-
PDF
- Split View
-
Views
-
Cite
Cite
M. I. DAWS, N. C. GARWOOD, H. W. PRITCHARD, Prediction of Desiccation Sensitivity in Seeds of Woody Species: A Probabilistic Model Based on Two Seed Traits and 104 Species, Annals of Botany, Volume 97, Issue 4, April 2006, Pages 667–674, https://doi.org/10.1093/aob/mcl022
Close - Share Icon Share
Abstract
• Background and Aims Seed desiccation sensitivity limits the ex situ conservation of up to 47 % of plant species, dependent on habitat. Whilst desirable, empirically determining desiccation tolerance levels in seeds of all species is unrealistic. A probabilistic model for the rapid identification of woody species at high risk of displaying seed desiccation sensitivity is presented.
• Methods The model was developed using binary logistic regression on seed trait data [seed mass, moisture content, seed coat ratio (SCR) and rainfall in the month of seed dispersal] for 104 species from 37 families from a semi-deciduous tropical forest in Panamá.
• Key Results For the Panamanian species, only seed mass and SCR were significantly related to the response to desiccation, with the desiccation-sensitive seeds being large and having a relatively low SCR (i.e. thin ‘seed’ coats). Application of this model to a further 38 species, of known seed storage behaviour, from two additional continents and differing vegetation types (dryland Africa and temperate Europe) correctly predicted the response to desiccation in all cases, and resolved conflicting published data for two species (Acer pseudoplatanus and Azadirachta indica).
• Conclusions This model may have application as a decision-making tool in the handling of species of unknown seed storage behaviour in species from three disparate habitats.
INTRODUCTION
Based on their response to desiccation, seeds can be divided into two main groups, orthodox (hereafter desiccation-tolerant) and recalcitrant (hereafter desiccation-sensitive). Desiccation-sensitive seeds cannot tolerate removal of bound water without viability loss and show signs of dehydration stress when ‘free’ water is being removed (Pritchard and Manger, 1998; Pammenter and Berjak, 1999; Black and Pritchard, 2002). In addition, since desiccation-sensitive seeds cannot be dried, storage is only possible for short periods of time (generally in the order of weeks to months) and they therefore pose a significant challenge for ex situ conservation.
The first challenge for the conservation of seeds of desiccation-sensitive species is to determine their response to desiccation. This can be achieved either passively by routine processing of seeds for long-term conservation and identifying species that fail to survive, or more actively by specific, targeted screening using, for example, 100 seeds (Pritchard et al., 2004a) or by fully characterizing the response to dehydration of individual species (e.g. Hong and Ellis, 1996). Using these approaches, approx. 540 species with desiccation-sensitive seeds have been identified to date (Flynn et al., 2004), although it has been estimated that this trait could be present in approx. 8 % (20 000 species) of the world's flowering plants (Dickie and Pritchard, 2002). As it is unlikely that all of these species will ever be identified through experimental determinations, a second approach to desiccation tolerance investigations is needed that identifies reliable and robust correlates of seed desiccation sensitivity, leading to the development of a predictive framework for seed storage responses.
A number of studies have investigated potential correlates of seed desiccation sensitivity, including seed mass (Hong and Ellis, 1998; Dickie and Pritchard, 2002; Pritchard et al., 2004b), seed shape (Tompsett, 1984, 1987; Hong and Ellis, 1997), seed moisture content at shedding (Hong and Ellis, 1998), seed germination rates (Pritchard et al., 2004b; Daws et al., 2005), seed allocation to physical defence [ratio of endocarp and testa mass to dispersal unit mass, i.e. ‘seed’ coat ratio (SCR); Pritchard et al., 2004b; Daws et al., 2005] and both gross and local-scale habitat variables (Hong and Ellis, 1998; Dussert et al., 2000; Tweddle et al., 2003; Pritchard et al., 2004b; Daws et al., 2005). These studies have generally shown that desiccation-sensitive seeds are large (greater than approx. 0·5 g), spherical to spheroid, shed at high water contents, germinate rapidly, have thin seed coats (low SCR) and are more frequent in wet habitats (e.g. tropical rain forests), or are shed in wetter periods in drier habitats. However, many of these studies have been based on species from a limited taxonomic grouping such as a single family (e.g. Meliaceae; Hong and Ellis, 1998) or genus (e.g. Coffea; Dussert et al., 2000) and therefore do not provide a general framework for predicting desiccation sensitivity in phylogenetically diverse species. More recently, Daws et al. (2005) have shown that seed mass, SCR and the timing of seed dispersal are all correlated with desiccation sensitivity across a range of diverse species from semi-deciduous tropical forest in Panamá. In addition, they demonstrated an evolutionary association between desiccation sensitivity and both large seed mass and ‘thin’ seed coats. Consequently, these traits may provide a framework for developing a widely applicable model for predicting the likelihood of desiccation sensitivity.
In this study, data for 104 woody plant species from 37 families from one habitat type, semi-deciduous tropical forest in Panamá, Central America, were used to assess the usefulness of several traits that have previously been suggested as correlates of desiccation sensitivity: seed mass, SCR, total rainfall in the month of seed shed and seed water content at shedding. These traits were selected because they are both readily comparable between species and can be easily determined. Subsequently, the wider applicability of this model was tested by validation using 38 tree species from two different continents and differing vegetation types, African dryland trees and European temperate deciduous forest species.
MATERIALS AND METHODS
Study site and species
Ripe fruits/seeds, at the point of natural dispersal, were collected from 104 woody species between 1985 and 1989 from Barro Colorado Island (BCI), Republic of Panamá (9 °10′N, 79 °51′W) (see Daws et al., 2005 for details). Nomenclature follows the Flora of Panama Checklist (D'Arcy, 1987). Vegetation on BCI consists of semi-deciduous tropical forest, and has been described in detail elsewhere (Leigh et al., 1982). Rainfall on BCI averages 2600 mm year−1, with a pronounced dry season between January and April (Dietrich et al., 1982). Precipitation on BCI was measured using a rain gauge for the period 1981 to 2002. Fleshy fruits were cleaned within 2 d of collection by removing the fleshy pulp: no cleaning was necessary for wind-dispersed seeds and those in dry, dehiscent pods. For each species, the month of seed collection was recorded and seed dry mass and water content determined by drying about 10 cleaned seeds per species (with fruit tissue removed) at 60 °C for 3 d.
Data on seed desiccation tolerance were collated from Release 6 of the Royal Botanic Gardens Kew's online Seed Information Database (SID; Flynn et al., 2004). Seed responses to desiccation are divided into three categories in SID: orthodox (desiccation-tolerant); recalcitrant (desiccation-sensitive); and intermediate (Ellis et al., 1990). Intermediate seeds, which account for just 2 % of the SID, tolerate the removal of all free water and consequently are, in this context, desiccation tolerant (see Black and Pritchard, 2002; Pritchard, 2004). Species in our data set were assigned to these two, broad seed storage categories, resulting in 75 desiccation-tolerant (none of which were listed as intermediate) and 29 desiccation-sensitive taxa.
Determination of allocation to defence
For species in the Panamanian data set and the 38 African and European species used for model validation, a minimum of eight individual seeds (dispersal unit) were dissected into their component parts: endocarp/testa and embryo/endosperm. These component parts were subsequently dried at 103 °C for 17 h (ISTA, 1999) followed by mass determinations. To calculate the allocation to defence (SCR), the ratio of the mass of covering structures (endocarp and testa) to the mass of the total dispersal unit was determined (Grubb and Burslem, 1998; Pritchard et al., 2004b). In addition, for the African and European species, these data were used to determine seed dry mass. Seed mass and SCR of the Panamanian species are reported in Appendix 1 (Daws et al., 2005); that of the African and European species are reported in Table 2.
Statistical analyses
For the Panamanian data set of 104 species, binary logistic regression implemented in Minitab 13 (Minitab Inc., State College, PA, USA) was used to examine the probability of seed desiccation tolerance or sensitivity with respect to: (a) log10 oven dry seed mass (g); (b) SCR; (c) mean (for the period 1981–2002) rainfall in the month of seed dispersal (mm); and (d) seed water content at shedding (% fresh mass basis). Since the relationship between water content on a fresh and dry mass basis is not linear, analyses were also conducted on a dry mass basis: this had no significant effect on the results and consequently data are only presented on a fresh mass basis.
Two approaches were used to develop the logistic model. First, the contribution of each main factor to the logistic model was tested by removing that term from the full model (the model including all four main terms). The significance of the difference between the log-likelihood of the reduced and full model was tested using a log-likelihood test where G = 2(logLfull – logLreduced), and G is distributed as χ2 with 1 d.f., to determine the significance of the change in log-likelihood after addition of each term (Tabachnick and Fidell, 2001). This approach is equivalent to testing for the significance in the change in residual deviance between regression models. Subsequently, for the two significant main terms, the significance of their interaction was tested by comparing the log-likelihood of the full model (two main terms and their interaction) with a reduced model including only the two main terms. Secondly, a forward selection approach to model development was followed. Thus, we assessed whether adding each of the factors in turn to the null model (i.e. a model containing none of the factors) had a significant effect (as assessed using log-likelihood tests). Subsequently, the effect of adding in second terms to the most significant single factor was tested, followed by the effect of adding in third terms to the most significant two-factor model, with a P < 0·05 criterion for inclusion until no further terms could be added. Having determined the logistic model, the significance of adding in the possible interaction term was tested. Since both approaches resulted in the same main terms in the final logistic model, only results for the first approach are presented.
Several approaches were used to validate the model generated from the logistic analysis. First, cross-validation of the model was conducted by comparing the prediction for the probability of desiccation sensitivity with the actual response to desiccation for each of the 104 species. This was achieved by computing the logistic model omitting the species in question and then entering the seed trait data for that species into the resulting model. Secondly, for all 104 species, we tested whether the logistic model correctly predicted their response to desiccation, by entering seed trait data for each species in turn into Equation 1 (i.e. using all 104 species to generate the logistic model). Thirdly, seed trait data for an additional 38 species from Africa and Europe with known responses to desiccation were entered into the model to generate a probability of each species having desiccation-sensitive seeds. In all three cases, the model was categorized as correctly predicting the response to drying for desiccation-sensitive species when the predicted probability was >0·5 (i.e. seeds are more likely to be desiccation sensitive than tolerant) and for desiccation-tolerant species when the probability was <0·5 (i.e. seeds are more likely to be desiccation tolerant than sensitive). Thus, in principle, a species with a P(D – S) = 0·50 is just as likely to be desiccation sensitive as tolerant.
RESULTS
Panamanian species
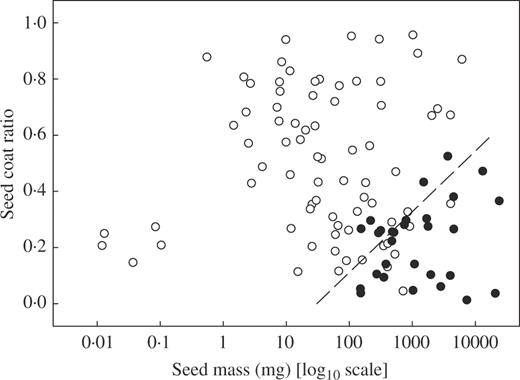
Logistic regression, using both forward- and reverse-fit approaches to model development, indicated that for this sample of Panamanian species only two traits, i.e. seed mass and SCR, significantly contributed to the logistic model explaining species' responses to desiccation (P < 0·001; Table 1). In addition, since there were no significant correlations between any of the four predictor variables (P > 0·05), these two variables are likely to be robust predictors of the response to desiccation. The desiccation-sensitive species had a mean seed mass of 1040 mg and an SCR of 0·209; in contrast, the desiccation-tolerant species had a mean seed mass and SCR of 10 mg and 0·509, respectively. Thus, the desiccation-sensitive species had large seeds with ‘thin’ seed coats (low SCR). In contrast, the desiccation-tolerant species had seeds that could span the entire seed mass range, and had SCRs spanning almost the entire observed range, except for the largest seeds which tended to have a higher SCR than desiccation-sensitive seeds of equivalent mass (Fig. 1).
The effect of seed mass and seed coat ratio on seed desiccation tolerance of 104 Panamanian woody (tree and shrub) species. Open symbols correspond to species with desiccation-tolerant seeds, closed symbols to species with desiccation-sensitive seeds. The dashed line corresponds to a probability of desiccation sensitivity of 0·5, i.e. the point at which, based on the logistic analysis (eqn 1), seeds are equally likely to be desiccation sensitive or tolerant.
