-
PDF
- Split View
-
Views
-
Cite
Cite
PETER BERNHARDT, TAMMY SAGE, PETER WESTON, HIROSHI AZUMA, MATHEW LAM, LEONARD B. THIEN, JEREMY BRUHL, The Pollination of Trimenia moorei (Trimeniaceae): Floral Volatiles, Insect/Wind Pollen Vectors and Stigmatic Self‐incompatibility in a Basal Angiosperm, Annals of Botany, Volume 92, Issue 3, September 2003, Pages 445–458, https://doi.org/10.1093/aob/mcg157
Close - Share Icon Share
Abstract
Trimenia moorei (Oliv.) Philipson is an andromonoecious liane with >0·40 of the total flower buds maturing as bisexual flowers. Male and bisexual flowers are strongly scented with pollen, anther sacs and receptacle scars testing positively for volatile emissions. Scent analyses detect over 20 components. The major fatty acid derivative is 8‐heptadecene, and 2‐phenylethanol dominates the benzenoids. While hover‐flies in the genera Melangyna and Triglyphus contact the stigma with their probosces, the stigma secretes no free‐flowing, edible fluids. Copious pollen is the only edible reward consumed by hover‐flies (Syprhidae), sawflies (Pergidae) and bees in the families Apidae, Colletidae and Halictidae. All these insects carried pollen of T. moorei on their heads, legs and thoraces and female bees in the genera Apis, Exoneura, Leioproctus and Lasioglossum stored pollen on their hind legs. Pollen traps also indicate that pollen is shed directly into the air, permitting wind pollination. When bisexual flower buds are bagged (isolated from insect foragers) on the liane then subjected to a series of hand‐pollination experiments after perianth segments open, the structural analyses of pollen–carpel interactions indicate that T. moorei has a trichome‐rich dry‐type stigma with an early‐acting self‐incompatibility (SI) system. Bicellular pollen grains deposited on stigmas belonging to the same plant germinate but fail to penetrate intercellular spaces, while grains deposited following cross‐pollination reach the ovule within 24 h. Fluorescence analyses of 76 carpels collected at random from unbagged (open‐pollinated) flowers on five plants indicates that at least 64 % of carpels are cross‐pollinated in situ. Trimenia moorei is the first species within the ANITA group, and second within reilictual‐basal angiosperm lineages, to exhibit stigmatic SI in combination with dry‐type stigma and bicellular pollen, a condition once considered to be atypical for angiosperms as a whole but now known to be present in numerous taxa.
Received: 20 Jan 2003; Returned for revision: 7 May 2003; Accepted: 2 June 2003
INTRODUCTION
The Trimeniaceae consists of eight species distributed in tropical and subtropical forests in eastern Australia and islands in the South Pacific (Endress and Sampson, 1983; Philipson, 1993; Wagner and Lorence, 1999). The family, comprising the monotypic genus Trimenia, was once included within the Laurales (sensu Mabberley, 1987). More recent molecular treatments have placed the Trimeniaceae as a discrete lineage within the ANITA grade, a paraphyletic group of seven families now placed at the base of the angiosperm phylogeny (Mathews and Donoghue, 2000; Qiu et al., 2000; Soltis, 2000; Sun et al., 2002). Knowledge of breeding systems and pollination mechanisms in Trimenia spp., as with other members of the ANITA group, remains limited (Endress, 2001). Floral sexuality within Trimenia is varied with some species exhibiting andromonoecy or potentially dioecy (Philipson, 1986; Wagner and Lorence, 1999). Protogyny, wind pollination and/or insect pollination are suspected (Wagner and Lorence, 1999; Endress, 2001), while reference to floral volatiles remains contradictory and anecdotal (Endress and Sampson, 1983; Endress, 2001). Previous studies have provided insights into the developmental anatomy of floral organs in Trimenia (Endress and Sampson, 1983; Endress, 2001) but structural information on pollen–carpel interactions are absent for the family, and it is not apparent whether self‐incompatibility (SI) occurs within any Trimenia sp.
The purpose of the present investigation was to examine three components of the breeding system of Trimenia moorei, an andromonoecious liane restricted to subtropical, north‐eastern Australia. Three basic questions were addressed: (1) Do flowers of T. moorei produce a scent, and if so, what are the constituent volatile components? (2) Does insect and/or wind pollination occur in T. moorei? (3) Is SI present in T. moorei and, if so, what is the site and structural features of carpellary cells where SI functions?
MATERIALS AND METHODS
Study site
Field studies of T. moorei were conducted in New South Wales in a region within the Northern Tablelands and North Coast (sensu Harden, 1993) within closed forest sensuSpecht et al. (1974). The primary site for the collection of floral scent, flower‐visiting insects, and studies on floral phenology and pollen–carpel interactions was on Boulder Flat road, 0·9 km ENE of Forest Way, Cunnawarra National Park (30°34′15S 152°16′10E, altitude 1040 m; Northern Tablelands). At this site, T. moorei was a common component of the vine flora including Smilax australis, Cissus antarctica, Cissus hypoglauca, Hibbertia scandens and Rubusnebulossus. These vines festooned understorey trees in forest dominated by Eucalyptusobliqua and E. cameronii (P. H. Weston 2533, 13/x/01, NSW 477096).
Floral lifespan and perfect/staminate floral ratios within inflorescences
Inflorescences with mature, closed buds were selected at random and marked with dated jeweller’s tags. These labelled buds were examined daily to record and compare the comparative number of perfect vs. staminate flowers and the collapse and/or abscission of their respective floral organs.
Scent analysis and structural characterization of scent‐producing tissue
To assess whether or not flowers produced scent distinct from vegetative tissue, volatiles from andromonoecious inflorescences and leaves of T. moorei (Table 1) were collected and analysed as described by Azuma et al. (1997). Controls were also conducted as described by Azuma et al. (1997). To determine which floral tissues were most likely to release volatiles, tepals, androecial and gynoecial segments were treated with a 1 % Neutral Red solution as in (Bernhardt, 1995), and were also prepared for light, scanning and transmission microscopy as described by Pontieri and Sage (1999).
Insect behaviour and pollen load analysis
Insect activity on floral organs was documented for about 40 h during daylight from 8 to 10 Oct. 2001. A Canon YC‐V200 video camera equipped with a Canon zoom lens was used to record insect visitations. Insects visiting T. moorei were collected and pollen grains carried by insects were removed, stained and identified following Bernhardt (1987). A pollen type was recorded as present on a slide if more than 25 individual grains, or tetrads or polyads of that type were counted (see Bernhardt, 1987; Kearns and Inouye, 1993). Pinned insect specimens were sent to the Entomology Department at the Australian Museum, Sydney for identification and voucher deposition.
Pollen traps
To determine the potential for pollen dispersal by wind, microscope slides covered with propylene jelly were placed for 2 d within five individual flowering plants and at 5 m intervals 2–3 m above ground between plants on a 60 m line transect bisecting the T. moorei population. The microscope slides were subsequently placed in slide boxes, sealed and transported to the University of Toronto where they were examined with a compound light microscope to quantify T. moorei pollen loads.
Pollen–carpel interactions following cross‐, self‐ and open pollination
Floral buds were enclosed in light‐weight pollination bags constructed from bridal veil approx. 48 h prior to the ‘female stage’ of anthesis as defined by Endress (2001) to exclude natural pollen vectors. Flowers were cross‐ or self‐pollinated during this female stage of anthesis prior to tepal abscission and anther dehiscence by tapping freshly dehisced anthers just above receptive stigmas at the time of anthesis of perfect (bisexual) flowers. Cross‐ and self‐pollinations of bagged flowers were conducted on five plants. As T. moorei is a long, rampant and rambling liane, cross‐pollination was performed using outcross pollen loads from stems separated by at least 50 m within the population. In contrast, self‐pollination was conducted using pollen from dehisced staminate flowers on the same inflorescence or branch as the self‐pollinated carpel. Pollination by hand was performed from 0700 to 1100 h, bags were then replaced and these flowers were harvested 24 and 48 h post‐pollination and prepared for fluorescence microscopy (n = 10 flowers/plant/pollination treatment; Martin, 1959) and light, scanning and transmission electron microscopy (n = 3–6 flowers per plant per microscopy technique used; Pontieri and Sage, 1999). Unpollinated perfect flowers and open‐pollinated flowers were also sampled from five plants at anthesis, 24 and 48 h post‐anthesis and prepared for light, scanning and transmission electron microscopy (n = 3–6 flowers per plant per microscopy technique used). Open‐pollinated, bisexual flowers (n = 76) were also collected over several days and prepared for light microscopy to determine the frequency of ovule penetration by pollen tubes. The frequency of ovule penetration by pollen tubes was also compared with the number of green, developing fruits on 91 inflorescences from seven plants collected on 2 Nov. 2001.
