel evolution towards ornithophily in Salvia. As to the staminal lever mechanism – this was already illustrated by the functional–morphological approaches of Zalewska (1928) and Himmelbaur and Stibal (1932–1934). To illustrate evolutionary tendencies within the ornithophilous species, Zalewska (1928) presented a morphological series from species with stiff stamens and free connectives (S. carduacea) to those with functional joints and fused connectives (via S. rhombifolia to S. patens and S. splendens). Even though not all the species involved are ornithophilous, we do not agree with her conclusions because fused connectives and functional joints are also present in almost all New World bee-pollinated sages. Himmelbaur and Stibal (1932–1934) emphasized the parallel evolution of the lever mechanism as well as its reduction. In general, we concur with their view, though they never discussed the reduction of the lever mechanism with respect to the shift from bees to birds. However, in this context, Reisfield (1987) mentioned that the reduction of the lever mechanism is likely induced by the ongoing shift from melittophily to ornithophily. He specified ‘transition species’ between the two syndromes. Though we also found species with character combinations being intermediate between the syndromes (Wester and Claßen-Bockhoff, 2007), we disagree with Reisfield (1987) who classified large flowers with a working lever mechanism as transitional stages (e.g. S. patens and S. fulgens).
From the systematic point of view, staminal levers (Lanceolata-type) occur in different groups in the Old World (e.g. S. lanceolata) and in the New World (Calosphace and S. penstemonoides; Table 2). Thus, parallel evolution in ornithophilous sages becomes apparent. In the New World, it is evident that the reduction of the staminal lever mechanism took place several times in parallel, i.e. in Calosphace (Haenkei-type, Elegans-type and Tubifera-type) and in Audibertia (Spathacea-type; Table 2).
The parallel evolution of the lever mechanism has recently been confirmed by the phylogenetic studies of Walker and Sytsma (2007). The authors found 15 stamen types based on connective widening, theca reduction, different modes of connective fusion and lever functionality, and mapped them on a tree based on molecular data. The tree includes three clades with monophyletic lineages and shows that Salvia is polyphyletic. Clade I is said to include bithecate anthers (stamen type A) and monothecate anthers with broad fused posterior lever arms (stamen type B), both with a lever mechanism. Clade II, an exclusively New World clade, includes five different stamen types and the Old World clade III includes two stamen types. Although the authors found special stamen types characterizing these clades, the grouping does not completely correspond to our classification. Ornithophilous representatives with a lever mechanism occur in two different clades, showing parallel evolution. In the authors' clade I, species are included which belong to our group I (S. penstemonoides), to our group I + III (S. sessilifolia, Old World) and to our group III (S. roemeriana). Their clade II includes two monophyletic lineages of subgenus Calosphace (e.g. S. patens in their stamen type F, S. oxyphora in their stamen type E; both our group I). The same is true for species with a reduced lever mechanism, which are found in the two Calosphace lineages (S. sagittata and S. haenkei) as well as in section Audibertia (S. spathacea; see Walker et al., 2004; all group II), again showing parallel evolution. However, we cannot confirm the authors' generalizations. For instance, the authors refer to their stamen type A as being bithecate, although, for example, the monothecate S. penstemonoides is included.
Although the study by Walker and Sytsma (2007) clearly illustrates the progress of knowledge gained by mapping phenotypic data on a molecular tree, we are far from reconstructing the evolutionary changes in Salvia in detail. In view of the great species number and the difficulties in identifying clearly the sister groups, we are only just beginning to understand the functional morphological, morphogenetic and ecological diversity in a phylogenetic way.
Bird-pollinated Salvia species are not only adequate examples illustrating morphological diversity under functional constraints, but they also confirm heterobathmy sensuTakhtajan (1959), i.e. the general view that characters evolve independently.
For instance, in S. roemeriana, presumably ancestral bithecate anthers and long filaments appear together with elongated and narrow tubular corollas. Further examples are species in which the joint is functional, but the movement is prevented due to lack of space. Werth (1956) already interpreted inactive levers in Salvia as a result of a general tendency in angiosperms to narrow flower tubes. Reisfield (1987) stated that a reduction in tube width (see also Himmelbaur and Stibal, 1932–1934) might occur if the lever becomes immovable and that the latter is virtually associated with ornithophily in Calosphace.
The staminal lever mechanism as a lost key innovation in bird-pollinated sages
The staminal lever mechanism has been regarded as a key innovation predominantly to release hidden pollen-sacs from the upper lip (Claßen-Bockhoff et al., 2004,b). Furthermore, it may contribute to an increase in the range of pollinators by compensating for different body sizes and behaviours (Wester and Claßen-Bockhoff, 2006,a, b). It may influence the breeding system by avoiding possible autogamy by herkogamy, and increasing male fitness by pollen portioning (Webb and Lloyd, 1986; Claßen-Bockhoff et al., 2004,b). Finally it may also allow pollinator-sharing among sympatric species by precise pollen deposition on the pollinators' body (Grant, 1994; Claßen-Bockhoff et al., 2004b).
As some of the ornithophilous species have reduced the lever mechanism in course of evolution, the question arises how these additional functions may be compensated in these species.
• The active lever mechanism might ensure pollen deposition on flower visitors with different body sizes and specific behaviour (Wester and Claßen-Bockhoff, 2006a, b). This is not necessary in ornithophilous species lacking a lever mechanism, which often have narrow (and long) corolla tubes, forcing the birds into a specific position (compare S. lanceolata and S. haenkei) (see Videos 1 and 2 in Supplementary information available online).