Log-likelihood ratio tests for each three-parameter reduced logistic model (B) compared with the full four-parameter model (A) for the probability of seed desiccation sensitivity. Subsequently, the reduced model (D), from which the one possible two-way interaction was removed, was compared with the full model (C), which included seed mass, SCR and their interaction term
| Test . | Term removed from the full model . | Log-likelihood . | Log-likelihood test: 2 (log Lfull – log Lreduced) . |
|---|---|---|---|
| A | None | −24·791 | N/A |
| B | SCR | −38·101 | 26·620*** |
| B | Rainfall | −24·846 | 0·110 NS |
| B | Seed mass | −35·739 | 21·896*** |
| B | Water content | −26·594 | 3·606 NS |
| C | None | −26·395 | N/A |
| D | SCR, seed mass | 27·729 | 2·668 NS |
| Test . | Term removed from the full model . | Log-likelihood . | Log-likelihood test: 2 (log Lfull – log Lreduced) . |
|---|---|---|---|
| A | None | −24·791 | N/A |
| B | SCR | −38·101 | 26·620*** |
| B | Rainfall | −24·846 | 0·110 NS |
| B | Seed mass | −35·739 | 21·896*** |
| B | Water content | −26·594 | 3·606 NS |
| C | None | −26·395 | N/A |
| D | SCR, seed mass | 27·729 | 2·668 NS |
Values for the log-likelihood test are presented for each reduced model, compared with the full model, in addition to the associated P-value for χ2 with d.f. = 1.
P < 0·001, NS = not significant (P > 0·05)·
Log-likelihood ratio tests for each three-parameter reduced logistic model (B) compared with the full four-parameter model (A) for the probability of seed desiccation sensitivity. Subsequently, the reduced model (D), from which the one possible two-way interaction was removed, was compared with the full model (C), which included seed mass, SCR and their interaction term
| Test . | Term removed from the full model . | Log-likelihood . | Log-likelihood test: 2 (log Lfull – log Lreduced) . |
|---|---|---|---|
| A | None | −24·791 | N/A |
| B | SCR | −38·101 | 26·620*** |
| B | Rainfall | −24·846 | 0·110 NS |
| B | Seed mass | −35·739 | 21·896*** |
| B | Water content | −26·594 | 3·606 NS |
| C | None | −26·395 | N/A |
| D | SCR, seed mass | 27·729 | 2·668 NS |
| Test . | Term removed from the full model . | Log-likelihood . | Log-likelihood test: 2 (log Lfull – log Lreduced) . |
|---|---|---|---|
| A | None | −24·791 | N/A |
| B | SCR | −38·101 | 26·620*** |
| B | Rainfall | −24·846 | 0·110 NS |
| B | Seed mass | −35·739 | 21·896*** |
| B | Water content | −26·594 | 3·606 NS |
| C | None | −26·395 | N/A |
| D | SCR, seed mass | 27·729 | 2·668 NS |
Values for the log-likelihood test are presented for each reduced model, compared with the full model, in addition to the associated P-value for χ2 with d.f. = 1.
P < 0·001, NS = not significant (P > 0·05)·
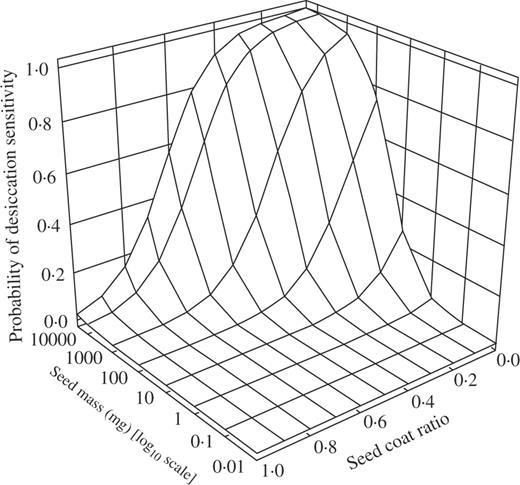
The probability of seed desiccation sensitivity in relation to seed mass and seed coat ratio based on the logistic analysis using 104 Panamanian woody species (Equation 1).
On a species by species basis, both cross-validation of our model, and the application of Equation 1 to the Panamanian species used to produce the model, resulted in 90 of the 104 species having their response to desiccation correctly assigned, a success rate of 87 % (see Appendix 1 for probabilities and species assignments). Both the models produced when dropping each species in turn and Equation 1 incorrectly assigned the same 14 species. This suggests that Equation 1 is robust and not unduly sensitive to the omission of particular species. In Fig. 1, the incorrectly assigned desiccation-sensitive and -tolerant species can be seen as closed symbols to the left of, and open symbols to the right of, the dashed P = 0·50 line, respectively.
Subsequently, this model (Equation 1) was applied to 10 species from Western Europe and 28 species from Africa (including a preponderance of dryland species). In all cases, including two species for which there has been uncertainty over their seed storage behaviour (Acer pseudoplatanus and Azadirachta indica), the model correctly predicted the seed response to desiccation when compared with published data on the physiological response of seeds to drying (Table 2).
African and European tree species used for model validation, including family (names lack the -aceae suffix), their response to desiccation and their probability of having desiccation-sensitive seeds [P(D–S)] based on their seed mass and seed coat ratio (SCR) usingEquation 1
| Species . | Family . | Country . | Seed mass (mg) . | SCR . | P(D – S) . | DS/DT? . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||||||
| Acer platanoides | Acer- | UK | 125 | 0·509 | 0·023 | DT1 | ||||||
| Acer pseudoplatanus | Acer- | Italy | 133 | 0·371 | 0·089 | DT2, DS2,3,4,5 | ||||||
| Aesculus hippocastanum | Hippocastan- | UK | 6437 | 0·190 | 0·958 | DS1 | ||||||
| Carpinus betulifolia | Betul- | UK | 44·3 | 0·799 | 0·001 | DT1 | ||||||
| Castanea sativa | Fag- | France | 3026 | 0·200 | 0·910 | DS1 | ||||||
| Corylus avellana | Betul- | UK | 1262 | 0·537 | 0·134 | DT1 | ||||||
| Fagus sylvatica | Fag- | UK | 206 | 0·321 | 0·196 | DT1 | ||||||
| Juglans regia | Jugland- | UK | 11100 | 0·656 | 0·265 | DT1 | ||||||
| Pinus sylvestris | Pin- | UK | 9·5 | 0·234 | 0·031 | DT1 | ||||||
| Quercus robur | Fag- | UK | 2285 | 0·190 | 0·895 | DS1 | ||||||
| Africa | ||||||||||||
| Anogeissus leiocarpus | Combret- | Burkina Faso | 7·3 | 0·630 | <0·001 | DT1 | ||||||
| Azadiracta indica | Meli- | Burkina Faso | 490 | 0·800 | 0·005 | DT6, DS7,8 | ||||||
| Azanza garckeana | Malv- | Kenya | 245 | 0·620 | 0·014 | DT1 | ||||||
| Carapa procera | Meli- | Mali | 1023 | 0·332 | 0·894 | DS1 | ||||||
| Cola cordifolia | Malv- | Burkina Faso | 1687 | 0·117 | 0·930 | DS1 | ||||||
| Cola nitida | Malv- | Côte d'Ivoire | 6472 | 0·075 | 0·986 | DS1 | ||||||
| Dovyalis caffra | Flacourti- | Kenya | 17 | 0·325 | 0·022 | DT1 | ||||||
| Erythroxylum emarginayum | Erythroxyl- | Malawi | 56·6 | 0·260 | 0·118 | DT1 | ||||||
| Garcinia huillensis | Clusi- | Malawi | 1630 | 0·359 | 0·536 | DS1 | ||||||
| Khaya senegalensis | Meli- | Burkina Faso | 178 | 0·319 | 0·178 | DT1 | ||||||
| Kigelia africana | Bignoni- | Burkina Faso | 71 | 0·710 | 0·002 | DT1 | ||||||
| Lannea microcarpa | Anacardi- | Burkina Faso | 135 | 0·369 | 0·092 | DT1 | ||||||
| Memecylon flavovirens | Melastomat- | Malawi | 289 | 0·495 | 0·056 | DT1 | ||||||
| Prunus africana | Ros- | Kenya | 113 | 0·220 | 0·276 | DT1 | ||||||
| Pterocarpus lucens | Fab- | Burkina Faso | 196 | 0·886 | <0·001 | DT1 | ||||||
| Sclerocarya birrea | Anacardi- | Kenya | 3380 | 0·881 | 0·012 | DT1 | ||||||
| Sterculia quinqueloba | Malv- | Malawi | 800 | 0·430 | 0·226 | DT1 | ||||||
| Strychnos cocculoides | Logani- | Tanzania | 191 | 0·272 | 0·270 | DT1 | ||||||
| Syzygium cumini | Myrt- | Tanzania | 681 | 0·076 | 0·896 | DS1 | ||||||
| Syzygium guineensis | Myrt- | Malawi | 415 | 0·090 | 0·825 | DS1 | ||||||
| Terminalia avicennoides | Combret- | Burkina Faso | 313 | 0·939 | <0·001 | DT1 | ||||||
| Terminalia macroptera | Combret- | Burkina Faso | 468 | 0·909 | 0·001 | DT1 | ||||||
| Trichilia emetica | Meli- | Kenya | 698 | 0·139 | 0·824 | DS1 | ||||||
| Vitellaria paradoxa | Sapot- | Burkina Faso | 4700 | 0·235 | 0·915 | DS1 | ||||||
| Widdringtonia whytei | Cupress- | Malawi | 12 | 0·260 | 0·030 | DT1 | ||||||
| Ximenia americana | Olac- | Tanzania | 621 | 0·358 | 0·321 | DT1 | ||||||
| Zanha africana | Sapind- | Malawi | 910 | 0·036 | 0·944 | DS1 | ||||||
| Ziziphus mauritania | Rhamn- | Burkina Faso | 298 | 0·844 | 0·002 | DT1 | ||||||
| Species . | Family . | Country . | Seed mass (mg) . | SCR . | P(D – S) . | DS/DT? . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||||||
| Acer platanoides | Acer- | UK | 125 | 0·509 | 0·023 | DT1 | ||||||
| Acer pseudoplatanus | Acer- | Italy | 133 | 0·371 | 0·089 | DT2, DS2,3,4,5 | ||||||
| Aesculus hippocastanum | Hippocastan- | UK | 6437 | 0·190 | 0·958 | DS1 | ||||||
| Carpinus betulifolia | Betul- | UK | 44·3 | 0·799 | 0·001 | DT1 | ||||||
| Castanea sativa | Fag- | France | 3026 | 0·200 | 0·910 | DS1 | ||||||
| Corylus avellana | Betul- | UK | 1262 | 0·537 | 0·134 | DT1 | ||||||
| Fagus sylvatica | Fag- | UK | 206 | 0·321 | 0·196 | DT1 | ||||||
| Juglans regia | Jugland- | UK | 11100 | 0·656 | 0·265 | DT1 | ||||||
| Pinus sylvestris | Pin- | UK | 9·5 | 0·234 | 0·031 | DT1 | ||||||
| Quercus robur | Fag- | UK | 2285 | 0·190 | 0·895 | DS1 | ||||||
| Africa | ||||||||||||
| Anogeissus leiocarpus | Combret- | Burkina Faso | 7·3 | 0·630 | <0·001 | DT1 | ||||||
| Azadiracta indica | Meli- | Burkina Faso | 490 | 0·800 | 0·005 | DT6, DS7,8 | ||||||
| Azanza garckeana | Malv- | Kenya | 245 | 0·620 | 0·014 | DT1 | ||||||
| Carapa procera | Meli- | Mali | 1023 | 0·332 | 0·894 | DS1 | ||||||
| Cola cordifolia | Malv- | Burkina Faso | 1687 | 0·117 | 0·930 | DS1 | ||||||
| Cola nitida | Malv- | Côte d'Ivoire | 6472 | 0·075 | 0·986 | DS1 | ||||||
| Dovyalis caffra | Flacourti- | Kenya | 17 | 0·325 | 0·022 | DT1 | ||||||
| Erythroxylum emarginayum | Erythroxyl- | Malawi | 56·6 | 0·260 | 0·118 | DT1 | ||||||
| Garcinia huillensis | Clusi- | Malawi | 1630 | 0·359 | 0·536 | DS1 | ||||||
| Khaya senegalensis | Meli- | Burkina Faso | 178 | 0·319 | 0·178 | DT1 | ||||||
| Kigelia africana | Bignoni- | Burkina Faso | 71 | 0·710 | 0·002 | DT1 | ||||||
| Lannea microcarpa | Anacardi- | Burkina Faso | 135 | 0·369 | 0·092 | DT1 | ||||||
| Memecylon flavovirens | Melastomat- | Malawi | 289 | 0·495 | 0·056 | DT1 | ||||||
| Prunus africana | Ros- | Kenya | 113 | 0·220 | 0·276 | DT1 | ||||||
| Pterocarpus lucens | Fab- | Burkina Faso | 196 | 0·886 | <0·001 | DT1 | ||||||
| Sclerocarya birrea | Anacardi- | Kenya | 3380 | 0·881 | 0·012 | DT1 | ||||||
| Sterculia quinqueloba | Malv- | Malawi | 800 | 0·430 | 0·226 | DT1 | ||||||
| Strychnos cocculoides | Logani- | Tanzania | 191 | 0·272 | 0·270 | DT1 | ||||||
| Syzygium cumini | Myrt- | Tanzania | 681 | 0·076 | 0·896 | DS1 | ||||||
| Syzygium guineensis | Myrt- | Malawi | 415 | 0·090 | 0·825 | DS1 | ||||||
| Terminalia avicennoides | Combret- | Burkina Faso | 313 | 0·939 | <0·001 | DT1 | ||||||
| Terminalia macroptera | Combret- | Burkina Faso | 468 | 0·909 | 0·001 | DT1 | ||||||
| Trichilia emetica | Meli- | Kenya | 698 | 0·139 | 0·824 | DS1 | ||||||
| Vitellaria paradoxa | Sapot- | Burkina Faso | 4700 | 0·235 | 0·915 | DS1 | ||||||
| Widdringtonia whytei | Cupress- | Malawi | 12 | 0·260 | 0·030 | DT1 | ||||||
| Ximenia americana | Olac- | Tanzania | 621 | 0·358 | 0·321 | DT1 | ||||||
| Zanha africana | Sapind- | Malawi | 910 | 0·036 | 0·944 | DS1 | ||||||
| Ziziphus mauritania | Rhamn- | Burkina Faso | 298 | 0·844 | 0·002 | DT1 | ||||||
Desiccation-sensitive (DS) species are shown in bold type font. DT, desiccation tolerant.