Histochemical features of the transmitting tissues pollen tubes encountered were characterized on fresh flowers using the following stains: (a) 1 % alcian blue in 3 % acetic acid for acidic polysacharides, pectins and mucilages (Jensen, 1962; Benes, 1968); (b) 0·05 % ruthenium red for pectic substances (Gurr, 1965); (c) 1 % aniline blue‐black (ABB) in 7 % acetic acid for proteins (Fisher, 1968); (d) sudan black B in 70 % ethanol for lipids (O’Brien and McCully, 1981), 0·01 % auramine‐O in 0·05 m Tris/HCl buffer at pH 7·2 observed under UV light for lipids (Heslop‐Harrison and Shivanna, 1977), and α‐Yarif reagent (Biosupplies, Australia, Parkville, Victoria) for arabinogalactans/ arabinogalactanproteins (AG/AGPs) with a control of β‐Yarif reagent (Biosupplies Australia, Parkville, Victoria, Australia). Antibodies were also used to detect pectins (JIM 5 and 7) and AG/AGPs (JIM 13) at the level of the TEM as described by Koehl (2002).
RESULTS
Floral lifespan and perfect/staminate floral ratios within inflorescences
Fifty‐three inflorescences (terminal and lateral inflorescences combined) derived from seven different lianes produced a total of 322 flowers with a mean of 6·4 ± 3·6 flowers per inflorescence (range = 1–17) of which 3·8 ± 2·8 were staminate flowers (range = 1–14). Only two living inflorescences examined at the site failed to produce male flowers. Only three inflorescences failed to produce a single bisexual flower.
The order in which buds opened on an inflorescence was acropetal to subacropetal. Within an inflorescence, buds of bisexual flowers were 84 % more likely to extend their stigmas above their opening tepals prior to the extension of stamens by buds of male flowers. Bisexual flowers remained in this ‘female phase’ an additional 2–4 d before extending their stamens. Male flowers on the same inflorescence invariably opened their perianths and extended their dehiscent stamens 1–2 d before the bisexual flowers in the female phase dropped their perianth segments and fully extended their stamens.
Scent analyses
GC‐MS analyses of floral and leaf volatiles detected a wide variety of compounds some of which were common or distinctive to both tissue types (Table 1). The dominant compounds unique to floral tissue sampled in the morning were 2‐phenylethanol and 8‐heptadecene. The timing of production of these compounds was coincident with floral insect activity. The floral odour of T. moorei was strong (detected at 2 m) and reminiscent of raw cucumber or the fresh rinds of watermelon. This fragrance was always detected at field sites in the morning but not in the afternoon. In contrast, crushed vegetative tissue yielded a different and distinctly spicy odour similar to commercial cinnamon bark or the crushed leaves of Lindera benzoin.
Neutral Red tests conducted on fully opened male and bisexual flowers over 2‐h periods indicated that several structures produced volatile compounds. The strongest and most immediate response was reserved for pollen, anther sacs, and abscission scars on the floral receptacle remaining after abscission of the tepals. The stigmas produced a partial response within 30 min of staining that was confined to the stigma margins regardless of whether the flower was in the female or male phase at the time of stain application.
Insect behaviour and pollen load analysis
A minimum of 16 different species of insects visited flowers of T. moorei (Table 2). Pollen load analysis indicated that many of these insects visited plant species other than T. moorei (Table 3).
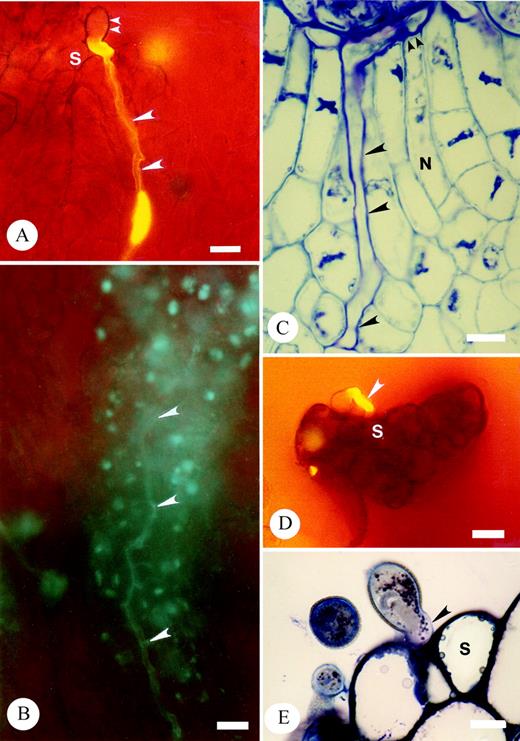
Hover‐flies (Fig. 1A and B; Syrphidae—Melangyna spp., 9–11 mm long; Triglyphusfulvicornis, 5–6 mm long) were always the first and most common floral visitors arriving at approx. 1000 h and remaining active until 1400 or 1600 h on both sunny, cloudless days and on cool cloudy days. Both male and female hover‐flies visited perfect and staminate flowers foraging for pollen on each floral type with the mouth parts, legs and thoraces of Melangyna spp. repeatedly contacting the stigmas of perfect flowers (Fig. 1A). Contact with stigmas was also established when Melangyna spp. either landed directly on stigmas or attempted to reach stamens of other flowers by crawling over the stigma. Microscopic analyses indicated that the heads, thoraces, legs and claws (tarsi) of Melangyna spp. had pollen depositions of T. moorei (Fig. 1C).
Three families of bees visited T. moorei; Colletidae (Amphylaeus nubilosellus, 9 mm long; Leioproctus advena, L. sp., 8–9 mm long; Meroglossa itamuca, 12 mm long; Fig. 1D), Apidae s.l. (Apis mellifera, 14 mm long; Exoneura bicolor and Exoneura spp., 6–8 mm long; Fig. 1E) and Halictidae (Lasioglossum speculatum, L. sp. nov.; 8–9 mm long; Fig. 1F). Bee activity commenced between 1030 and 1100 h but rarely persisted beyond 1300 h.
Worker Apis mellifera and female Lasioglossum, Leioproctus and Exoneura spp. landed directly on perfect and staminate flowers with dehiscent anthers collecting pollen with their forelegs by scraping anthers then transferring grains to their corbiculae (A. mellifera) or scopae (all remaining species). In contrast to the hover‐flies, bees were not observed contacting stigmas with their mouth parts, but their legs and abdomens consistently brushed the stigma during foraging activity along an inflorescence or between flowers at the same level in an inflorescence. The branched pollen‐retaining hairs of halictids and Leioproctus, located on both hind legs and ventral abdominal surfaces, came into direct contact with stigmas.
Males and females of Amphylaeus nubillosellus and Meroglossa itamuca placed their mouth parts on anthers while foraging for pollen. Visits to inflorescences by these colletid bees were brief and they launched themselves into the air after visiting two or three flowers on one inflorescence. It was not apparent if these bees contacted stigmas while they foraged as their activities were always confined to inflorescences on those vines confined to light gaps blooming 1–3 m over the heads of collectors.
Unidentified sawflies (Pergidae; Hymenoptera, 5 mm in length) were also observed foraging for pollen on flowers of T. moorei. The sawflies foraged in a slow methodical manner visiting every flower on the inflorescence and they contacted stigmas while foraging in a manner similar to hover‐flies.
A male Aporocera sp. Nov. (Chrysomelidae), 4 mm in length was the only beetle collected on the flowers of T. moorei. Similar beetles were seen foraging on leaves but not collected.
Pollen traps
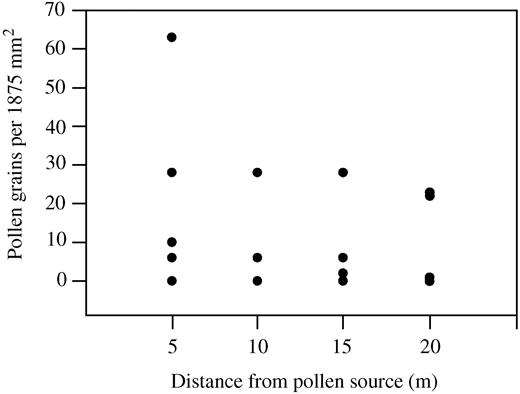
Pollen of T. moorei was dispersed onto the petroleum jelly‐covered microscope slides placed within plants and along the transect within the population (Fig. 2).