• Herkogamy decreases possible autogamy either in species with a lever mechanism or in those lacking one. For S. greggii, a species with a working lever mechanism, Webb and Lloyd (1986) described that a pollen-loaded visitor first transfers pollen to the stigma being in front of the thecae. Then, the visitor releases the lever mechanism, getting dusted with pollen (‘movement herkogamy’). We confirm this observation, but the stigma can also be touched after a dusting with pollen (see S. guaranitica; Wester and Claßen-Bockhoff, 2006,b). In ornithophilous species lacking a lever mechanism, the approaching birds usually are forced into a specific position. Thus, in general, the birds would touch the stigma first, which is usually positioned in front of the thecae [see Webb and Lloyd, 1986 (‘approach herkogamy’); Wester and Claßen-Bockhoff, 2006a].
• Though pollen portioning is observed in sages with a working lever mechanism, due to the pollen morphology of the genus, it is also observed in species lacking a lever mechanism (authors' unpubl. res.; Claßen-Bockhoff et al., 2004,b; compare Vogel and Coccuci, 1988; Castellanos et al., 2006).
• Pollen deposition in species with a working lever mechanism might be precise enough to cause mechanical isolation (Grant, 1994; Claßen-Bockhoff et al., 2004,b). In ornithophilous species, pollen might be transferred precisely, especially in species with a working lever mechanism. Pollen might be also transferred via a ‘smear effect’ on a larger part of the bird's body, especially in species with long and narrow corolla tubes, lacking a lever mechanism. Both modes might be observed within the same species (Wester and Claßen-Bockhoff, 2006,a, b; compare S. guaranitica). Probably, mechanical isolation is more effective in melittophilous species as the bees' mouthparts are more fixed to a given corolla length in comparison to the birds' tongues that can extend longer to reach the nectar.
CONCLUSIONS
The staminal lever mechanism in Salvia is a fascinating example of an adaptation that becomes less important and even reduced in some of the bird-pollinated species. Once released from the need for pollination, the posterior connective arms might undergo a transfer of function, acting as nectar-retaining structures (at least in 28 species: Haenkei-type, Elegans-type and S. tubifera).
Although Salvia illustrates a remarkable reduction of a sophisticated pollination mechanism due to a pollinator shift, it is not an exclusive feature. In the Australian genus Hemigenia (Westringieae, Lamiaceae), the bee-pollinated species generally have lever-like stamens as in Salvia, whereas the two ornithophilous species have immovable stamens (Guerin, 2005). In Fabaceae, some of the ornithophilous species lack the intricate mechanisms for pollen transfer observed in many bee-pollinated keel blossoms (Westerkamp, 1997) and instead have simple tubular flowers or brush-like flowers (e.g. Erythrina spp.; Westerkamp, 1990, 1997). In Microcorys (Westringieae, Lamiaceae), the staminodes guide bees into the flower entrance to allow precise pollen transfer in melittophilous species, whereas they are reduced or nonfunctional in the ornithophilous Microcorys eremophiloides (Guerin, 2005). In melittophilous Penstemon species (Plantaginaceae), staminodes contribute to better contact of the pollinator's body with the reproductive organs, whereas in ornithophilous flowers the staminodes lack this function (Walker-Larsen and Harder, 2001).
Given that the staminal lever mechanism is a key innovation in Salvia, we come to the surprising conclusion that this novelty has undergone many changes due to the pollinator shift from bees to birds. Salvia is a large genus that most probably radiated due to this key innovation, which itself evolved and finally became lost.
ACKNOWLEDGEMENTS
Our grateful thanks are extended to the many people who helped with plant material, logistic support, permits for research and collection, access to herbarium material and bird skins, identification of birds and sages as well as providing stimulating discussions, especially to J. A. Balderrama, J. C. Crespo and V. García (Universidad Mayor de San Simón, Cochabamba, Bolivia), A. Espejo (UAMIZ), C. Froissart (Olivet, France), C. González and J. F. Ornelas (Instituto de Ecología, Xalapa, Mexico), N. Newfield (Metairie, USA), H. Oliva and F. Ramón (CORU), M. Ordano (UNAM, Mexico), O. Reyna (Universidad de Guadalajara, Mexico), M. Sazima (Universidade Estadual de Campinas, Brazil), A. Schmidt-Lebuhn (University of Halle, Germany), G. Stiles (Universidad Nacional de Colombia, Bogotá, Colombia), A. Vázquez (IBUG), M. Véliz (BIGU), H. Vibrans (CHAPA), S. Vogel (University of Vienna, Austria), J. B. Walker (University of Wisconsin-Madison, USA), A. Weller (ZFMK, Bonn, Germany), C. Westerkamp (Universidade Federal do Ceará, Crato, Brazil), M. Wetherwax (JEPS) and J. Wood (OXF). We are also grateful to D. Franke and L. Klöckner for refining our illustrations, H. Bahmann and F. Hoppe for taking care of the plants in the Botanic Garden of Mainz and E. Groot (all University of Mainz) for proofreading a draft of the paper. We thank the Deutsche Forschungsgemeinschaft (DFG Cl 81/10-1) and the Feldbausch-Stiftung (Mainz) for financial support. This paper is part of the doctoral thesis of Petra Wester (Fachbereich Biologie, University of Mainz, Germany).