African and European tree species used for model validation, including family (names lack the -aceae suffix), their response to desiccation and their probability of having desiccation-sensitive seeds [P(D–S)] based on their seed mass and seed coat ratio (SCR) usingEquation 1
| Species . | Family . | Country . | Seed mass (mg) . | SCR . | P(D – S) . | DS/DT? . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||||||
| Acer platanoides | Acer- | UK | 125 | 0·509 | 0·023 | DT1 | ||||||
| Acer pseudoplatanus | Acer- | Italy | 133 | 0·371 | 0·089 | DT2, DS2,3,4,5 | ||||||
| Aesculus hippocastanum | Hippocastan- | UK | 6437 | 0·190 | 0·958 | DS1 | ||||||
| Carpinus betulifolia | Betul- | UK | 44·3 | 0·799 | 0·001 | DT1 | ||||||
| Castanea sativa | Fag- | France | 3026 | 0·200 | 0·910 | DS1 | ||||||
| Corylus avellana | Betul- | UK | 1262 | 0·537 | 0·134 | DT1 | ||||||
| Fagus sylvatica | Fag- | UK | 206 | 0·321 | 0·196 | DT1 | ||||||
| Juglans regia | Jugland- | UK | 11100 | 0·656 | 0·265 | DT1 | ||||||
| Pinus sylvestris | Pin- | UK | 9·5 | 0·234 | 0·031 | DT1 | ||||||
| Quercus robur | Fag- | UK | 2285 | 0·190 | 0·895 | DS1 | ||||||
| Africa | ||||||||||||
| Anogeissus leiocarpus | Combret- | Burkina Faso | 7·3 | 0·630 | <0·001 | DT1 | ||||||
| Azadiracta indica | Meli- | Burkina Faso | 490 | 0·800 | 0·005 | DT6, DS7,8 | ||||||
| Azanza garckeana | Malv- | Kenya | 245 | 0·620 | 0·014 | DT1 | ||||||
| Carapa procera | Meli- | Mali | 1023 | 0·332 | 0·894 | DS1 | ||||||
| Cola cordifolia | Malv- | Burkina Faso | 1687 | 0·117 | 0·930 | DS1 | ||||||
| Cola nitida | Malv- | Côte d'Ivoire | 6472 | 0·075 | 0·986 | DS1 | ||||||
| Dovyalis caffra | Flacourti- | Kenya | 17 | 0·325 | 0·022 | DT1 | ||||||
| Erythroxylum emarginayum | Erythroxyl- | Malawi | 56·6 | 0·260 | 0·118 | DT1 | ||||||
| Garcinia huillensis | Clusi- | Malawi | 1630 | 0·359 | 0·536 | DS1 | ||||||
| Khaya senegalensis | Meli- | Burkina Faso | 178 | 0·319 | 0·178 | DT1 | ||||||
| Kigelia africana | Bignoni- | Burkina Faso | 71 | 0·710 | 0·002 | DT1 | ||||||
| Lannea microcarpa | Anacardi- | Burkina Faso | 135 | 0·369 | 0·092 | DT1 | ||||||
| Memecylon flavovirens | Melastomat- | Malawi | 289 | 0·495 | 0·056 | DT1 | ||||||
| Prunus africana | Ros- | Kenya | 113 | 0·220 | 0·276 | DT1 | ||||||
| Pterocarpus lucens | Fab- | Burkina Faso | 196 | 0·886 | <0·001 | DT1 | ||||||
| Sclerocarya birrea | Anacardi- | Kenya | 3380 | 0·881 | 0·012 | DT1 | ||||||
| Sterculia quinqueloba | Malv- | Malawi | 800 | 0·430 | 0·226 | DT1 | ||||||
| Strychnos cocculoides | Logani- | Tanzania | 191 | 0·272 | 0·270 | DT1 | ||||||
| Syzygium cumini | Myrt- | Tanzania | 681 | 0·076 | 0·896 | DS1 | ||||||
| Syzygium guineensis | Myrt- | Malawi | 415 | 0·090 | 0·825 | DS1 | ||||||
| Terminalia avicennoides | Combret- | Burkina Faso | 313 | 0·939 | <0·001 | DT1 | ||||||
| Terminalia macroptera | Combret- | Burkina Faso | 468 | 0·909 | 0·001 | DT1 | ||||||
| Trichilia emetica | Meli- | Kenya | 698 | 0·139 | 0·824 | DS1 | ||||||
| Vitellaria paradoxa | Sapot- | Burkina Faso | 4700 | 0·235 | 0·915 | DS1 | ||||||
| Widdringtonia whytei | Cupress- | Malawi | 12 | 0·260 | 0·030 | DT1 | ||||||
| Ximenia americana | Olac- | Tanzania | 621 | 0·358 | 0·321 | DT1 | ||||||
| Zanha africana | Sapind- | Malawi | 910 | 0·036 | 0·944 | DS1 | ||||||
| Ziziphus mauritania | Rhamn- | Burkina Faso | 298 | 0·844 | 0·002 | DT1 | ||||||
| Species . | Family . | Country . | Seed mass (mg) . | SCR . | P(D – S) . | DS/DT? . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||||||
| Acer platanoides | Acer- | UK | 125 | 0·509 | 0·023 | DT1 | ||||||
| Acer pseudoplatanus | Acer- | Italy | 133 | 0·371 | 0·089 | DT2, DS2,3,4,5 | ||||||
| Aesculus hippocastanum | Hippocastan- | UK | 6437 | 0·190 | 0·958 | DS1 | ||||||
| Carpinus betulifolia | Betul- | UK | 44·3 | 0·799 | 0·001 | DT1 | ||||||
| Castanea sativa | Fag- | France | 3026 | 0·200 | 0·910 | DS1 | ||||||
| Corylus avellana | Betul- | UK | 1262 | 0·537 | 0·134 | DT1 | ||||||
| Fagus sylvatica | Fag- | UK | 206 | 0·321 | 0·196 | DT1 | ||||||
| Juglans regia | Jugland- | UK | 11100 | 0·656 | 0·265 | DT1 | ||||||
| Pinus sylvestris | Pin- | UK | 9·5 | 0·234 | 0·031 | DT1 | ||||||
| Quercus robur | Fag- | UK | 2285 | 0·190 | 0·895 | DS1 | ||||||
| Africa | ||||||||||||
| Anogeissus leiocarpus | Combret- | Burkina Faso | 7·3 | 0·630 | <0·001 | DT1 | ||||||
| Azadiracta indica | Meli- | Burkina Faso | 490 | 0·800 | 0·005 | DT6, DS7,8 | ||||||
| Azanza garckeana | Malv- | Kenya | 245 | 0·620 | 0·014 | DT1 | ||||||
| Carapa procera | Meli- | Mali | 1023 | 0·332 | 0·894 | DS1 | ||||||
| Cola cordifolia | Malv- | Burkina Faso | 1687 | 0·117 | 0·930 | DS1 | ||||||
| Cola nitida | Malv- | Côte d'Ivoire | 6472 | 0·075 | 0·986 | DS1 | ||||||
| Dovyalis caffra | Flacourti- | Kenya | 17 | 0·325 | 0·022 | DT1 | ||||||
| Erythroxylum emarginayum | Erythroxyl- | Malawi | 56·6 | 0·260 | 0·118 | DT1 | ||||||
| Garcinia huillensis | Clusi- | Malawi | 1630 | 0·359 | 0·536 | DS1 | ||||||
| Khaya senegalensis | Meli- | Burkina Faso | 178 | 0·319 | 0·178 | DT1 | ||||||
| Kigelia africana | Bignoni- | Burkina Faso | 71 | 0·710 | 0·002 | DT1 | ||||||
| Lannea microcarpa | Anacardi- | Burkina Faso | 135 | 0·369 | 0·092 | DT1 | ||||||
| Memecylon flavovirens | Melastomat- | Malawi | 289 | 0·495 | 0·056 | DT1 | ||||||
| Prunus africana | Ros- | Kenya | 113 | 0·220 | 0·276 | DT1 | ||||||
| Pterocarpus lucens | Fab- | Burkina Faso | 196 | 0·886 | <0·001 | DT1 | ||||||
| Sclerocarya birrea | Anacardi- | Kenya | 3380 | 0·881 | 0·012 | DT1 | ||||||
| Sterculia quinqueloba | Malv- | Malawi | 800 | 0·430 | 0·226 | DT1 | ||||||
| Strychnos cocculoides | Logani- | Tanzania | 191 | 0·272 | 0·270 | DT1 | ||||||
| Syzygium cumini | Myrt- | Tanzania | 681 | 0·076 | 0·896 | DS1 | ||||||
| Syzygium guineensis | Myrt- | Malawi | 415 | 0·090 | 0·825 | DS1 | ||||||
| Terminalia avicennoides | Combret- | Burkina Faso | 313 | 0·939 | <0·001 | DT1 | ||||||
| Terminalia macroptera | Combret- | Burkina Faso | 468 | 0·909 | 0·001 | DT1 | ||||||
| Trichilia emetica | Meli- | Kenya | 698 | 0·139 | 0·824 | DS1 | ||||||
| Vitellaria paradoxa | Sapot- | Burkina Faso | 4700 | 0·235 | 0·915 | DS1 | ||||||
| Widdringtonia whytei | Cupress- | Malawi | 12 | 0·260 | 0·030 | DT1 | ||||||
| Ximenia americana | Olac- | Tanzania | 621 | 0·358 | 0·321 | DT1 | ||||||
| Zanha africana | Sapind- | Malawi | 910 | 0·036 | 0·944 | DS1 | ||||||
| Ziziphus mauritania | Rhamn- | Burkina Faso | 298 | 0·844 | 0·002 | DT1 | ||||||
Desiccation-sensitive (DS) species are shown in bold type font. DT, desiccation tolerant.
DISCUSSION
These results demonstrate that two seed traits, seed mass and SCR correlate with seed responses to desiccation. Furthermore, using these two traits to develop a predictive model, based on the responses of Panamanian tropical forest woody species, is sufficient to predict the response to drying for seeds of trees from a further two biomes and continents.
Whilst it is unsurprising that seed mass correlates with desiccation sensitivity since larger seeds will desiccate more slowly, the adaptive significance of a low SCR for desiccation-sensitive species is less clear. Desiccation-sensitive seeds are at high water contents at shedding, are metabolically active and in some instances are actively progressing towards germination (e.g. Avicennia marina; Berjak et al., 1984). Consequently, desiccation-sensitive species have the potential to germinate rapidly, which may further reduce the opportunity for seed drying by facilitating access to soil water (Pritchard et al., 2004b; Daws et al., 2005). Such responses could be delayed by a ‘thick’ seed coat. In addition, rapid germination may help minimize seed consumption by vertebrate seed predators; since seedlings are often less appealing than seeds (Curran and Webb, 2000), the need to ‘invest’ in thick covering structures for defensive purposes may be reduced. Consequently, for desiccation-sensitive species, there may be little benefit in having a thick covering structure that is both inefficient in terms of seed provisioning and delays germination (Pritchard et al., 2004b; Daws et al., 2005). However, a number of temperate desiccation-sensitive species, such as Aesculus hippocastanum (Pritchard et al., 1996) and Quercus robur (Pritchard and Manger, 1990), have delayed germination or dormancy to ensure germination occurs in spring rather than at the time of seed dispersal (autumn). Both these species also have a low SCR (Table 2), suggesting that their ‘attractiveness’ to seed predators may be advantageous; indeed, many seed predators, e.g. squirrels and jays, actively cache these seeds, which will both reduce the risk of desiccation (Garcia et al., 2002) and contribute to seed dispersal in space (den Ouden et al., 2005).