Structural and histochemical features of the pollen tube pathway of unpollinated flowers
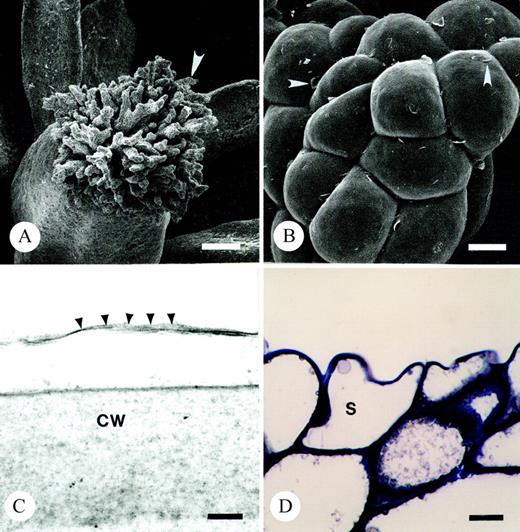
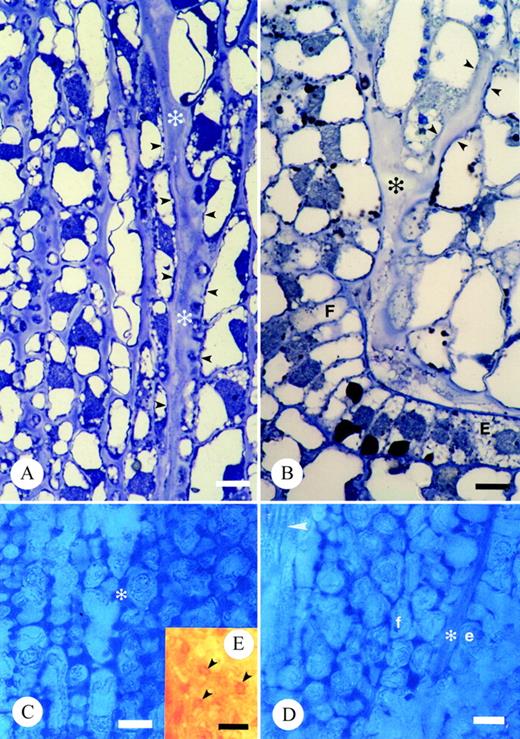
The terminally situated stigma is composed of pluricellular, pluriseriate trichomes (Fig. 3A and B; see also, Endress and Sampson, 1983). The stigma of T. moorei is of the dry‐type. The tightly packed cells of T. moorei are covered by a thin cuticle (Fig. 3C) histochemically positive for lipids and proteins. The cuticle is punctately uplifted in some areas and histochemically indistinct (Fig. 3B). The protoplast of tightly packed stigmatic trichome cells at the distal regions of the stigma are occupied by a predominant vacuole (Fig. 3D), a nucleus, very few organelles, and a minimal endomembrane system represented only by endoplasmic reticulum. The cell walls are slightly histochemically positive and immunopositive for pectins and AGPs. An extracellular matrix (ECM) is noticeably absent in these distal regions of the stigma. A large core of stigmatic tissue continuous with the stigma/ovary junction and ovule funiculus is composed of loosely packed, elongate cells with a thick extracellular matrix (Fig. 4; see also Endress and Sampson, 1983) histochemically positive primarily for pectins (Fig. 4C and D) and to a lesser degree, AG/AGPs (Fig. 4E). Immunolabelling also indicated that the ECM contains pectins and AGPs, although localization events were low due to loss of these coumpounds during chemical fixation. The ECM was continuous with an ECM lining the ovary wall and micropyle (Fig. 4B and D).
Pollen–carpel interactions following bagged cross‐ and open pollination
All bagged, and hand cross‐pollinated stigmas (n = 50) resulted in pollen tube growth to the ovary locule by 24 h post‐pollination and entry into the ovule by 48 h. Germinated cross‐pollen tubes of T. moorei grew initially between the tightly packed cells of the stigmatic trichomes (Fig. 5A) and subsequently grew intercellulary (Fig. 5B) within the extensive ECM prior to entry into the ovary locule. Within the ovary, pollen tubes also grew in the ECM to penetrate the micropyle and subsequently grew intercellularly within the nucellus up to the embryo sac (Fig. 5C). Sixty‐four per cent of ovules from open (insect and/or wind) pollinated flowers sectioned contained pollen tubes in the nucellus.
Pollen–carpel interactions following self‐pollination
All bagged, hand self‐pollinated stigmas (n = 50) resulted either in failure of pollen germination or, if germination ensued, pollen tubes produced only a short tube that failed to penetrate the tightly packed stigmatic cells (Fig. 5D and E). Observations were the same at 24 and 48 h following self‐pollination.
Conversion of flowers into fruit
A mean of 1·6 fruits per inflorescence ± 1·2 (range 0–5) were produced from open‐pollinated flowers. Eight inflorescences carried one or two abortive ovaries that turned yellow, instead of green, and dropped off when touched with a probe.
DISCUSSION
The present study provides novel information on specific components of the floral biology of Trimenia moorei. Most notably, evidence is provided for floral scent production, insect pollination and wind dispersal of pollen, and the presence of self‐pollen recognition and rejection reactions at the stigmatic epidermis. As well, characterization of the nature of cells that pollen tubes encounter en route to the embryo sac indicates the presence of an extensive ECM that extends from the base of the stigma to the micropyle. The discussion begins by placing the results on floral scent analysis in the context of what is known about floral scent production and function in other angiosperms. The significance of insect/wind pollination, as well as self‐pollen recognition and rejection, in this species is then discussed. In conclusion, the functional significance of data on transmitting tissue exudates in T. moorei is addressed.
Scent production
The spicy odour of bruised vegetation of T. moorei explains one reason why the otherwise, unpleasantly bitter tasting leaves (P. Bernhardt, unpubl. res.) were collected in the 19th century to flavour some beverages (Mayden, 1894). Of greater importance, the flowers of T. moorei produce two dominant compounds, 2‐phenylethanol and 8‐heptadecene, coincident with insect activity in the morning. The first compound is one of the most common floral scent volatiles in angiosperms, having been reported in over 34 genera (Knudsen et al., 1993). For example, it is a major component in the floral scent of Abelia grandiflora (Haynes et al., 1991), Nigritella niga (Kaiser, 1993) and Magnolia virginiana (northern population; Azuma et al., 1997). In contrast, 8‐heptadecene is an uncommon floral scent volatile and is usually identified as a minor component when present (Thien et al., 1975; Azuma et al., 1997). Taxa in which 8‐heptadecene has been identified as a minor component include Magnolia acuminata (Thien et al., 1975; Azuma et al., 1997). Within the ANITA grade, both 2‐phenylethanol and 8‐heptadecene are volatiles produced by flowers in the Nymphaeaceae, the only other taxon within the ANITA grade documented currently as producers of known floral volatiles (Knudsen et al., 1993). Recent studies examining floral volatile production by Amborellatrichopoda indicate an absence of such activity (Thien et al., 2003)
The compound 2‐phenylethanol is known to attract insects and elicit behavioural responses such as reflex extension of the proboscis and antennae detected by an electroantennogram (EAG) in some butterflies (Luehdorfiajaponica, Omura et al., 1999; Pieris rapae, Honda et al., 1998), a beetle (Hoplia communis, Imai et al., 1998), the two‐spotted stinkbug (Perillus bioculatus, Weissbecker et al., 1999), the twelve‐spotted lady beetle, Coleomegilla maculata, and the green lacewing, Chrysoperla carnea (Zhu et al., 1999). In flowers where this volatile has been identified, it is produced in pulses that are temporally coordinated with insect activity. Notably, in the present study, the majority of insect activity was coincident with production of 2‐phenylethanol, and hover‐flies visiting T. moorei were observed extending their probosces to touch the stigmas in spite of the absence of free‐flowing exudates. Whether or not this compound is important in the pollination biology of other Trimenia spp., and thus common to the taxon, possibly originating early in the history of the family, remains to be characterized.
Insect/wind pollination
Observations and analyses of floral foragers on T. moorei illustrate that this species incorporates both Australian native species of Diptera and Hymenoptera without much pollen dispersal by Coleoptera. Therefore, T. moorei appears to conform to the generalist model of insect‐pollination exhibited by many relictual‐basal angiosperms, some belonging to the ANITA group, and posited to exist in protoangiosperms (Bernhardt and Thien, 1987; Grimaldi 1999; Bernhardt, 2000). Diptera, an ancient order of insects, fossils of which first appear in Triassic strata (Rohdendorf, 1974; Grimaldi, 1999), are common pollinators amongst the ANITA group (Schneider and Jeter, 1982; Thien et al., 2000), other relictual‐basal taxa (Bernhardt and Thien, 1987; Tosaki et al., 2001) and the gymnosperm genus, Ephedra (Meeuse et al., 1990). However, the pollination of T. moorei by hover‐flies should not be construed as model evidence of an early association between the family, Syrphidae, and the first angiosperms. While the family, Syrphidae, does belong to the suborder Brachyneura, (Jurassic, minimum age; Ren, 1998) which probably co‐radiated with the early angiosperms (Grimaldi, 1999), fossils representative of the family, Syrphidae, occur no earlier than late Cretaceous strata long after the initial diversification of the angiosperms (Kovalev, 1985).
In contrast to reports of Diptera serving as pollinators of members of the ANITA grade, some species in the genera Nymphaea and Nuphar (Nymphaeaceae) are currently the only other taxa, in addition to T. moorei, with bee pollinators (Bernhardt and Thien, 1987; Lippok et al., 2000). Recent reports do indicate that, Sarcandra glabra, a species from the Chloranthaceae, a taxon that differentiated after the main lineages of the ANITA grade in combined structural and molecular analyses (Doyle and Endress, 2000), is also visited by bees and other insects (Tosaki et al., 2001). Fossil evidence shows that some Hymenoptera also radiated through the late Jurassic, and true bees were present in the Cretaceous (Grimaldi, 1999). Notably, some floral foragers of T. moorei belong to the Colletidae, still regarded as the ‘most primitive’ (i.e. wasp‐like) of bee families. However, there is reluctantance to ascribe a long‐term association between colletid bees and the Trimeniaceae or between colletids and the ANITA grade, and no comparative evidence supports any specific associations. Michener (1979) notes that Australia is the centre of diversity for the Colletidae, so the presence of Amyphylaeus,Leioproctus and Meroglossa spp. on flowers of T. moorei may merely coincide with bee geography, phenology and population densities. It is more important to note that, as the flower of T. moorei lacks nectar concealed within an elongated floral tube, both long‐tongue Exoneura spp. (Apidae) and short‐tongue halictids and colletids must be of equal importance in the active transport of pollen (Bernhardt, 1987, 1989, 1996).
Pollen was the primary reward for all insects visiting flowers of T. moorei as nectar was not produced by any parts of the flower, including the stigma. When placed in an evolutionary context, these results are significant because pollen as a reward represents an ancient character trait with the consumption of pollen by insects established by the Carboniferous (Labandeira, 1997; Grimaldi, 1999). This report of a stigma that does not produce secretions (dry‐type stigma) is in conflict with earlier reports that T. moorei develops a wet‐type stigma (Endress and Igersheim, 2001). The stigma of T. moorei lacks nectarioles (sensuVogel, 1998) and fails to secrete a protonectar as an edible reward (see also review in Bernhardt, 1996). Similar observations have been made for two other members of the ANITA group, Amborella trichopoda (Amborellaceae; Thien et al., 2002) and Illicium floridanum (Illiciaceae; Koehl, 2002). These combined analyses call into question conclusions that the wet‐type stigma, and hence stigmatic secretions functioning as a reward, was the plesiomorphic condition of angiosperms (Endress and Igersheim, 2000).
In the absence of either a nectar or free‐flowing lipid reward, the floral presentation of T. moorei follows an important character of the classic ‘Mess and Soil’ mode of pollination as described in Faegri and van der Pijl (1979). In addition, it is characteristic that a flower with a Mess and Soil system often bears many anthers that shed or extrude pollen thereby ‘dusting or encrusting’ relatively unspecialized foragers with their grains while insects forage. This Mess and Soil mode is most often associated with beetle pollinators (Faegri and van der Pijl, 1979). However, T. moorei diverges from this mode of floral presentation in one important character. Its flowers lack the large, broad and persistent perianth segments or enveloping bract(s) required to form an urn‐like chamber over extrusive stamens as in the Cyclanthaceae, Araceae and so many magnoliid families that are beetle‐pollinated (Bernhardt, 2000). The absence of such ‘floral walls’ in T, moorei may discourage many beetles while encouraging visits from Diptera and Hymenoptera with short probosces (Syrphidae, Colletidae, Halictidae), which are well documented as generalist foragers in eastern Australia (Armstrong, 1979; Bernhardt, 1987, 1989, 1995, 1996; Bernhardt and Weston, 1996).
The Trimeniaceae represents the third taxon within the ANITA grade to employ both wind and insects to disperse pollen as combined wind and insect pollination has been reported for Brasenia schreberi (Cabombaceae; Osborn and Schneider, 1988) and Amborellatrichopoda (Thien et al., 2003). These results are significant because wind pollination in relictual‐basal angiosperms has been heretofore considered rare (Thien et al., 2000), although Philipson (1993) speculated that Trimenia papuana was likely to be wind‐pollinated. Other basal angiosperms outside the ANITA group employing wind/insect pollination or wind only are Saururus cernuus (Saururaceae; Thien et al., 1983) and Hydyosmum (Chloranthaceae; Endress, 1987), respectively. The dry, broad and branched stigma of T. moorei may be best interpreted as convergent with stigmas of other wind‐pollinated systems distributed through the angiosperms (Faegri and van der Pijl, 1979; Knox, 1979).
While the relative contribution of wind vs. insect pollination in T. moorei remains to be determined, there are two overlapping reasons why the non‐directional mode of pollen dispersal by air currents may be present in this species. First, pollen load analyses indicate that the nectarless flowers of T. moorei ‘share’ their floral foragers with the nectarless (P. Bernhardt, unpubl. res.) male flowers of the co‐blooming liane, Smilax australis. All of the 13 insect taxa found to carry the pollen of T. moorei had at least one forager that carried Smilax pollen mixed with Trimenia pollen. Cross‐pollination in T. moorei is essential for seed set but it will not occur if a bee or fly forages for pollen on a single plant of T. moorei and then switches over to flowers of Smilax. Secondly, T. moorei blooms in early–mid‐spring, a wet cool season in this montane region. Late frosts are not uncommon in this region so there were days when there were no field observations or collections of foraging insects due to insect‐inclement weather. Wind pollination is retained in T. moorei as a ‘failsafe mechanism’ ensuring some level of cross‐pollination when the flowers of competing plants or climatic conditions restrict or terminate the movements of insect pollinators.
Self‐pollen recognition and rejection in T. moorei
The principal finding of structural observations following cross‐ and self‐pollination is that T. moorei exhibits SI reactions at a dry‐stigmatic surface in association with bicellular pollen. These results are noteworthy because most dry stigmatic SI systems were traditionally thought to be associated with tricellular pollen grains (de Nettancourt, 1997). T. moorei joins a growing number of angiosperm taxa with these combined character traits once considered anomalous (Franklin et al., 1995; Pontieri and Sage, 1999; Sage et al., 2000, 2001). The nature of pollen and stigmatic epidermal cells involved in SI will have important implications for the type of self‐recognition and rejection mechanisms present in T. moorei. To date, SI in plants with dry‐type stigmas have been documented to operate via signal‐transduction mechanisms (reviewed in Stone and Goring, 2001). Stigmatic SI, in association with a dry‐type stigma and bicellular pollen grains, is also present in the less basal but nevertheless early‐diverging angiosperm taxon, Saururaceae. In contrast to SI in T. moorei, self‐pollen grains in three of five species within the Saururaceae fail to germinate (Pontieri and Sage, 1997, 1999). However, self‐pollen rejection reactions in T. moorei are similar macroscopically to those documented in other phylogenetically younger lineages with bicellular pollen grains. These include the eudicot families, Papaveraceae (Franklin‐Tong et al., 1994), Onagraceae (Hecht, 1964) and such monocotyledons as the family, Commelinaceae (Owens, 1981), and the genus, Trillium spp. (Sage et al., 2001) in which bicellular self‐pollen grains germinate but fail to grow between stigma surface cells as do successful, cross‐pollen tubes.
Is the presence of stigmatic SI in association with bicellular pollen in T. moorei of evolutionary significance? Recent molecular data supports the hypothesis that SI has evolved on multiple occasions throughout the evolutionary history of flowering plants (Matton et al., 1994; Franklin et al., 1995; de Nettancourt, 1997). The timing of the evolution of SI with respect to angiosperm origins is an unanswered question, as is the nature of archaic SI mechanisms and site‐specific origins of SI (Sage et al., 1994, 2000; Read et al., 1995; Weller and Sakai, 1999). Some have suggested that SI was an important physiological process tied to the early evolution of flowering plants while others have suggested the alternative that it was absent in the first carpels (Whitehouse, 1950; Weller et al., 1995). Both stylar gametophytic SI, stigmatic sporophytic SI and late‐acting ovarian SI have been suggested to be the first SI mechanisms to evolve (Bernhardt and Thien, 1987; Zavada, 1984, 1990; Gibbs, 1986, 1991; Sage et al., 1998). Bell (1995) noted that SI reactions evolved in association with carpel closure and penetration of pollen tubes into ground tissues as observed in the present study for T. moorei. Endress and Igersheim (2000) suggest that the site of carpel closure is the site of incompatibility reactions.