As a function of the logistic model, there is a tendency for larger seeded desiccation-sensitive species to have a higher SCR (see Fig. 1). Whilst, on average, SCR is lower in desiccation-sensitive than -tolerant species, this finding potentially adds an additional level of complexity to our understanding of the trade-offs between seed physical defence and seed mass as they interact with the response to desiccation.
Seed water content at dispersal was not a useful predictor of response to drying in our logistic model for the Panamanian species (Table 1) even though this feature was shown to correlate with the response to desiccation in the Meliaceae (Hong and Ellis, 1998). Nonetheless, in agreement with Hong and Ellis (1998), all the desiccation-sensitive species in this study were shed at comparatively high water contents (20·3–52·5 %). However, the desiccation-tolerant species had a similar range of water contents from 9·1 to 61·6 %. Desiccation-sensitive seeds must be shed at high water contents because drying below approx. 20 % water content results in desiccation-induced mortality. In contrast, desiccation-tolerant seeds can be shed wet or dry: desiccation-tolerant seeds in fleshy fruits are often shed at high water contents (e.g. papaya, 43·3 % water content, Wood et al., 2000), whilst wind-dispersed seeds in dry capsules are typically shed at lower water contents (e.g. Trichospermum galeottii, 14·7 % water content).
A further proposed correlate of desiccation sensitivity is habitat, in terms of both local habitat, either at the time of seed dispersal or following dispersal (Dussert et al., 2000; Pritchard et al., 2004), or gross habitat type such as wet vs. dry (Tweddle et al., 2003). Desiccation-sensitive species are more frequent in wet tropical forests and decrease in abundance with increasing aridity (Tweddle et al., 2003). In dry environments, the time of seed dispersal coincides with the wettest months of the year (Pritchard et al., 2004b). However, for our Panamanian data set, rainfall in the month of seed shed did not make a significant contribution to the logistic model. Pritchard et al. (2004b) reported that desiccation-sensitive seeds of African dryland trees were typically shed when rainfall exceeded approx. 60 mm. However, even in the 4 month dry season on BCI, rainfall only falls below 60 mm for 2 months and averages 35 mm even in the driest months. Thus, dry season rainfall may not be sufficiently low to result in high mortality for desiccation-sensitive seeds in the short dry season. Alternatively, total monthly rainfall may be an inappropriate scale for considering this effect, and micro-site characteristics may be more useful. For example, one of the species with desiccation-sensitive seeds dispersed in the dry season on BCI (Virola sebeifera) is confined to wet micro-sites (Daws et al., 2002, 2005). Although we have been able to predict the risk of desiccation sensitivity while ignoring this variable, a consideration of climate and in particular micro-climatic variables in future models may improve their predictive power.
Whilst the predictions of the response to desiccation were accurate for 38 African and European woody species, 14 of the Panamanian species appear to have been misclassified by the model. A practical implication of this is that detailed characterization of the response to desiccation should be conducted on these species. The model correctly predicted the response for all 38 species from Africa and Europe including two species for which there has been debate over their seed storage classification (A. pseudoplatanus and A. indica). Acer pseudoplatanus has been classified as recalcitrant by a number of authors (e.g. Hong and Ellis, 1990; Dickie et al., 1991; Greggains et al., 2000) but as desiccation tolerant when harvested within the species native range (Daws et al., 2006). Our model predicts that seeds of this species are desiccation tolerant [probability of desiccation tolerance = 1 – P(desiccation sensitivity) = 0·911]. Previous reports of desiccation sensitivity are likely to reflect the use of seeds that developed under sub-optimal conditions and hence failed to display their maximum potential level of desiccation tolerance (MPDT; Daws et al., 2004, 2006). Similarly, there have been conflicting reports of the level of desiccation tolerance for A. indica seeds (Berjak et al., 1995; Poulsen, 1996; Sacandé, 2000), probably associated with difficulties in identifying seed maturity and optimal seed handling methodologies (Sacandé, 2000). Nonetheless, our model correctly predicts the MPDT response for this species (probability of desiccation tolerance = 0·995). Consequently our model may be of use in reclassifying woody species for which their current seed storage behaviour is in dispute.
Here we have shown how a model based on just two readily obtainable seed traits can predict the probability of a woody species having desiccation-sensitive seeds. Although based on species from one vegetation type, this model also correctly predicts the response to drying of species from both drier tropical and temperate vegetation types and has been developed with species whose seed masses span six orders of magnitude. Consequently, for unknown species with a seed mass value in this range (0·01 mg to 24 g), this model may provide the first step in a decision-making framework for the application of ex situ seed conservation strategies to a diverse range of species from many habitats.
APPENDIX 1
Details of the 104 Panamanian species used in the study
Classification to family level (names lack the -aceae suffix), seed mass, seed coat ratio (SCR), rainfall in the month of seed dispersal and seed water content (WC) at shedding (species with desiccation-sensitive seeds are shown in bold type face). Seed dry mass and seed coat ratio data are taken fromDaws et al. (2005). Also shown is the predicted probability of seeds of each species exhibiting desiccation sensitivity P (D – S) based on entering species-specific values for SCR and dry mass intoEquation 1
| Species . | Family . | Dry mass (mg) . | SCR . | Monthly rainfall at seed shed (mm) . | WC at dispersal (% f. wt. basis) . | P(D – S) . |
|---|---|---|---|---|---|---|
| Adelia triloba (Müll.Arg.) Hemsle | Euphorbi- | 26 | 0·354 | 34·2 | 10·5 | 0·025 |
| Adenopodia polystachya (L.) J.R.Dixon ex Croat | Fab- | 298 | 0·942 | 32·9 | 29·6 | 0·001 |
| Albizia guachapele (H.B. & K.) Dugand | Fab- | 32 | 0·433 | 280 | 17·1 | 0·012 |
| Alchornea costaricensis Pax & K.Hoffm. | Euphorbi- | 34 | 0·799 | 280 | 20·5 | <0·001 |
| Alibertia edulis (Rich.) A.Rich. ex DC. | Rubi- | 15 | 0·114 | 32·9 | 24·4 | 0·142 |
| Anacardium excelsum (Bertero & Balb. Ex Kunth) Skeels | Anacardi- | 1507 | 0·433 | 280 | 43·8 | 0·339* |
| Andira inermis (Sw.) Kunth | Fab- | 792 | 0·297 | 280 | 41·7 | 0·522 |
| Annona glabra L. | Annon- | 229 | 0·358 | 271·8 | 21·7 | 0·157 |
| Annona muricata L. | Annon- | 322 | 0·706 | 275 | 22·3 | 0·008 |
| Anthodon panamense A.C.Sm. | Celastr- | 81 | 0·438 | 310·4 | 61·1 | 0·031 |
| Apeiba membranacea Spruce ex Benth. | Malv- | 69 | 0·776 | 280 | 52·7 | 0·001 |
| Apeiba tibourbou Aubl. | Malv- | 17 | 0·584 | 280 | 25·2 | 0·002 |
| Astronium graveolens Jacq. | Anacardi- | 30 | 0·368 | 280 | 30·2 | 0·024 |
| Bactris gasipaes Kunth | Arec- | 1680 | 0·303 | 275 | 42·5 | 0·675 |
| Beilschmiedia pendula (Sw.) Hemsl. | Laur- | 3987 | 0·100 | 280 | 33·2 | 0·973 |
| Brosimum alicastrum Sw. | Mor- | 712 | 0·045 | 280 | 58·7 | 0·924# |
| Byrsonima spicata (Cav.) Kunth | Malphigi- | 108 | 0·953 | 93 | 21·7 | <0·001 |
| Callichlamys latifolia (Rich.) K.Schum. | Bignoni- | 134 | 0·328 | 93 | 29·6 | 0·132 |
| Calophyllum longifolium Willd. | Clusi- | 4532 | 0·266 | 93 | 40·7 | 0·884 |
| Capparis frondosa Jacq. | Brassic- | 97 | 0·262 | 275 | 19·8 | 0·178 |
| Carica papaya L. | Caric- | 12 | 0·459 | 349·3 | 43·3 | 0·004 |
| Cecropia obtusifolia Bertol. | Mor- | 0·5 | 0·878 | 310·4 | 36·2 | <0·001 |
| Chamaedorea tepejilote Liebm. | Arec- | 150 | 0·053 | 275 | 20·3 | 0·724 |
| Chrysophyllum cainito L. | Sapot- | 210 | 0·562 | 68·1 | 39·4 | 0·022 |
| Clidemia octona (Bonpl.) L.O.Williams | Melastomat- | 0·01 | 0·250 | 271·8 | 29·4 | <0·001 |
| Cochlospermum vitifolium (willd.) Sprenq. | Cochlosperm- | 26 | 0·741 | 93 | 25·5 | 0·001 |
| Connarus turczaninowii Triana & Planch. | Connar- | 405 | 0·214 | 280 | 18·6 | 0·572# |
| Cordia panamensis L.Riley | Boragin- | 59 | 0·720 | 93 | 20·7 | 0·001 |
| Cupania cinerea Poepp. | Sapind- | 510 | 0·254 | 275·6 | 41·0 | 0·526 |
| Cydista aequinoctalis (L.) Miers. | Bignoni- | 112 | 0·547 | 32·9 | 58·1 | 0·014 |