The present report of stigmatic SI in association with a dry‐type stigma in the ANITA group offers the exciting possibility that SI with these spatial properties may have been operative in the earliest angiosperms. This is potentiality significant because stigmatic SI in combination with dry‐type stigmas has been noted to occur primarily in association with phylogenetically younger taxa, and has been thought to represent a relatively derived type of SI (Dickinson, 1995). Significantly, Austrobaileya scandens, another member of the ITA clade of the ANITA group, reportedly exhibits stigmatic SI (Prakash and Alexander, 1984). Notably, however, early reports of stigmatic SI in another member of the ITA clade, Illicium floridanum (Thien et al., 1983), have been discounted recently and self‐sterility in I. floridanum is probably due to early‐acting inbreeding depression (Koehl, 2002). Assessment of the evolutionary significance of SI in T. moorei in the context of the early history of angiosperms will require an examination of the compatibility status of remaining species in the family as well as other members of ANITA. Although SI may have been present in the dioecious species A. trichopoda due to the possibility of lability in sex expression (Endress and Igersheim, 2000), recent observations indicate that sexual expression in the species is not labile (Thien et al., 2003). Controlled cross‐ and self‐pollinations in the Nymphaea ceae indicate that some species are self‐compatible (Ervik et al., 1995; Orban and Bouharmont, 1995).
Patterns of cross‐pollen tube growth
In addition to the aforementioned observations on self pollen tube growth, it is also noted that cross‐pollen tubes of T. moorei penetrate and grow intercellularly, first, amongst the multicellular trichomes comprising the stigma and, second, within a large core of solid transmitting tissue en route to the ovary after stigmatic germination. These results are significant as previous workers have posited that pollen tube growth in Trimenia spp. was probably confined to the region where the carpellary margins are juxtaposed (Endress and Sampson, 1983). Similar growth patterns of pollen tubes in a solid transmitting tract en route to the ovary have been reported for another species within the ANITA grade, Nymphaeacapensis (Nympheaeceae; Orban and Bouharmount, 1995) and other less basal but nevertheless ancient taxa to include the Winteraceae (Sage et al., 1998; Sage and Sampson, 2003) and Saururaceae (Pontieri and Sage, 1997, 1999). Growth of pollen tubes following stigmatic germination within a solid transmitting tract in basal angiosperms may be of importance in the evolution of SI as discussed by Bell (1995) in addition to other pollen–carpel signalling phenomena (Pontieri and Sage, 1999) and may have significant implications for the reconstruction of the early evolution of the pollen‐tube pathway, a topic of long‐standing interest (Bailey and Swamy, 1951; Lloyd and Wells, 1992; Endress and Igersheim, 2000).
Transmitting tissue exudate
The most outstanding characteristic of the transmitting tissue of T. moorei is the presence of a well‐developed ECM extending from the base of the stigma to the micropyle that is histochemically positive for pectins and AG/AGPs. These ECM components are well known for their hydrophilic nature (Carpita and Gibeaut, 1993; Showalter, 2001) and hence their ability to provide a richly hydrated transmitting tissue for pollen tube growth. As well, both pectins and AGPs have been implicated in pollen tube nutrition and guidance from the stigma to the embryo sac in some angiosperms (Sanders and Lord, 1992; Cheung et al., 1995; Hulskamp et al., 1995; Lennon et al., 1998). Notably, these two components are also present in the ECM from the stigma to the micropyle of two other ANITA species, I. floridanum (Koehl, 2002) and A. trichopoda (Thien et al., 2003), and pectins and/or AG/AGPs are present in other relictual‐basal groups (Sage et al., 1998; Pontieri and Sage, 1999) suggesting that such components may have been important in the early evolution of pollen–carpel interactions of angiosperms. The role of these components in pollen tube growth in any of the species of ANITA and the early evolution of pollen–carpel interactions in angiosperms remains to be clarified.
The presence of pectins and AG/AGPs in the transmitting tissue of T. moorei is important for the phenomenon of angiospermy. Endress and Igersheim (2000) note that members of ANITA accomplish angiospermy by secretion. The present study and studies on I. floridanum (Koehl, 2002) and A. trichopoda (Thien et al., 2003) indicate that pectins and AG/AGPs function in angiospermy by secretion. The presence of these molecules at the site of carpel closure is significant because these molecules form tight molecular bonds cementing cells together as noted to occur in the middle lamella (Carpita and Gibeaut, 1993; Showalter 2001). Thus, these compounds effectively provide protection to both growing pollen tubes and enclosed ovules via angiospermy. Such features may have been important in the early evolution of angiosperms where insects were actively crawling over floral organs in search of rewards (possibly pollen; see Bernhardt and Thien, 1987) as reported here for T. moorei and elsewhere for A. trichopoda (Thien et al., 2003).
ACKNOWLEDGEMENTS
Research was funded by both a National Geographic Grant (No. 69741‐01) to P.B., L.B.T. and T.L.S. and a Natural Sciences and Engineering Research Council of Canada grant to T.L.S. The authors are indebted to P. H. Raven for his encouragement and support. We thank the Royal Botanic Gardens, Sydney, the Missouri Botanical Gardens, St Louis and the University of New England, Armidale, for additional laboratory facilities, storage space and extra equipment.
Fig. 1. Insect pollinators of T. moorei. A, Melangyna sp. (Syrphidae) forages for pollen on a lateral inflorescence. Note extended proboscis of the hover fly touching the stigma. Small arrowhead marks male flower. Large arrowhead marks bisexual flower. Bar = 4 mm. B, Triglyphus fulvicornis (Syrphidae) forages for pollen on a female flower borne on a lateral inflorescence. Middle leg is touching stigma. Bar = 2·5 mm. C, Tarsal claw of Melangyna sp. Arrowhead indicates biapeturate grain of T. moorei. The remaining grains are combinations of T. moorei and Smilax. Bar = 0·05 mm. D, Meroglossa itamuca (Colletidae). Note the short, bilobed proboscis (arrowhead). Bar = 1 mm. E, Exoneura sp. (Apidae). Note the long proboscis (arrowhead). Bar = 2 mm. F, Female Lasioglossum sp. nov. The short proboscis is not visible as it is folded under the head. Bar = 1 mm.
Fig. 2. Graph illustrating pollen dispersal on petroleum jelly‐covered slides placed along a transect within a population of flowering plants of T. moorei. Each point represents the number of pollen grains from one slide.
Fig. 3. Light and scanning and transmission electron micrographs illustrating the dry stigma of T. moorei during the ‘female’ phase of floral ontogeny. A, Arrow marks one branch of stigma. Bar = 84 µm. B, Arrowheads denote uplifted cuticle on stigma. Bar = 10 µm. C, Arrowheads illustrate cuticular boundary of stigma. Bar =100 nm. D, Thin section of resin embedded stigma stained with toluidine blue. Note tightly packed vacuolate cells comprising terminal regions of stigma. Bar = 10 µm. CW, cell wall; S, stigmatic epidermal cell.
Fig. 4. Light micrographs illustrating transmitting tissue and associated extracellular matrix of T. moorei. A and B, Thin sections of resin embedded tissue stained with toluidine blue. Bar = 10 µm: A, ECM of stigma base and carpel margin (*); B, ECM adjacent placenta, funiculus (*) and within micropyle. Small arrowheads mark integument cells lining the micropyle. C–E, Hand‐sections of unfixed carpels stained with alcian blue to detect acidic pectins in ECM (*; C and D) and Yariv’s antigen to detect AGs/AGPs in ECM (E). Bar = 20 µm. C, Stigma base and carpel margin. D, Funiculus adjacent placenta. Arrowhead denotes vascular trace in funiculus. E, Arrowheads denote presence of AG/AGPs in stigma base. E and e, placenta epidermal cell; F and f, funiculus.
Fig. 5. Micrographs of aniline blue fluorescence and thin sections of resin‐embedded pollinated carpels, stained with toluidine blue, illustrating cross‐ and self‐pollen tube growth in T. moorei. A and B, aniline blue fluorescence of intercellular growth of cross‐pollen tubes within the stigma following germination (A) and within the stigma base (B). Carpel tissues have not been squashed. Large arrowheads denote pollen tubes. Small arrowheads mark pollen grain. Bar = 20 µm. C, Thin sections of intercellular growth of cross‐pollen within the nucellus (large arrowheads) up to the embryo sac (small arrowheads). Bar = 10 µm. D and E, Cessation of self‐pollen tube germination prior to intercellular growth within the stigma. Arrowhead denotes self‐pollen tube at stigma surface. D, ABF. Bar = 20 µm. E, TS. Bar = 10 µm. S, stigma.
Chemical composition (µg sample–1 2 h–1) of floral scent and leaf volatiles emitted from Trimenia moorei
| Source: | Flower 1 | Flower 2 | Leaves | Control* |
| Sampling time: | 1235–1435 h | 1500–1700 h | 1800–2400 h | 1500–1700 h |
| No. of flowers sampled: | 37 | 69 | – | – |
| No. of inflorescences sampled: | 4 | 6 | – | – |
| Flower‐visitor activity: | Active? | Non‐active? | – | – |
| Fatty acid derivatives | ||||
| Decane | +† | + | 0·58 | + |
| Undecane | + | + | 1·37 | + |
| Dodecane | 0·12 | + | 2·46 | + |
| Tridecane | + | + | 2·20 | + |
| Tetradecane | 0·16 | + | 0·49 | + |
| Pentadecane | 0·23 | –‡ | 0·10 | – |
| Hexadecane | 0·13 | – | + | – |
| Heptadecane | 0·17 | – | – | – |
| 8‐Heptadecene | 0·85 | – | – | – |
| 3‐Hexenol | – | 0·47 | – | – |
| 3‐Hexenyl acetate | + | 1·12 | 4·37 | + |
| 2‐Heptyl acetate | 0·08 | 0·18 | 3·70 | – |
| Octanal | + | + | 0·66 | + |
| Nonanal | + | + | 3·38 | + |
| Decanal | 0·13 | + | 0·64 | + |
| 2,6‐Nonadienal | 0·13 | – | – | – |
| 6‐Methyl‐5‐hepten‐2‐one | + | + | 0·44 | + |
| Subtotal | 2·00 | 1·77 | 20·39 | Trace |
| % | 35·1 | 85·9 | 91·6 | – |
| Terpenoids | ||||
| Limonene | + | 0·15 | 0·98 | + |
| Monoterpene | – | + | + | + |
| Subtotal | Trace | 0·15 | 0·98 | Trace |
| % | – | 7·3 | 4·4 | – |
| Benzenoids | ||||
| 2‐Phenylethanol | 2·38 | – | – | – |
| Methyl salicylate | 0·29 | + | 0·13 | + |
| Pentyl benzoate | 0·17 | – | + | – |
| Subtotal | 2·84 | Trace | 0·13 | Trace |
| % | 49·8 | – | 0·6 | – |
| Unknowns | ||||
| Unknown‐1 (41 55 67 79 123) | 0·46 | 0·14 | 0·14 | – |
| Unknown‐2 (71 111 159) | 0·18 | – | – | – |
| Unknown‐3 (57 85 99 113) | 0·22 | + | 0·61 | + |
| Subtotal | 0·86 | 0·14 | 0·75 | Trace |
| % | 15·1 | 6·8 | 3·4 | – |
| Total (µg sample–1 2 h–1) | 5·70 | 2·06 | 22·25 | Trace |
| % | 100 | 100 | 100 | – |
| Source: | Flower 1 | Flower 2 | Leaves | Control* |
| Sampling time: | 1235–1435 h | 1500–1700 h | 1800–2400 h | 1500–1700 h |
| No. of flowers sampled: | 37 | 69 | – | – |
| No. of inflorescences sampled: | 4 | 6 | – | – |
| Flower‐visitor activity: | Active? | Non‐active? | – | – |
| Fatty acid derivatives | ||||
| Decane | +† | + | 0·58 | + |
| Undecane | + | + | 1·37 | + |
| Dodecane | 0·12 | + | 2·46 | + |
| Tridecane | + | + | 2·20 | + |
| Tetradecane | 0·16 | + | 0·49 | + |
| Pentadecane | 0·23 | –‡ | 0·10 | – |
| Hexadecane | 0·13 | – | + | – |
| Heptadecane | 0·17 | – | – | – |
| 8‐Heptadecene | 0·85 | – | – | – |
| 3‐Hexenol | – | 0·47 | – | – |
| 3‐Hexenyl acetate | + | 1·12 | 4·37 | + |
| 2‐Heptyl acetate | 0·08 | 0·18 | 3·70 | – |
| Octanal | + | + | 0·66 | + |
| Nonanal | + | + | 3·38 | + |
| Decanal | 0·13 | + | 0·64 | + |
| 2,6‐Nonadienal | 0·13 | – | – | – |
| 6‐Methyl‐5‐hepten‐2‐one | + | + | 0·44 | + |
| Subtotal | 2·00 | 1·77 | 20·39 | Trace |
| % | 35·1 | 85·9 | 91·6 | – |
| Terpenoids | ||||
| Limonene | + | 0·15 | 0·98 | + |
| Monoterpene | – | + | + | + |
| Subtotal | Trace | 0·15 | 0·98 | Trace |
| % | – | 7·3 | 4·4 | – |
| Benzenoids | ||||
| 2‐Phenylethanol | 2·38 | – | – | – |
| Methyl salicylate | 0·29 | + | 0·13 | + |
| Pentyl benzoate | 0·17 | – | + | – |
| Subtotal | 2·84 | Trace | 0·13 | Trace |
| % | 49·8 | – | 0·6 | – |
| Unknowns | ||||
| Unknown‐1 (41 55 67 79 123) | 0·46 | 0·14 | 0·14 | – |
| Unknown‐2 (71 111 159) | 0·18 | – | – | – |
| Unknown‐3 (57 85 99 113) | 0·22 | + | 0·61 | + |
| Subtotal | 0·86 | 0·14 | 0·75 | Trace |
| % | 15·1 | 6·8 | 3·4 | – |
| Total (µg sample–1 2 h–1) | 5·70 | 2·06 | 22·25 | Trace |
| % | 100 | 100 | 100 | – |
* Empty bag.
† +, Trace amount.
‡ –, Not detected.
Chemical composition (µg sample–1 2 h–1) of floral scent and leaf volatiles emitted from Trimenia moorei
| Source: | Flower 1 | Flower 2 | Leaves | Control* |
| Sampling time: | 1235–1435 h | 1500–1700 h | 1800–2400 h | 1500–1700 h |
| No. of flowers sampled: | 37 | 69 | – | – |
| No. of inflorescences sampled: | 4 | 6 | – | – |
| Flower‐visitor activity: | Active? | Non‐active? | – | – |
| Fatty acid derivatives | ||||
| Decane | +† | + | 0·58 | + |
| Undecane | + | + | 1·37 | + |
| Dodecane | 0·12 | + | 2·46 | + |
| Tridecane | + | + | 2·20 | + |
| Tetradecane | 0·16 | + | 0·49 | + |
| Pentadecane | 0·23 | –‡ | 0·10 | – |
| Hexadecane | 0·13 | – | + | – |
| Heptadecane | 0·17 | – | – | – |
| 8‐Heptadecene | 0·85 | – | – | – |
| 3‐Hexenol | – | 0·47 | – | – |
| 3‐Hexenyl acetate | + | 1·12 | 4·37 | + |
| 2‐Heptyl acetate | 0·08 | 0·18 | 3·70 | – |
| Octanal | + | + | 0·66 | + |
| Nonanal | + | + | 3·38 | + |
| Decanal | 0·13 | + | 0·64 | + |
| 2,6‐Nonadienal | 0·13 | – | – | – |
| 6‐Methyl‐5‐hepten‐2‐one | + | + | 0·44 | + |
| Subtotal | 2·00 | 1·77 | 20·39 | Trace |
| % | 35·1 | 85·9 | 91·6 | – |
| Terpenoids | ||||
| Limonene | + | 0·15 | 0·98 | + |
| Monoterpene | – | + | + | + |
| Subtotal | Trace | 0·15 | 0·98 | Trace |
| % | – | 7·3 | 4·4 | – |
| Benzenoids | ||||
| 2‐Phenylethanol | 2·38 | – | – | – |
| Methyl salicylate | 0·29 | + | 0·13 | + |
| Pentyl benzoate | 0·17 | – | + | – |
| Subtotal | 2·84 | Trace | 0·13 | Trace |
| % | 49·8 | – | 0·6 | – |
| Unknowns | ||||
| Unknown‐1 (41 55 67 79 123) | 0·46 | 0·14 | 0·14 | – |
| Unknown‐2 (71 111 159) | 0·18 | – | – | – |
| Unknown‐3 (57 85 99 113) | 0·22 | + | 0·61 | + |
| Subtotal | 0·86 | 0·14 | 0·75 | Trace |
| % | 15·1 | 6·8 | 3·4 | – |
| Total (µg sample–1 2 h–1) | 5·70 | 2·06 | 22·25 | Trace |
| % | 100 | 100 | 100 | – |
| Source: | Flower 1 | Flower 2 | Leaves | Control* |
| Sampling time: | 1235–1435 h | 1500–1700 h | 1800–2400 h | 1500–1700 h |
| No. of flowers sampled: | 37 | 69 | – | – |
| No. of inflorescences sampled: | 4 | 6 | – | – |
| Flower‐visitor activity: | Active? | Non‐active? | – | – |
| Fatty acid derivatives | ||||
| Decane | +† | + | 0·58 | + |
| Undecane | + | + | 1·37 | + |
| Dodecane | 0·12 | + | 2·46 | + |
| Tridecane | + | + | 2·20 | + |
| Tetradecane | 0·16 | + | 0·49 | + |
| Pentadecane | 0·23 | –‡ | 0·10 | – |
| Hexadecane | 0·13 | – | + | – |
| Heptadecane | 0·17 | – | – | – |
| 8‐Heptadecene | 0·85 | – | – | – |
| 3‐Hexenol | – | 0·47 | – | – |
| 3‐Hexenyl acetate | + | 1·12 | 4·37 | + |
| 2‐Heptyl acetate | 0·08 | 0·18 | 3·70 | – |
| Octanal | + | + | 0·66 | + |
| Nonanal | + | + | 3·38 | + |
| Decanal | 0·13 | + | 0·64 | + |
| 2,6‐Nonadienal | 0·13 | – | – | – |
| 6‐Methyl‐5‐hepten‐2‐one | + | + | 0·44 | + |
| Subtotal | 2·00 | 1·77 | 20·39 | Trace |
| % | 35·1 | 85·9 | 91·6 | – |
| Terpenoids | ||||
| Limonene | + | 0·15 | 0·98 | + |
| Monoterpene | – | + | + | + |
| Subtotal | Trace | 0·15 | 0·98 | Trace |
| % | – | 7·3 | 4·4 | – |
| Benzenoids | ||||
| 2‐Phenylethanol | 2·38 | – | – | – |
| Methyl salicylate | 0·29 | + | 0·13 | + |
| Pentyl benzoate | 0·17 | – | + | – |
| Subtotal | 2·84 | Trace | 0·13 | Trace |
| % | 49·8 | – | 0·6 | – |
| Unknowns | ||||
| Unknown‐1 (41 55 67 79 123) | 0·46 | 0·14 | 0·14 | – |
| Unknown‐2 (71 111 159) | 0·18 | – | – | – |
| Unknown‐3 (57 85 99 113) | 0·22 | + | 0·61 | + |
| Subtotal | 0·86 | 0·14 | 0·75 | Trace |
| % | 15·1 | 6·8 | 3·4 | – |
| Total (µg sample–1 2 h–1) | 5·70 | 2·06 | 22·25 | Trace |
| % | 100 | 100 | 100 | – |
* Empty bag.