| Dalbergia retusa Hamsl· | Fab- | 130 | 0·792 | 280 | 14·7 | 0·001 |
| Davilla aspera (Aubl.) Benoist | Dilleni- | 24 | 0·337 | 280 | 10·2 | 0·027 |
| Dendropanax arboreus (L.) Decne. & Planch. | Arali- | 8 | 0·790 | 275 | 42·7 | <0·001 |
| Desmoncus isthmius L.H.Bailey | Arec- | 314 | 0·261 | 280 | 33·6 | 0·397* |
| Didymopanax morototoni (Aubl.) | Arali- | 14 | 0·642 | 68·1 | 16·5 | 0·001 |
| Dioclea reflexa Hook. f. | Fab- | 4013 | 0·672 | 93 | 11·8 | 0·106 |
| Dipteryx panamensis (Pittier) Record & mell | Fab- | 6127 | 0·870 | 68·1 | 29·7 | 0·024 |
| Elaeis oleifera (Kunth) Cortés | Arec- | 2507 | 0·694 | 280 | 19·4 | 0·058 |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Fab- | 545 | 0·470 | 280 | 10·6 | 0·121 |
| Ficus obtusifolia Kunth | Mor- | 1 | 0·635 | 271·8 | 29·9 | <0·001 |
| Garcinia mangostana L. | Clusi- | 748 | 0·281 | – | 0·548 | |
| Guarea guidonia (L.) Sleumer | Meli- | 153 | 0·267 | 271·8 | 27·8 | 0·240* |
| Guazuma ulmifolia Lam. | Malv- | 4 | 0·488 | 34·2 | 52·5 | 0·001 |
| Gustavia superba (Kunth.) O. Berq. | Lecythid- | 2815 | 0·061 | 271·8 | 42·6 | 0·974 |
| Hampea appendiculata (Donn. Sm.) Standl. | Malv- | 60 | 0·187 | 310·4 | 10·3 | 0·226 |
| Herrania purpurea (Pittier) R.E.Schult | Malv- | 217 | 0·296 | 93 | 42·7 | 0·247* |
| Hevea brasiliense Müll.Arg | Euphorbi- | 3630 | 0·525 | 275 | – | 0·319* |
| Hura crepitans L. | Euphorbi- | 845 | 0·327 | 280 | 18·1 | 0·463 |
| Hybanthus prunifolius (Humb. & Bonpl. Ex Roem. & Schult.) Schulze-Menz | Viol- | 12 | 0·268 | 280 | 16·6 | 0·028 |
| Hyeronima laxiflora (Tul.) Müll. Arg | Euphorbi- | 7 | 0·699 | 34·2 | 16·4 | <0·001 |
| Hylenaea praecelsa (Miers) A.C.Sm. | Celastr- | 2027 | 0·670 | 310·4 | 16·5 | 0·060 |
| Inga minutula (Schery) T.S.Elias. | Fab- | 380 | 0·141 | 32·9 | 41·3 | 0·722 |
| Laetia procera (Poepp.) Eichler | Salic- | 5 | 0·762 | 32·9 | 42·5 | <0·001 |
| Lafoensia punicifolia DC. | Lythr- | 36 | 0·516 | 34·2 | 19·8 | 0·007 |
| Lonchocarpus pentaphyllus (Poir.) Kunth | Fab- | 161 | 0·156 | 310·4 | 19·6 | 0·501# |
| Luehea seemannii Triana & Planch. | Malv- | 3 | 0·784 | 34·2 | 20·2 | <0·001 |
| Macfadyena unguis-cati (L.) A.H.Gentry | Bignon- | 29 | 0·633 | 32·9 | 56·9 | 0·002 |
| Mangifera indica L. | Anacardi- | 4500 | 0·381 | 271·8 | 38·3 | 0·706 |
| Margaritaria nobilis L. f. | Euphorbi- | 8 | 0·756 | 310·4 | 32·2 | <0·001 |
| Maripa panamensis Hemsl. | Convolvul- | 353 | 0·094 | 275·6 | 22·1 | 0·795 |
| Mesechites trifida (Jacq.) Müll. Arg. | Apocyn- | 3 | 0·429 | 271·8 | 32·4 | 0·002 |
| Miconia argentea (Sw.) DC. | Melastomat- | 0·08 | 0·274 | 93 | 40·2 | <0·001 |
| Mouriri myrtilloides subsp. parvifolia (Benth.) Morley | Melastomat- | 68 | 0·116 | 271·8 | 37·2 | 0·400 |
| Ochroma pyramidale (Cav. Ex Lam.) Urb. | Malv- | 10 | 0·940 | 93 | 22·0 | <0·001 |
| Ocotea whitei Woodson | Laur- | 7300 | 0·013 | 271·8 | 33·7 | 0·993 |
| Odontadenia macrantha (Roem. & Schutt.) Markgr. | Apocyn- | 172 | 0·379 | 32·9 | 29·2 | 0·103 |
| Ormosia macrocalyx Ducke | Fab- | 401 | 0·131 | 271·8 | 46·8 | 0·751# |
| Ossaea quinquenervia (Mill.) Coqn. | Melastomat- | 0·01 | 0·207 | 271·8 | 36·6 | <0·001 |
| Pachira sessilis (Bentham) Pittier. | Malv- | 349 | 0·206 | 271·8 | 13·6 | 0·557# |
| Passiflora foetida L. | Passiflor- | 8 | 0·650 | 34·2 | 30·4 | <0·001 |
| Persea americana Mill. | Laur- | 20 670 | 0·037 | 271·8 | 52·5 | 0·997 |
| Piper marginatum Jacq. | Piper- | 0·1 | 0·208 | 32·9 | 35·5 | <0·001 |
| Piper peltatum L. | Piper- | 0·04 | 0·147 | 275 | 31·2 | <0·001 |
| Platymiscium pinnatum (Jacq.) Dugand | Fab- | 313 | 0·791 | 34·2 | 24·5 | 0·003 |
| Platypodium elegans Vogel | Fab- | 1219 | 0·892 | 93 | 10·8 | 0·004 |
| Pouteria sapota (Jacq.) H.E.Moore & Stearn | Sapot- | 13 040 | 0·472 | 275 | 41·5 | 0·724 |
| Prionostemma aspera (Lam.) Miers | Celastr- | 182 | 0·431 | 275·6 | 38·9 | 0·068 |
| Prioria copaifera Griseb. | Fab- | 23 840 | 0·366 | 271·8 | 42·1 | 0·930 |
| Protium panamense (Rose) I.M.Johnst. | Burser- | 4800 | 0·256 | 280 | 26·4 | 0·899 |
| Pseudobombax septenatum (Jacq.) Dugand | Malv- | 60 | 0·246 | 32·9 | 11·3 | 0·140 |
| Psidium guajava L. | Myrt- | 8 | 0·861 | 280 | 24·8 | <0·001 |
| Psychotria micrantha Kunth. | Rubi- | 10 | 0·575 | 238·4 | 38·3 | 0·001 |
| Quararibea pterocalyx Hemsl. | Malv- | 4040 | 0·356 | 349·3 | 61·6 | 0·736# |
| Randia formosa (Jacq.) Schum. | Rubi- | 31 | 0·523 | 271·8 | 43·4 | 0·005 |
| Rheedia edulis (Seem.) Planch. | Clusi- | 289 | 0·252 | 280 | 45·7 | 0·400* |
| Serjania rhombea Radlk. | Sapind- | 11 | 0·829 | 349·3 | 37·7 | <0·001 |
| Siparuna guianensis Aubl. | Monimi- | 20 | 0·618 | 275·6 | 16·0 | 0·001 |
| Solanum hayesii Fernald | Solan- | 2 | 0·807 | 93 | 18·0 | <0·001 |
| Spondias mombin L. | Anacardi- | 1160 | 0·957 | 28 | 54·1 | 0·002 |
| Stizolobium pruriens (L. in Stickm.) Medik. | Fab- | 529 | 0·176 | 93 | 10·0 | 0·715# |
| Stylogyne standleyi Lundell | Myrsin- | 66 | 0·278 | 93 | 38·2 | 0·114 |
| Swartzia simplex ‘ochnacea’ (Sw.) Sprenq. | Fab- | 1025 | 0·048 | 280 | 38·8 | 0·943 |
| Swietenia macrophylla King | Meli- | 470 | 0·290 | 32·9 | 43·2 | 0·418 |
| Synechanthus warscewiczianus H.Wendl. | Arec- | 272 | 0·105 | 238·4 | 41·1 | 0·732 |
| Syzygium jambos L. | Myrt- | 2380 | 0·076 | 32·9 | – | 0·965 |
| Tabebuia guayacan (Seem.) Hemsl. | Bignoni- | 26 | 0·204 | 32·9 | 9·1 | 0·101 |
| Tachigali versicolor Standl. & L.O.Williams | Fab- | 910 | 0·275 | 271·8 | 18·7 | 0·608# |
| Theobroma cacao L. | Malv- | 1771 | 0·275 | 28 | – | 0·743 |
| Trema micrantha (L.) Blume | Ulm- | 2 | 0·682 | 275 | 31·7 | <0·001 |
| Trichilia tuberculata C. DC. | Meli- | 151 | 0·038 | 310·4 | 40·2 | 0·754 |
| Trichospermum galeottii (Turcz.) Kosterm. | Malv- | 3 | 0·571 | 34·2 | 14·7 | <0·001 |
| Virola sebeifera Aubl. | Myristic- | 472 | 0·223 | 68·1 | 22·1 | 0·585 |
| Virola surinamensis (Rol.) Warb. | Myristic- | 1952 | 0·103 | 280 | 25·9 | 0·946 |
| Zanthoxylum panamense P.Wilson | Rut- | 29 | 0·791 | 349·3 | 14·6 | <0·001 |
| Species . | Family . | Dry mass (mg) . | SCR . | Monthly rainfall at seed shed (mm) . | WC at dispersal (% f. wt. basis) . | P(D – S) . |
|---|---|---|---|---|---|---|
| Adelia triloba (Müll.Arg.) Hemsle | Euphorbi- | 26 | 0·354 | 34·2 | 10·5 | 0·025 |
| Adenopodia polystachya (L.) J.R.Dixon ex Croat | Fab- | 298 | 0·942 | 32·9 | 29·6 | 0·001 |
| Albizia guachapele (H.B. & K.) Dugand | Fab- | 32 | 0·433 | 280 | 17·1 | 0·012 |
| Alchornea costaricensis Pax & K.Hoffm. | Euphorbi- | 34 | 0·799 | 280 | 20·5 | <0·001 |
| Alibertia edulis (Rich.) A.Rich. ex DC. | Rubi- | 15 | 0·114 | 32·9 | 24·4 | 0·142 |
| Anacardium excelsum (Bertero & Balb. Ex Kunth) Skeels | Anacardi- | 1507 | 0·433 | 280 | 43·8 | 0·339* |
| Andira inermis (Sw.) Kunth | Fab- | 792 | 0·297 | 280 | 41·7 | 0·522 |
| Annona glabra L. | Annon- | 229 | 0·358 | 271·8 | 21·7 | 0·157 |
| Annona muricata L. | Annon- | 322 | 0·706 | 275 | 22·3 | 0·008 |
| Anthodon panamense A.C.Sm. | Celastr- | 81 | 0·438 | 310·4 | 61·1 | 0·031 |
| Apeiba membranacea Spruce ex Benth. | Malv- | 69 | 0·776 | 280 | 52·7 | 0·001 |
| Apeiba tibourbou Aubl. | Malv- | 17 | 0·584 | 280 | 25·2 | 0·002 |
| Astronium graveolens Jacq. | Anacardi- | 30 | 0·368 | 280 | 30·2 | 0·024 |
| Bactris gasipaes Kunth | Arec- | 1680 | 0·303 | 275 | 42·5 | 0·675 |
| Beilschmiedia pendula (Sw.) Hemsl. | Laur- | 3987 | 0·100 | 280 | 33·2 | 0·973 |
| Brosimum alicastrum Sw. | Mor- | 712 | 0·045 | 280 | 58·7 | 0·924# |
| Byrsonima spicata (Cav.) Kunth | Malphigi- | 108 | 0·953 | 93 | 21·7 | <0·001 |
| Callichlamys latifolia (Rich.) K.Schum. | Bignoni- | 134 | 0·328 | 93 | 29·6 | 0·132 |
| Calophyllum longifolium Willd. | Clusi- | 4532 | 0·266 | 93 | 40·7 | 0·884 |
| Capparis frondosa Jacq. | Brassic- | 97 | 0·262 | 275 | 19·8 | 0·178 |
| Carica papaya L. | Caric- | 12 | 0·459 | 349·3 | 43·3 | 0·004 |
| Cecropia obtusifolia Bertol. | Mor- | 0·5 | 0·878 | 310·4 | 36·2 | <0·001 |
| Chamaedorea tepejilote Liebm. | Arec- | 150 | 0·053 | 275 | 20·3 | 0·724 |
| Chrysophyllum cainito L. | Sapot- | 210 | 0·562 | 68·1 | 39·4 | 0·022 |
| Clidemia octona (Bonpl.) L.O.Williams | Melastomat- | 0·01 | 0·250 | 271·8 | 29·4 | <0·001 |
| Cochlospermum vitifolium (willd.) Sprenq. | Cochlosperm- | 26 | 0·741 | 93 | 25·5 | 0·001 |
| Connarus turczaninowii Triana & Planch. | Connar- | 405 | 0·214 | 280 | 18·6 | 0·572# |
| Cordia panamensis L.Riley | Boragin- | 59 | 0·720 | 93 | 20·7 | 0·001 |
| Cupania cinerea Poepp. | Sapind- | 510 | 0·254 | 275·6 | 41·0 | 0·526 |
| Cydista aequinoctalis (L.) Miers. | Bignoni- | 112 | 0·547 | 32·9 | 58·1 | 0·014 |
| Dalbergia retusa Hamsl· | Fab- | 130 | 0·792 | 280 | 14·7 | 0·001 |
| Davilla aspera (Aubl.) Benoist | Dilleni- | 24 | 0·337 | 280 | 10·2 | 0·027 |