† +, Trace amount.
‡ –, Not detected.
Analyses of pollen loads carried by insects collected on flowers of Trimenia moorei
| Insect taxon | Trimenia pollen only | Trimenia + other spp. | Other species only | No pollen |
| Coleoptera | ||||
| Aporocera sp. | . | . | . | 1 |
| Diptera | ||||
| Melangyna spp. | 3 | 6 | . | 1 |
| Sapromyza sp. | . | 1 | . | . |
| Triglyphus fulvicornis | 3 | 1 | . | 2 |
| Hymenoptera | ||||
| Amphylaeus nubilosellus | . | 1 | . | 1 |
| Apis mellifera | . | 2 | . | |
| Exoneura bicolor | . | . | 1 | . |
| Exoneura spp. | 3 | 3 | 2 | . |
| Lasioglossum rufocollare | . | 1 | . | . |
| L. speculatum | . | 1 | . | . |
| L. sp. (Chilalictus) | . | 1 | . | |
| Leioproctus advena | . | 1 | . | . |
| Leioproctus sp. (Euryglossidia) | . | 1 | . | . |
| Merogsloss itamuca | 1 | 1 | . | 3 |
| Pergid spp. (sawflies) | 3 | 2 | . | . |
| Totals | 13 | 22 | 3 | 8 |
| Insect taxon | Trimenia pollen only | Trimenia + other spp. | Other species only | No pollen |
| Coleoptera | ||||
| Aporocera sp. | . | . | . | 1 |
| Diptera | ||||
| Melangyna spp. | 3 | 6 | . | 1 |
| Sapromyza sp. | . | 1 | . | . |
| Triglyphus fulvicornis | 3 | 1 | . | 2 |
| Hymenoptera | ||||
| Amphylaeus nubilosellus | . | 1 | . | 1 |
| Apis mellifera | . | 2 | . | |
| Exoneura bicolor | . | . | 1 | . |
| Exoneura spp. | 3 | 3 | 2 | . |
| Lasioglossum rufocollare | . | 1 | . | . |
| L. speculatum | . | 1 | . | . |
| L. sp. (Chilalictus) | . | 1 | . | |
| Leioproctus advena | . | 1 | . | . |
| Leioproctus sp. (Euryglossidia) | . | 1 | . | . |
| Merogsloss itamuca | 1 | 1 | . | 3 |
| Pergid spp. (sawflies) | 3 | 2 | . | . |
| Totals | 13 | 22 | 3 | 8 |
Analyses of pollen loads carried by insects collected on flowers of Trimenia moorei
| Insect taxon | Trimenia pollen only | Trimenia + other spp. | Other species only | No pollen |
| Coleoptera | ||||
| Aporocera sp. | . | . | . | 1 |
| Diptera | ||||
| Melangyna spp. | 3 | 6 | . | 1 |
| Sapromyza sp. | . | 1 | . | . |
| Triglyphus fulvicornis | 3 | 1 | . | 2 |
| Hymenoptera | ||||
| Amphylaeus nubilosellus | . | 1 | . | 1 |
| Apis mellifera | . | 2 | . | |
| Exoneura bicolor | . | . | 1 | . |
| Exoneura spp. | 3 | 3 | 2 | . |
| Lasioglossum rufocollare | . | 1 | . | . |
| L. speculatum | . | 1 | . | . |
| L. sp. (Chilalictus) | . | 1 | . | |
| Leioproctus advena | . | 1 | . | . |
| Leioproctus sp. (Euryglossidia) | . | 1 | . | . |
| Merogsloss itamuca | 1 | 1 | . | 3 |
| Pergid spp. (sawflies) | 3 | 2 | . | . |
| Totals | 13 | 22 | 3 | 8 |
| Insect taxon | Trimenia pollen only | Trimenia + other spp. | Other species only | No pollen |
| Coleoptera | ||||
| Aporocera sp. | . | . | . | 1 |
| Diptera | ||||
| Melangyna spp. | 3 | 6 | . | 1 |
| Sapromyza sp. | . | 1 | . | . |
| Triglyphus fulvicornis | 3 | 1 | . | 2 |
| Hymenoptera | ||||
| Amphylaeus nubilosellus | . | 1 | . | 1 |
| Apis mellifera | . | 2 | . | |
| Exoneura bicolor | . | . | 1 | . |
| Exoneura spp. | 3 | 3 | 2 | . |
| Lasioglossum rufocollare | . | 1 | . | . |
| L. speculatum | . | 1 | . | . |
| L. sp. (Chilalictus) | . | 1 | . | |
| Leioproctus advena | . | 1 | . | . |
| Leioproctus sp. (Euryglossidia) | . | 1 | . | . |
| Merogsloss itamuca | 1 | 1 | . | 3 |
| Pergid spp. (sawflies) | 3 | 2 | . | . |
| Totals | 13 | 22 | 3 | 8 |
Number of insects carrying pollen types derived from mixed load analyses of insects collected on flowers of Trimenia moorei
| Pollen taxa* | ||||||||
| Insect taxon | Ac | Am | Le | M1 | M2 | Sm | So | Tr |
| Diptera | ||||||||
| Melangyna spp. | . | . | . | . | . | 6 | . | 6 |
| Sapromyza sp. | . | . | . | . | . | 1 | . | 1 |
| Triglyphus fulvicornis | . | . | . | 1 | . | . | . | 1 |
| Hymenoptera | ||||||||
| Amphylaeus nubilosellus | . | . | . | . | 1 | . | 1 | |
| Apis mellifera | . | . | 1 | . | . | 1 | . | 2 |
| Exoneura spp. | . | . | . | . | . | 3 | . | 3 |
| Lasioglossum rufocollare | . | 1 | . | . | . | 1 | . | 1 |
| L. speculatum | . | . | . | . | . | 1 | . | 1 |
| L. sp. (Chilalictus) | . | . | . | . | . | 1 | . | 1 |
| Leioproctus advena | . | . | . | 1 | 1 | . | 1 | 1 |
| Leioproctus sp. (Euryglossidia) | 1 | . | . | . | . | 1 | . | 1 |
| Meroglossa itamuca | . | . | . | 1 | . | 1 | . | 1 |
| Pergid spp. (sawflies) | . | . | 1 | . | . | 1 | . | 2 |
| Totals | 1 | 1 | 2 | 3 | 1 | 18 | 1 | 22 |
| Pollen taxa* | ||||||||
| Insect taxon | Ac | Am | Le | M1 | M2 | Sm | So | Tr |
| Diptera | ||||||||
| Melangyna spp. | . | . | . | . | . | 6 | . | 6 |
| Sapromyza sp. | . | . | . | . | . | 1 | . | 1 |
| Triglyphus fulvicornis | . | . | . | 1 | . | . | . | 1 |
| Hymenoptera | ||||||||
| Amphylaeus nubilosellus | . | . | . | . | 1 | . | 1 | |
| Apis mellifera | . | . | 1 | . | . | 1 | . | 2 |
| Exoneura spp. | . | . | . | . | . | 3 | . | 3 |
| Lasioglossum rufocollare | . | 1 | . | . | . | 1 | . | 1 |
| L. speculatum | . | . | . | . | . | 1 | . | 1 |
| L. sp. (Chilalictus) | . | . | . | . | . | 1 | . | 1 |
| Leioproctus advena | . | . | . | 1 | 1 | . | 1 | 1 |
| Leioproctus sp. (Euryglossidia) | 1 | . | . | . | . | 1 | . | 1 |
| Meroglossa itamuca | . | . | . | 1 | . | 1 | . | 1 |
| Pergid spp. (sawflies) | . | . | 1 | . | . | 1 | . | 2 |
| Totals | 1 | 1 | 2 | 3 | 1 | 18 | 1 | 22 |
* Ac, Acacia; Am, Amyema; Le, Leucopogon lanceolatus; M1, Myrtaceae (Eucalyptus‐type); M2, Myrtaceae (Leptospermum, Syncarpia‐types); Sm, Smilax australis, So, Solanum; Tr, Trimenia moorei.