| Dendropanax arboreus (L.) Decne. & Planch. | Arali- | 8 | 0·790 | 275 | 42·7 | <0·001 |
| Desmoncus isthmius L.H.Bailey | Arec- | 314 | 0·261 | 280 | 33·6 | 0·397* |
| Didymopanax morototoni (Aubl.) | Arali- | 14 | 0·642 | 68·1 | 16·5 | 0·001 |
| Dioclea reflexa Hook. f. | Fab- | 4013 | 0·672 | 93 | 11·8 | 0·106 |
| Dipteryx panamensis (Pittier) Record & mell | Fab- | 6127 | 0·870 | 68·1 | 29·7 | 0·024 |
| Elaeis oleifera (Kunth) Cortés | Arec- | 2507 | 0·694 | 280 | 19·4 | 0·058 |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Fab- | 545 | 0·470 | 280 | 10·6 | 0·121 |
| Ficus obtusifolia Kunth | Mor- | 1 | 0·635 | 271·8 | 29·9 | <0·001 |
| Garcinia mangostana L. | Clusi- | 748 | 0·281 | – | 0·548 | |
| Guarea guidonia (L.) Sleumer | Meli- | 153 | 0·267 | 271·8 | 27·8 | 0·240* |
| Guazuma ulmifolia Lam. | Malv- | 4 | 0·488 | 34·2 | 52·5 | 0·001 |
| Gustavia superba (Kunth.) O. Berq. | Lecythid- | 2815 | 0·061 | 271·8 | 42·6 | 0·974 |
| Hampea appendiculata (Donn. Sm.) Standl. | Malv- | 60 | 0·187 | 310·4 | 10·3 | 0·226 |
| Herrania purpurea (Pittier) R.E.Schult | Malv- | 217 | 0·296 | 93 | 42·7 | 0·247* |
| Hevea brasiliense Müll.Arg | Euphorbi- | 3630 | 0·525 | 275 | – | 0·319* |
| Hura crepitans L. | Euphorbi- | 845 | 0·327 | 280 | 18·1 | 0·463 |
| Hybanthus prunifolius (Humb. & Bonpl. Ex Roem. & Schult.) Schulze-Menz | Viol- | 12 | 0·268 | 280 | 16·6 | 0·028 |
| Hyeronima laxiflora (Tul.) Müll. Arg | Euphorbi- | 7 | 0·699 | 34·2 | 16·4 | <0·001 |
| Hylenaea praecelsa (Miers) A.C.Sm. | Celastr- | 2027 | 0·670 | 310·4 | 16·5 | 0·060 |
| Inga minutula (Schery) T.S.Elias. | Fab- | 380 | 0·141 | 32·9 | 41·3 | 0·722 |
| Laetia procera (Poepp.) Eichler | Salic- | 5 | 0·762 | 32·9 | 42·5 | <0·001 |
| Lafoensia punicifolia DC. | Lythr- | 36 | 0·516 | 34·2 | 19·8 | 0·007 |
| Lonchocarpus pentaphyllus (Poir.) Kunth | Fab- | 161 | 0·156 | 310·4 | 19·6 | 0·501# |
| Luehea seemannii Triana & Planch. | Malv- | 3 | 0·784 | 34·2 | 20·2 | <0·001 |
| Macfadyena unguis-cati (L.) A.H.Gentry | Bignon- | 29 | 0·633 | 32·9 | 56·9 | 0·002 |
| Mangifera indica L. | Anacardi- | 4500 | 0·381 | 271·8 | 38·3 | 0·706 |
| Margaritaria nobilis L. f. | Euphorbi- | 8 | 0·756 | 310·4 | 32·2 | <0·001 |
| Maripa panamensis Hemsl. | Convolvul- | 353 | 0·094 | 275·6 | 22·1 | 0·795 |
| Mesechites trifida (Jacq.) Müll. Arg. | Apocyn- | 3 | 0·429 | 271·8 | 32·4 | 0·002 |
| Miconia argentea (Sw.) DC. | Melastomat- | 0·08 | 0·274 | 93 | 40·2 | <0·001 |
| Mouriri myrtilloides subsp. parvifolia (Benth.) Morley | Melastomat- | 68 | 0·116 | 271·8 | 37·2 | 0·400 |
| Ochroma pyramidale (Cav. Ex Lam.) Urb. | Malv- | 10 | 0·940 | 93 | 22·0 | <0·001 |
| Ocotea whitei Woodson | Laur- | 7300 | 0·013 | 271·8 | 33·7 | 0·993 |
| Odontadenia macrantha (Roem. & Schutt.) Markgr. | Apocyn- | 172 | 0·379 | 32·9 | 29·2 | 0·103 |
| Ormosia macrocalyx Ducke | Fab- | 401 | 0·131 | 271·8 | 46·8 | 0·751# |
| Ossaea quinquenervia (Mill.) Coqn. | Melastomat- | 0·01 | 0·207 | 271·8 | 36·6 | <0·001 |
| Pachira sessilis (Bentham) Pittier. | Malv- | 349 | 0·206 | 271·8 | 13·6 | 0·557# |
| Passiflora foetida L. | Passiflor- | 8 | 0·650 | 34·2 | 30·4 | <0·001 |
| Persea americana Mill. | Laur- | 20 670 | 0·037 | 271·8 | 52·5 | 0·997 |
| Piper marginatum Jacq. | Piper- | 0·1 | 0·208 | 32·9 | 35·5 | <0·001 |
| Piper peltatum L. | Piper- | 0·04 | 0·147 | 275 | 31·2 | <0·001 |
| Platymiscium pinnatum (Jacq.) Dugand | Fab- | 313 | 0·791 | 34·2 | 24·5 | 0·003 |
| Platypodium elegans Vogel | Fab- | 1219 | 0·892 | 93 | 10·8 | 0·004 |
| Pouteria sapota (Jacq.) H.E.Moore & Stearn | Sapot- | 13 040 | 0·472 | 275 | 41·5 | 0·724 |
| Prionostemma aspera (Lam.) Miers | Celastr- | 182 | 0·431 | 275·6 | 38·9 | 0·068 |
| Prioria copaifera Griseb. | Fab- | 23 840 | 0·366 | 271·8 | 42·1 | 0·930 |
| Protium panamense (Rose) I.M.Johnst. | Burser- | 4800 | 0·256 | 280 | 26·4 | 0·899 |
| Pseudobombax septenatum (Jacq.) Dugand | Malv- | 60 | 0·246 | 32·9 | 11·3 | 0·140 |
| Psidium guajava L. | Myrt- | 8 | 0·861 | 280 | 24·8 | <0·001 |
| Psychotria micrantha Kunth. | Rubi- | 10 | 0·575 | 238·4 | 38·3 | 0·001 |
| Quararibea pterocalyx Hemsl. | Malv- | 4040 | 0·356 | 349·3 | 61·6 | 0·736# |
| Randia formosa (Jacq.) Schum. | Rubi- | 31 | 0·523 | 271·8 | 43·4 | 0·005 |
| Rheedia edulis (Seem.) Planch. | Clusi- | 289 | 0·252 | 280 | 45·7 | 0·400* |
| Serjania rhombea Radlk. | Sapind- | 11 | 0·829 | 349·3 | 37·7 | <0·001 |
| Siparuna guianensis Aubl. | Monimi- | 20 | 0·618 | 275·6 | 16·0 | 0·001 |
| Solanum hayesii Fernald | Solan- | 2 | 0·807 | 93 | 18·0 | <0·001 |
| Spondias mombin L. | Anacardi- | 1160 | 0·957 | 28 | 54·1 | 0·002 |
| Stizolobium pruriens (L. in Stickm.) Medik. | Fab- | 529 | 0·176 | 93 | 10·0 | 0·715# |
| Stylogyne standleyi Lundell | Myrsin- | 66 | 0·278 | 93 | 38·2 | 0·114 |
| Swartzia simplex ‘ochnacea’ (Sw.) Sprenq. | Fab- | 1025 | 0·048 | 280 | 38·8 | 0·943 |
| Swietenia macrophylla King | Meli- | 470 | 0·290 | 32·9 | 43·2 | 0·418 |
| Synechanthus warscewiczianus H.Wendl. | Arec- | 272 | 0·105 | 238·4 | 41·1 | 0·732 |
| Syzygium jambos L. | Myrt- | 2380 | 0·076 | 32·9 | – | 0·965 |
| Tabebuia guayacan (Seem.) Hemsl. | Bignoni- | 26 | 0·204 | 32·9 | 9·1 | 0·101 |
| Tachigali versicolor Standl. & L.O.Williams | Fab- | 910 | 0·275 | 271·8 | 18·7 | 0·608# |
| Theobroma cacao L. | Malv- | 1771 | 0·275 | 28 | – | 0·743 |
| Trema micrantha (L.) Blume | Ulm- | 2 | 0·682 | 275 | 31·7 | <0·001 |
| Trichilia tuberculata C. DC. | Meli- | 151 | 0·038 | 310·4 | 40·2 | 0·754 |
| Trichospermum galeottii (Turcz.) Kosterm. | Malv- | 3 | 0·571 | 34·2 | 14·7 | <0·001 |
| Virola sebeifera Aubl. | Myristic- | 472 | 0·223 | 68·1 | 22·1 | 0·585 |
| Virola surinamensis (Rol.) Warb. | Myristic- | 1952 | 0·103 | 280 | 25·9 | 0·946 |
| Zanthoxylum panamense P.Wilson | Rut- | 29 | 0·791 | 349·3 | 14·6 | <0·001 |
Desiccation-tolerant species incorrectly assigned both by our model (Equation 1) and in the cross-validation as desiccation sensitive.
Desiccation-sensitive species incorrectly assigned both by our model (Equation 1) and in the cross-validation as desiccation tolerant.
Classification to family level (names lack the -aceae suffix), seed mass, seed coat ratio (SCR), rainfall in the month of seed dispersal and seed water content (WC) at shedding (species with desiccation-sensitive seeds are shown in bold type face). Seed dry mass and seed coat ratio data are taken fromDaws et al. (2005). Also shown is the predicted probability of seeds of each species exhibiting desiccation sensitivity P (D – S) based on entering species-specific values for SCR and dry mass intoEquation 1
| Species . | Family . | Dry mass (mg) . | SCR . | Monthly rainfall at seed shed (mm) . | WC at dispersal (% f. wt. basis) . | P(D – S) . |
|---|---|---|---|---|---|---|
| Adelia triloba (Müll.Arg.) Hemsle | Euphorbi- | 26 | 0·354 | 34·2 | 10·5 | 0·025 |
| Adenopodia polystachya (L.) J.R.Dixon ex Croat | Fab- | 298 | 0·942 | 32·9 | 29·6 | 0·001 |
| Albizia guachapele (H.B. & K.) Dugand | Fab- | 32 | 0·433 | 280 | 17·1 | 0·012 |
| Alchornea costaricensis Pax & K.Hoffm. | Euphorbi- | 34 | 0·799 | 280 | 20·5 | <0·001 |
| Alibertia edulis (Rich.) A.Rich. ex DC. | Rubi- | 15 | 0·114 | 32·9 | 24·4 | 0·142 |
| Anacardium excelsum (Bertero & Balb. Ex Kunth) Skeels | Anacardi- | 1507 | 0·433 | 280 | 43·8 | 0·339* |
| Andira inermis (Sw.) Kunth | Fab- | 792 | 0·297 | 280 | 41·7 | 0·522 |
| Annona glabra L. | Annon- | 229 | 0·358 | 271·8 | 21·7 | 0·157 |
| Annona muricata L. | Annon- | 322 | 0·706 | 275 | 22·3 | 0·008 |
| Anthodon panamense A.C.Sm. | Celastr- | 81 | 0·438 | 310·4 | 61·1 | 0·031 |
| Apeiba membranacea Spruce ex Benth. | Malv- | 69 | 0·776 | 280 | 52·7 | 0·001 |
| Apeiba tibourbou Aubl. | Malv- | 17 | 0·584 | 280 | 25·2 | 0·002 |
| Astronium graveolens Jacq. | Anacardi- | 30 | 0·368 | 280 | 30·2 | 0·024 |
| Bactris gasipaes Kunth | Arec- | 1680 | 0·303 | 275 | 42·5 | 0·675 |
| Beilschmiedia pendula (Sw.) Hemsl. | Laur- | 3987 | 0·100 | 280 | 33·2 | 0·973 |
| Brosimum alicastrum Sw. | Mor- | 712 | 0·045 | 280 | 58·7 | 0·924# |
| Byrsonima spicata (Cav.) Kunth | Malphigi- | 108 | 0·953 | 93 | 21·7 | <0·001 |
| Callichlamys latifolia (Rich.) K.Schum. | Bignoni- | 134 | 0·328 | 93 | 29·6 | 0·132 |
| Calophyllum longifolium Willd. | Clusi- | 4532 | 0·266 | 93 | 40·7 | 0·884 |
| Capparis frondosa Jacq. | Brassic- | 97 | 0·262 | 275 | 19·8 | 0·178 |
| Carica papaya L. | Caric- | 12 | 0·459 | 349·3 | 43·3 | 0·004 |
| Cecropia obtusifolia Bertol. | Mor- | 0·5 | 0·878 | 310·4 | 36·2 | <0·001 |
| Chamaedorea tepejilote Liebm. | Arec- | 150 | 0·053 | 275 | 20·3 | 0·724 |