Number of insects carrying pollen types derived from mixed load analyses of insects collected on flowers of Trimenia moorei
| Pollen taxa* | ||||||||
| Insect taxon | Ac | Am | Le | M1 | M2 | Sm | So | Tr |
| Diptera | ||||||||
| Melangyna spp. | . | . | . | . | . | 6 | . | 6 |
| Sapromyza sp. | . | . | . | . | . | 1 | . | 1 |
| Triglyphus fulvicornis | . | . | . | 1 | . | . | . | 1 |
| Hymenoptera | ||||||||
| Amphylaeus nubilosellus | . | . | . | . | 1 | . | 1 | |
| Apis mellifera | . | . | 1 | . | . | 1 | . | 2 |
| Exoneura spp. | . | . | . | . | . | 3 | . | 3 |
| Lasioglossum rufocollare | . | 1 | . | . | . | 1 | . | 1 |
| L. speculatum | . | . | . | . | . | 1 | . | 1 |
| L. sp. (Chilalictus) | . | . | . | . | . | 1 | . | 1 |
| Leioproctus advena | . | . | . | 1 | 1 | . | 1 | 1 |
| Leioproctus sp. (Euryglossidia) | 1 | . | . | . | . | 1 | . | 1 |
| Meroglossa itamuca | . | . | . | 1 | . | 1 | . | 1 |
| Pergid spp. (sawflies) | . | . | 1 | . | . | 1 | . | 2 |
| Totals | 1 | 1 | 2 | 3 | 1 | 18 | 1 | 22 |
| Pollen taxa* | ||||||||
| Insect taxon | Ac | Am | Le | M1 | M2 | Sm | So | Tr |
| Diptera | ||||||||
| Melangyna spp. | . | . | . | . | . | 6 | . | 6 |
| Sapromyza sp. | . | . | . | . | . | 1 | . | 1 |
| Triglyphus fulvicornis | . | . | . | 1 | . | . | . | 1 |
| Hymenoptera | ||||||||
| Amphylaeus nubilosellus | . | . | . | . | 1 | . | 1 | |
| Apis mellifera | . | . | 1 | . | . | 1 | . | 2 |
| Exoneura spp. | . | . | . | . | . | 3 | . | 3 |
| Lasioglossum rufocollare | . | 1 | . | . | . | 1 | . | 1 |
| L. speculatum | . | . | . | . | . | 1 | . | 1 |
| L. sp. (Chilalictus) | . | . | . | . | . | 1 | . | 1 |
| Leioproctus advena | . | . | . | 1 | 1 | . | 1 | 1 |
| Leioproctus sp. (Euryglossidia) | 1 | . | . | . | . | 1 | . | 1 |
| Meroglossa itamuca | . | . | . | 1 | . | 1 | . | 1 |
| Pergid spp. (sawflies) | . | . | 1 | . | . | 1 | . | 2 |
| Totals | 1 | 1 | 2 | 3 | 1 | 18 | 1 | 22 |
* Ac, Acacia; Am, Amyema; Le, Leucopogon lanceolatus; M1, Myrtaceae (Eucalyptus‐type); M2, Myrtaceae (Leptospermum, Syncarpia‐types); Sm, Smilax australis, So, Solanum; Tr, Trimenia moorei.
References
ArmstrongJA.
AzumaH, Toyota M, Asakawa Y, Yamaoka R, García‐Franco JG, Dieringer G, Thien LB, Kawano S.
BaileyIW, Swamy BGL.
BellPR.
BenesK.
BernhardtP.
BernhardtP.
BernhardtP.
BernhardtP.
BernhardtP.
BernhardtP, Thien LB.
BernhardtP, Weston PH.
CarpitaNC, Gibeaut DM.
CheungAY, Wang H, Wu HM.
DickinsonHG.
DoyleJA, Endress PK.
EndressP.
EndressP.
EndressP, Igersheim A.
EndressP, Sampson FB.
ErvikF,Renner S., Johanson KA
FaegriK, van der Pijl L.
FisherDB.
FranklinFCH, Lawrence MJ, Franklin‐Tong VE.
Franklin‐TongVE, Lawrence MJ, Franklin FCH.
GibbsPE.
GrimaldiD.
HardenG. (ed).
HaynesKF, Zhao JZ, Latif A.
HechtA.
Heslop‐HarrisonJ, Shivanna KR.
HondaK, Omura H, Hayashi N.
HulskampM, Schneitz K, Pruitt RE.
ImaiT, Maekawa M, Tsuchiya S, Fujimori,T.
KearnsCA, Inouye DW.
KnudsenJT, Tollsten L, Bergström LG.
KoehlV.
KovalevVG.
LabandeiraC.
LennonK, Stephance A. Hepler PK, Lord EM.
LippokB, Gardine A, Williamson P, Renner S.
LloydDG, Wells MS.
MabberleyDJ.
MartinFW.
MathewsS, Donoghue MJ.
MattonDP, Nass N, Clarke AE, Newbigin E.
MaydenJH.
MeeuseADJ, de Meijer AH, Mohr OWP, Wellinga SM.
O’BrienTP, McCully ME.
OmuraH, Honda K, Nakagawa A, Hayashi N.
OrbanI, Bourharmount J.
OsbornJM, Schneider EI.
PhilipsonWR.
PontieriV, Sage TL.
PontieriV, Sage TL.
PrakashN, Alexander III JH.
QiuYL, Lee J, Bernasconi‐Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Salvolainen V, Chase MW.
ReadSM, Newbigin E, Clarke AE, McClure BA, Kao T.
RenD.
SageTL,Sampson FB.
SageTL, Bertin RI, Williams EG.
SageTL, Griffin SR, Pontieri V, Drobac P, Cole WW, Barrett SCH.
SageTL, Pontieri V, Christopher R.
SageTL, Sampson FB, Bayliss P, Gordon MG, Heij EG.
SandersLC, Lord EM.
SchneiderEL.
SchneiderE, Jeter JM.
ShowalterAM.
SoltisPS, Soltis DE, Zanis MJ, Kim S.
SpechtRA, Rae EM, Boughton VH.
StoneSL, Goring DR.
SunG, Ji Q, Dilcher DL, Zheng S, Nixon KC, Wan X.
ThienLB, Azuma H, Kawano S.
ThienLB, Heimermann WH, Holman RT.
ThienLB, Sage TL, Jaffre T, Bernhardt P, Pontieri V, Weston P, Malloch D, Azuma H, Graham SW, McPherson MAet al.
ThienLB, White DA, Yatsu LY.
TosakiY, Renner C. Takahasi H.
VogelS.
WagnerWL, Lorence DH.
WeissbeckerB, Van Loon J JA, Dicke M.
WellerSG, Sakai AK.
WellerSG, Donoghue MJ, Charlesworth D.
WhitehouseHLK.
ZavadaJS.
ZavadaJS.
ZhuJ, Cosse AA, Obrycki JJ, Boo‐Kyung S, Baker TC.
Author notes
1Department of Biology, St Louis University, St Louis, MO 63103, USA, 2Department of Botany, University of Toronto, Toronto, Ontario, Canada M5S 3B2, 3Royal Botanic Gardens, Sydney, Mrs Macquaries Road, Sydney, NSW 2000, Australia, 4Department of Botany, Graduate School of Science, Kyoto University, Kyoto 606‐8502, Japan, 5Department of Cell and Molecular Biology, Tulane University, New Orleans, LA 70118, USA and 6NCW Beadle Herbarium, School of Environmental Sciences and Natural Resources Management, University of New England, Armidale, NSW 2351, Australia