| Chrysophyllum cainito L. | Sapot- | 210 | 0·562 | 68·1 | 39·4 | 0·022 |
| Clidemia octona (Bonpl.) L.O.Williams | Melastomat- | 0·01 | 0·250 | 271·8 | 29·4 | <0·001 |
| Cochlospermum vitifolium (willd.) Sprenq. | Cochlosperm- | 26 | 0·741 | 93 | 25·5 | 0·001 |
| Connarus turczaninowii Triana & Planch. | Connar- | 405 | 0·214 | 280 | 18·6 | 0·572# |
| Cordia panamensis L.Riley | Boragin- | 59 | 0·720 | 93 | 20·7 | 0·001 |
| Cupania cinerea Poepp. | Sapind- | 510 | 0·254 | 275·6 | 41·0 | 0·526 |
| Cydista aequinoctalis (L.) Miers. | Bignoni- | 112 | 0·547 | 32·9 | 58·1 | 0·014 |
| Dalbergia retusa Hamsl· | Fab- | 130 | 0·792 | 280 | 14·7 | 0·001 |
| Davilla aspera (Aubl.) Benoist | Dilleni- | 24 | 0·337 | 280 | 10·2 | 0·027 |
| Dendropanax arboreus (L.) Decne. & Planch. | Arali- | 8 | 0·790 | 275 | 42·7 | <0·001 |
| Desmoncus isthmius L.H.Bailey | Arec- | 314 | 0·261 | 280 | 33·6 | 0·397* |
| Didymopanax morototoni (Aubl.) | Arali- | 14 | 0·642 | 68·1 | 16·5 | 0·001 |
| Dioclea reflexa Hook. f. | Fab- | 4013 | 0·672 | 93 | 11·8 | 0·106 |
| Dipteryx panamensis (Pittier) Record & mell | Fab- | 6127 | 0·870 | 68·1 | 29·7 | 0·024 |
| Elaeis oleifera (Kunth) Cortés | Arec- | 2507 | 0·694 | 280 | 19·4 | 0·058 |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Fab- | 545 | 0·470 | 280 | 10·6 | 0·121 |
| Ficus obtusifolia Kunth | Mor- | 1 | 0·635 | 271·8 | 29·9 | <0·001 |
| Garcinia mangostana L. | Clusi- | 748 | 0·281 | – | 0·548 | |
| Guarea guidonia (L.) Sleumer | Meli- | 153 | 0·267 | 271·8 | 27·8 | 0·240* |
| Guazuma ulmifolia Lam. | Malv- | 4 | 0·488 | 34·2 | 52·5 | 0·001 |
| Gustavia superba (Kunth.) O. Berq. | Lecythid- | 2815 | 0·061 | 271·8 | 42·6 | 0·974 |
| Hampea appendiculata (Donn. Sm.) Standl. | Malv- | 60 | 0·187 | 310·4 | 10·3 | 0·226 |
| Herrania purpurea (Pittier) R.E.Schult | Malv- | 217 | 0·296 | 93 | 42·7 | 0·247* |
| Hevea brasiliense Müll.Arg | Euphorbi- | 3630 | 0·525 | 275 | – | 0·319* |
| Hura crepitans L. | Euphorbi- | 845 | 0·327 | 280 | 18·1 | 0·463 |
| Hybanthus prunifolius (Humb. & Bonpl. Ex Roem. & Schult.) Schulze-Menz | Viol- | 12 | 0·268 | 280 | 16·6 | 0·028 |
| Hyeronima laxiflora (Tul.) Müll. Arg | Euphorbi- | 7 | 0·699 | 34·2 | 16·4 | <0·001 |
| Hylenaea praecelsa (Miers) A.C.Sm. | Celastr- | 2027 | 0·670 | 310·4 | 16·5 | 0·060 |
| Inga minutula (Schery) T.S.Elias. | Fab- | 380 | 0·141 | 32·9 | 41·3 | 0·722 |
| Laetia procera (Poepp.) Eichler | Salic- | 5 | 0·762 | 32·9 | 42·5 | <0·001 |
| Lafoensia punicifolia DC. | Lythr- | 36 | 0·516 | 34·2 | 19·8 | 0·007 |
| Lonchocarpus pentaphyllus (Poir.) Kunth | Fab- | 161 | 0·156 | 310·4 | 19·6 | 0·501# |
| Luehea seemannii Triana & Planch. | Malv- | 3 | 0·784 | 34·2 | 20·2 | <0·001 |
| Macfadyena unguis-cati (L.) A.H.Gentry | Bignon- | 29 | 0·633 | 32·9 | 56·9 | 0·002 |
| Mangifera indica L. | Anacardi- | 4500 | 0·381 | 271·8 | 38·3 | 0·706 |
| Margaritaria nobilis L. f. | Euphorbi- | 8 | 0·756 | 310·4 | 32·2 | <0·001 |
| Maripa panamensis Hemsl. | Convolvul- | 353 | 0·094 | 275·6 | 22·1 | 0·795 |
| Mesechites trifida (Jacq.) Müll. Arg. | Apocyn- | 3 | 0·429 | 271·8 | 32·4 | 0·002 |
| Miconia argentea (Sw.) DC. | Melastomat- | 0·08 | 0·274 | 93 | 40·2 | <0·001 |
| Mouriri myrtilloides subsp. parvifolia (Benth.) Morley | Melastomat- | 68 | 0·116 | 271·8 | 37·2 | 0·400 |
| Ochroma pyramidale (Cav. Ex Lam.) Urb. | Malv- | 10 | 0·940 | 93 | 22·0 | <0·001 |
| Ocotea whitei Woodson | Laur- | 7300 | 0·013 | 271·8 | 33·7 | 0·993 |
| Odontadenia macrantha (Roem. & Schutt.) Markgr. | Apocyn- | 172 | 0·379 | 32·9 | 29·2 | 0·103 |
| Ormosia macrocalyx Ducke | Fab- | 401 | 0·131 | 271·8 | 46·8 | 0·751# |
| Ossaea quinquenervia (Mill.) Coqn. | Melastomat- | 0·01 | 0·207 | 271·8 | 36·6 | <0·001 |
| Pachira sessilis (Bentham) Pittier. | Malv- | 349 | 0·206 | 271·8 | 13·6 | 0·557# |
| Passiflora foetida L. | Passiflor- | 8 | 0·650 | 34·2 | 30·4 | <0·001 |
| Persea americana Mill. | Laur- | 20 670 | 0·037 | 271·8 | 52·5 | 0·997 |
| Piper marginatum Jacq. | Piper- | 0·1 | 0·208 | 32·9 | 35·5 | <0·001 |
| Piper peltatum L. | Piper- | 0·04 | 0·147 | 275 | 31·2 | <0·001 |
| Platymiscium pinnatum (Jacq.) Dugand | Fab- | 313 | 0·791 | 34·2 | 24·5 | 0·003 |
| Platypodium elegans Vogel | Fab- | 1219 | 0·892 | 93 | 10·8 | 0·004 |
| Pouteria sapota (Jacq.) H.E.Moore & Stearn | Sapot- | 13 040 | 0·472 | 275 | 41·5 | 0·724 |
| Prionostemma aspera (Lam.) Miers | Celastr- | 182 | 0·431 | 275·6 | 38·9 | 0·068 |
| Prioria copaifera Griseb. | Fab- | 23 840 | 0·366 | 271·8 | 42·1 | 0·930 |
| Protium panamense (Rose) I.M.Johnst. | Burser- | 4800 | 0·256 | 280 | 26·4 | 0·899 |
| Pseudobombax septenatum (Jacq.) Dugand | Malv- | 60 | 0·246 | 32·9 | 11·3 | 0·140 |
| Psidium guajava L. | Myrt- | 8 | 0·861 | 280 | 24·8 | <0·001 |
| Psychotria micrantha Kunth. | Rubi- | 10 | 0·575 | 238·4 | 38·3 | 0·001 |
| Quararibea pterocalyx Hemsl. | Malv- | 4040 | 0·356 | 349·3 | 61·6 | 0·736# |
| Randia formosa (Jacq.) Schum. | Rubi- | 31 | 0·523 | 271·8 | 43·4 | 0·005 |
| Rheedia edulis (Seem.) Planch. | Clusi- | 289 | 0·252 | 280 | 45·7 | 0·400* |
| Serjania rhombea Radlk. | Sapind- | 11 | 0·829 | 349·3 | 37·7 | <0·001 |
| Siparuna guianensis Aubl. | Monimi- | 20 | 0·618 | 275·6 | 16·0 | 0·001 |
| Solanum hayesii Fernald | Solan- | 2 | 0·807 | 93 | 18·0 | <0·001 |
| Spondias mombin L. | Anacardi- | 1160 | 0·957 | 28 | 54·1 | 0·002 |
| Stizolobium pruriens (L. in Stickm.) Medik. | Fab- | 529 | 0·176 | 93 | 10·0 | 0·715# |
| Stylogyne standleyi Lundell | Myrsin- | 66 | 0·278 | 93 | 38·2 | 0·114 |
| Swartzia simplex ‘ochnacea’ (Sw.) Sprenq. | Fab- | 1025 | 0·048 | 280 | 38·8 | 0·943 |
| Swietenia macrophylla King | Meli- | 470 | 0·290 | 32·9 | 43·2 | 0·418 |
| Synechanthus warscewiczianus H.Wendl. | Arec- | 272 | 0·105 | 238·4 | 41·1 | 0·732 |
| Syzygium jambos L. | Myrt- | 2380 | 0·076 | 32·9 | – | 0·965 |
| Tabebuia guayacan (Seem.) Hemsl. | Bignoni- | 26 | 0·204 | 32·9 | 9·1 | 0·101 |
| Tachigali versicolor Standl. & L.O.Williams | Fab- | 910 | 0·275 | 271·8 | 18·7 | 0·608# |
| Theobroma cacao L. | Malv- | 1771 | 0·275 | 28 | – | 0·743 |
| Trema micrantha (L.) Blume | Ulm- | 2 | 0·682 | 275 | 31·7 | <0·001 |
| Trichilia tuberculata C. DC. | Meli- | 151 | 0·038 | 310·4 | 40·2 | 0·754 |
| Trichospermum galeottii (Turcz.) Kosterm. | Malv- | 3 | 0·571 | 34·2 | 14·7 | <0·001 |
| Virola sebeifera Aubl. | Myristic- | 472 | 0·223 | 68·1 | 22·1 | 0·585 |
| Virola surinamensis (Rol.) Warb. | Myristic- | 1952 | 0·103 | 280 | 25·9 | 0·946 |
| Zanthoxylum panamense P.Wilson | Rut- | 29 | 0·791 | 349·3 | 14·6 | <0·001 |
| Species . | Family . | Dry mass (mg) . | SCR . | Monthly rainfall at seed shed (mm) . | WC at dispersal (% f. wt. basis) . | P(D – S) . |
|---|---|---|---|---|---|---|
| Adelia triloba (Müll.Arg.) Hemsle | Euphorbi- | 26 | 0·354 | 34·2 | 10·5 | 0·025 |
| Adenopodia polystachya (L.) J.R.Dixon ex Croat | Fab- | 298 | 0·942 | 32·9 | 29·6 | 0·001 |
| Albizia guachapele (H.B. & K.) Dugand | Fab- | 32 | 0·433 | 280 | 17·1 | 0·012 |
| Alchornea costaricensis Pax & K.Hoffm. | Euphorbi- | 34 | 0·799 | 280 | 20·5 | <0·001 |
| Alibertia edulis (Rich.) A.Rich. ex DC. | Rubi- | 15 | 0·114 | 32·9 | 24·4 | 0·142 |
| Anacardium excelsum (Bertero & Balb. Ex Kunth) Skeels | Anacardi- | 1507 | 0·433 | 280 | 43·8 | 0·339* |
| Andira inermis (Sw.) Kunth | Fab- | 792 | 0·297 | 280 | 41·7 | 0·522 |
| Annona glabra L. | Annon- | 229 | 0·358 | 271·8 | 21·7 | 0·157 |
| Annona muricata L. | Annon- | 322 | 0·706 | 275 | 22·3 | 0·008 |
| Anthodon panamense A.C.Sm. | Celastr- | 81 | 0·438 | 310·4 | 61·1 | 0·031 |
| Apeiba membranacea Spruce ex Benth. | Malv- | 69 | 0·776 | 280 | 52·7 | 0·001 |
| Apeiba tibourbou Aubl. | Malv- | 17 | 0·584 | 280 | 25·2 | 0·002 |
| Astronium graveolens Jacq. | Anacardi- | 30 | 0·368 | 280 | 30·2 | 0·024 |
| Bactris gasipaes Kunth | Arec- | 1680 | 0·303 | 275 | 42·5 | 0·675 |
| Beilschmiedia pendula (Sw.) Hemsl. | Laur- | 3987 | 0·100 | 280 | 33·2 | 0·973 |
| Brosimum alicastrum Sw. | Mor- | 712 | 0·045 | 280 | 58·7 | 0·924# |
| Byrsonima spicata (Cav.) Kunth | Malphigi- | 108 | 0·953 | 93 | 21·7 | <0·001 |
| Callichlamys latifolia (Rich.) K.Schum. | Bignoni- | 134 | 0·328 | 93 | 29·6 | 0·132 |
| Calophyllum longifolium Willd. | Clusi- | 4532 | 0·266 | 93 | 40·7 | 0·884 |
| Capparis frondosa Jacq. | Brassic- | 97 | 0·262 | 275 | 19·8 | 0·178 |
| Carica papaya L. | Caric- | 12 | 0·459 | 349·3 | 43·3 | 0·004 |
| Cecropia obtusifolia Bertol. | Mor- | 0·5 | 0·878 | 310·4 | 36·2 | <0·001 |
| Chamaedorea tepejilote Liebm. | Arec- | 150 | 0·053 | 275 | 20·3 | 0·724 |
| Chrysophyllum cainito L. | Sapot- | 210 | 0·562 | 68·1 | 39·4 | 0·022 |
| Clidemia octona (Bonpl.) L.O.Williams | Melastomat- | 0·01 | 0·250 | 271·8 | 29·4 | <0·001 |
| Cochlospermum vitifolium (willd.) Sprenq. | Cochlosperm- | 26 | 0·741 | 93 | 25·5 | 0·001 |
| Connarus turczaninowii Triana & Planch. | Connar- | 405 | 0·214 | 280 | 18·6 | 0·572# |
| Cordia panamensis L.Riley | Boragin- | 59 | 0·720 | 93 | 20·7 | 0·001 |
| Cupania cinerea Poepp. | Sapind- | 510 | 0·254 | 275·6 | 41·0 | 0·526 |
| Cydista aequinoctalis (L.) Miers. | Bignoni- | 112 | 0·547 | 32·9 | 58·1 | 0·014 |
| Dalbergia retusa Hamsl· | Fab- | 130 | 0·792 | 280 | 14·7 | 0·001 |
| Davilla aspera (Aubl.) Benoist | Dilleni- | 24 | 0·337 | 280 | 10·2 | 0·027 |
| Dendropanax arboreus (L.) Decne. & Planch. | Arali- | 8 | 0·790 | 275 | 42·7 | <0·001 |
| Desmoncus isthmius L.H.Bailey | Arec- | 314 | 0·261 | 280 | 33·6 | 0·397* |
| Didymopanax morototoni (Aubl.) | Arali- | 14 | 0·642 | 68·1 | 16·5 | 0·001 |
| Dioclea reflexa Hook. f. | Fab- | 4013 | 0·672 | 93 | 11·8 | 0·106 |
| Dipteryx panamensis (Pittier) Record & mell | Fab- | 6127 | 0·870 | 68·1 | 29·7 | 0·024 |
| Elaeis oleifera (Kunth) Cortés | Arec- | 2507 | 0·694 | 280 | 19·4 | 0·058 |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Fab- | 545 | 0·470 | 280 | 10·6 | 0·121 |
| Ficus obtusifolia Kunth | Mor- | 1 | 0·635 | 271·8 | 29·9 | <0·001 |
| Garcinia mangostana L. | Clusi- | 748 | 0·281 | – | 0·548 | |
| Guarea guidonia (L.) Sleumer | Meli- | 153 | 0·267 | 271·8 | 27·8 | 0·240* |
| Guazuma ulmifolia Lam. | Malv- | 4 | 0·488 | 34·2 | 52·5 | 0·001 |
| Gustavia superba (Kunth.) O. Berq. | Lecythid- | 2815 | 0·061 | 271·8 | 42·6 | 0·974 |
| Hampea appendiculata (Donn. Sm.) Standl. | Malv- | 60 | 0·187 | 310·4 | 10·3 | 0·226 |
| Herrania purpurea (Pittier) R.E.Schult | Malv- | 217 | 0·296 | 93 | 42·7 | 0·247* |
| Hevea brasiliense Müll.Arg | Euphorbi- | 3630 | 0·525 | 275 | – | 0·319* |
| Hura crepitans L. | Euphorbi- | 845 | 0·327 | 280 | 18·1 | 0·463 |
| Hybanthus prunifolius (Humb. & Bonpl. Ex Roem. & Schult.) Schulze-Menz | Viol- | 12 | 0·268 | 280 | 16·6 | 0·028 |
| Hyeronima laxiflora (Tul.) Müll. Arg | Euphorbi- | 7 | 0·699 | 34·2 | 16·4 | <0·001 |
| Hylenaea praecelsa (Miers) A.C.Sm. | Celastr- | 2027 | 0·670 | 310·4 | 16·5 | 0·060 |
| Inga minutula (Schery) T.S.Elias. | Fab- | 380 | 0·141 | 32·9 | 41·3 | 0·722 |
| Laetia procera (Poepp.) Eichler | Salic- | 5 | 0·762 | 32·9 | 42·5 | <0·001 |
| Lafoensia punicifolia DC. | Lythr- | 36 | 0·516 | 34·2 | 19·8 | 0·007 |
| Lonchocarpus pentaphyllus (Poir.) Kunth | Fab- | 161 | 0·156 | 310·4 | 19·6 | 0·501# |
| Luehea seemannii Triana & Planch. | Malv- | 3 | 0·784 | 34·2 | 20·2 | <0·001 |
| Macfadyena unguis-cati (L.) A.H.Gentry | Bignon- | 29 | 0·633 | 32·9 | 56·9 | 0·002 |
| Mangifera indica L. | Anacardi- | 4500 | 0·381 | 271·8 | 38·3 | 0·706 |
| Margaritaria nobilis L. f. | Euphorbi- | 8 | 0·756 | 310·4 | 32·2 | <0·001 |
| Maripa panamensis Hemsl. | Convolvul- | 353 | 0·094 | 275·6 | 22·1 | 0·795 |
| Mesechites trifida (Jacq.) Müll. Arg. | Apocyn- | 3 | 0·429 | 271·8 | 32·4 | 0·002 |
| Miconia argentea (Sw.) DC. | Melastomat- | 0·08 | 0·274 | 93 | 40·2 | <0·001 |
| Mouriri myrtilloides subsp. parvifolia (Benth.) Morley | Melastomat- | 68 | 0·116 | 271·8 | 37·2 | 0·400 |
| Ochroma pyramidale (Cav. Ex Lam.) Urb. | Malv- | 10 | 0·940 | 93 | 22·0 | <0·001 |
| Ocotea whitei Woodson | Laur- | 7300 | 0·013 | 271·8 | 33·7 | 0·993 |
| Odontadenia macrantha (Roem. & Schutt.) Markgr. | Apocyn- | 172 | 0·379 | 32·9 | 29·2 | 0·103 |
| Ormosia macrocalyx Ducke | Fab- | 401 | 0·131 | 271·8 | 46·8 | 0·751# |
| Ossaea quinquenervia (Mill.) Coqn. | Melastomat- | 0·01 | 0·207 | 271·8 | 36·6 | <0·001 |
| Pachira sessilis (Bentham) Pittier. | Malv- | 349 | 0·206 | 271·8 | 13·6 | 0·557# |
| Passiflora foetida L. | Passiflor- | 8 | 0·650 | 34·2 | 30·4 | <0·001 |
| Persea americana Mill. | Laur- | 20 670 | 0·037 | 271·8 | 52·5 | 0·997 |
| Piper marginatum Jacq. | Piper- | 0·1 | 0·208 | 32·9 | 35·5 | <0·001 |
| Piper peltatum L. | Piper- | 0·04 | 0·147 | 275 | 31·2 | <0·001 |
| Platymiscium pinnatum (Jacq.) Dugand | Fab- | 313 | 0·791 | 34·2 | 24·5 | 0·003 |
| Platypodium elegans Vogel | Fab- | 1219 | 0·892 | 93 | 10·8 | 0·004 |
| Pouteria sapota (Jacq.) H.E.Moore & Stearn | Sapot- | 13 040 | 0·472 | 275 | 41·5 | 0·724 |
| Prionostemma aspera (Lam.) Miers | Celastr- | 182 | 0·431 | 275·6 | 38·9 | 0·068 |
| Prioria copaifera Griseb. | Fab- | 23 840 | 0·366 | 271·8 | 42·1 | 0·930 |
| Protium panamense (Rose) I.M.Johnst. | Burser- | 4800 | 0·256 | 280 | 26·4 | 0·899 |
| Pseudobombax septenatum (Jacq.) Dugand | Malv- | 60 | 0·246 | 32·9 | 11·3 | 0·140 |
| Psidium guajava L. | Myrt- | 8 | 0·861 | 280 | 24·8 | <0·001 |
| Psychotria micrantha Kunth. | Rubi- | 10 | 0·575 | 238·4 | 38·3 | 0·001 |
| Quararibea pterocalyx Hemsl. | Malv- | 4040 | 0·356 | 349·3 | 61·6 | 0·736# |
| Randia formosa (Jacq.) Schum. | Rubi- | 31 | 0·523 | 271·8 | 43·4 | 0·005 |
| Rheedia edulis (Seem.) Planch. | Clusi- | 289 | 0·252 | 280 | 45·7 | 0·400* |
| Serjania rhombea Radlk. | Sapind- | 11 | 0·829 | 349·3 | 37·7 | <0·001 |
| Siparuna guianensis Aubl. | Monimi- | 20 | 0·618 | 275·6 | 16·0 | 0·001 |
| Solanum hayesii Fernald | Solan- | 2 | 0·807 | 93 | 18·0 | <0·001 |
| Spondias mombin L. | Anacardi- | 1160 | 0·957 | 28 | 54·1 | 0·002 |
| Stizolobium pruriens (L. in Stickm.) Medik. | Fab- | 529 | 0·176 | 93 | 10·0 | 0·715# |
| Stylogyne standleyi Lundell | Myrsin- | 66 | 0·278 | 93 | 38·2 | 0·114 |
| Swartzia simplex ‘ochnacea’ (Sw.) Sprenq. | Fab- | 1025 | 0·048 | 280 | 38·8 | 0·943 |
| Swietenia macrophylla King | Meli- | 470 | 0·290 | 32·9 | 43·2 | 0·418 |
| Synechanthus warscewiczianus H.Wendl. | Arec- | 272 | 0·105 | 238·4 | 41·1 | 0·732 |
| Syzygium jambos L. | Myrt- | 2380 | 0·076 | 32·9 | – | 0·965 |
| Tabebuia guayacan (Seem.) Hemsl. | Bignoni- | 26 | 0·204 | 32·9 | 9·1 | 0·101 |
| Tachigali versicolor Standl. & L.O.Williams | Fab- | 910 | 0·275 | 271·8 | 18·7 | 0·608# |
| Theobroma cacao L. | Malv- | 1771 | 0·275 | 28 | – | 0·743 |
| Trema micrantha (L.) Blume | Ulm- | 2 | 0·682 | 275 | 31·7 | <0·001 |
| Trichilia tuberculata C. DC. | Meli- | 151 | 0·038 | 310·4 | 40·2 | 0·754 |
| Trichospermum galeottii (Turcz.) Kosterm. | Malv- | 3 | 0·571 | 34·2 | 14·7 | <0·001 |
| Virola sebeifera Aubl. | Myristic- | 472 | 0·223 | 68·1 | 22·1 | 0·585 |
| Virola surinamensis (Rol.) Warb. | Myristic- | 1952 | 0·103 | 280 | 25·9 | 0·946 |
| Zanthoxylum panamense P.Wilson | Rut- | 29 | 0·791 | 349·3 | 14·6 | <0·001 |
Desiccation-tolerant species incorrectly assigned both by our model (Equation 1) and in the cross-validation as desiccation sensitive.
Desiccation-sensitive species incorrectly assigned both by our model (Equation 1) and in the cross-validation as desiccation tolerant.
We thank Professor Norman Pammenter for comments on the manuscript. Steven Paton of the Smithsonian Tropical Research Institute, Panamá kindly provided rainfall data. Financial support to N.C.G. was provided by the NSF (BSR-8517395), NERC (GR3-69511) and the Smithsonian Tropical Research Institute. Financial support to M.I.D. and H.W.P. was provided by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew receives grant-aided support from Defra, UK. Funding to pay the Open Access publication charges for this article was provided by the Royal Botanic Gardens, Kew.
LITERATURE CITED
Berjak P, Dini M, Pammenter NW.
Berjak P, Campbell GK, Farrant JM, Omondi Oloo W, Pammenter NW.
Black M, Pritchard HW. (eds).
Curran LM, Webb CO.
D'Arcy WG.
Daws MI, Mullins CE, Burslem DFRP, Paton SR, Dalling JW.
Daws MI, Lydall E, Chmielarz P, Leprince O, Matthews S, Thanos CA, Pritchard HW.
Daws MI, Garwood NC, Pritchard HW.
Daws MI, Cleland H, Chmielarz P, Gorian F, Leprince O, Mullins CE, Thanos CA, Vandvik V, Pritchard HW.
Den Ouden J, Jansen PA, Smit R.
Dickie JB, Bowyer JT.
Dickie JB, Pritchard HW.
Dickie JB, May K, Morris SVA, Titley SE.
Dietrich WE, Windsor DM, Dunne T.
Dussert S, Chabrillange N, Engelmann F, Anthony F, Louarn J, Hamon S.
Ellis RH, Hong TD, Roberts EH.
Flynn S, Turner RM, Dickie JB.
Garcia D, Banuelos MJ, Houle G.
Greggains V, Finch-Savage WE, Quick WP, Atherton NM.
Grubb PJ, Burslem DFRP.
Hong TD, Ellis RH.
Hong TD, Ellis RH.
Hong TD, Ellis RH.
Hong TD, Ellis RH.
Leigh EG Jr, Rand AS, Windsor DM. (eds).
Pammenter NW, Berjak P.
Poulsen K.
Pritchard HW.
Pritchard HW, Manger KR.
Pritchard HW, Wood CB, Hodges S, Vautier HJ.
Pritchard HW, Daws MI, Fletcher BJ, Gaméné CS, Msanga HP, Omondi W.
Sacandé M.
Tompsett PB.
Tompsett PB.
Tweddle JC, Dickie JB, Baskin CC, Baskin JM.
Author notes
1Seed Conservation Department, Royal Botanic Gardens Kew, Wakehurst Place, Ardingly, West Sussex RH17 6TN, UK and 2Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901-6509, USA