-
PDF
- Split View
-
Views
-
Cite
Cite
VINOOD B. PATEL, SIMON WORRALL, PETER W. EMERY, VICTOR R. PREEDY, PROTEIN ADDUCT SPECIES IN MUSCLE AND LIVER OF RATS FOLLOWING ACUTE ETHANOL ADMINISTRATION, Alcohol and Alcoholism, Volume 40, Issue 6, November/December 2005, Pages 485–493, https://doi.org/10.1093/alcalc/agh196
Close - Share Icon Share
Abstract
Aims: Previous immunohistochemical studies have shown that the post-translational formation of aldehyde–protein adducts may be an important process in the aetiology of alcohol-induced muscle disease. However, other studies have shown that in a variety of tissues, alcohol induces the formation of various other adduct species, including hybrid acetaldehyde–malondialdehyde–protein adducts and adducts with free radicals themselves, e.g. hydroxyethyl radical (HER)–protein adducts. Furthermore, acetaldehyde–protein adducts may be formed in reducing or non-reducing environments resulting in distinct molecular entities, each with unique features of stability and immunogenicity. Some in vitro studies have also suggested that unreduced adducts may be converted to reduced adducts in situ. Our objective was to test the hypothesis that in muscle a variety of different adduct species are formed after acute alcohol exposure and that unreduced adducts predominate. Methods: Rabbit polyclonal antibodies were raised against unreduced and reduced aldehydes and the HER–protein adducts. These were used to assay different adduct species in soleus (type I fibre-predominant) and plantaris (type II fibre-predominant) muscles and liver in four groups of rats administered acutely with either [A] saline (control); [B] cyanamide (an aldehyde dehydrogenase inhibitor); [C] ethanol; [D] cyanamide+ethanol. Results: Amounts of unreduced acetaldehyde and malondialdehyde adducts were increased in both muscles of alcohol-dosed rats. However there was no increase in the amounts of reduced acetaldehyde adducts, as detected by both the rabbit polyclonal antibody and the RT1.1 mouse monoclonal antibody. Furthermore, there was no detectable increase in malondialdehyde–acetaldehyde and HER–protein adducts. Similar results were obtained in the liver. Conclusions: Adducts formed in skeletal muscle and liver of rats exposed acutely to ethanol are mainly unreduced acetaldehyde and malondialdehyde species.
(Received 2 March 2005; first review notified 29 March 2005; in revised form 15 July 2005; accepted 26 July 2005)
INTRODUCTION
Alcohol-induced muscle disease is one of the most prevalent skeletal muscle disorders in the western hemisphere affecting between 40–60% of all chronic alcohol misusers (Estruch et al., 1993; Fernandez-Sola et al., 1995; Sacanella et al., 1995; Preedy et al., 2001c). This disease is characterized by selective atrophy of type II fibres (glycolytic, fast-twitch, anaerobic) whereas the type I (oxidative, slow twitch, aerobic) fibres are relatively protected (Preedy et al., 2001b, c, d).
Although the causative agent is known, the steps between alcohol exposure and development of biochemical and functional abnormalities in muscle have not been fully elucidated. However, there is increasing evidence that the development of alcohol-related diseases may involve the formation of protein adducts, which are post-translationally modified proteins formed by covalent linkage of reactive nucleophiles (e.g. aldehydes) to parent proteins (Worrall et al., 1993; Niemela et al., 1999; Thiele et al., 2001; Worrall and Thiele, 2001). Protein adducts may perturb tissue biochemistry via neoantigen formation and concomitant antibody generation (Rolla et al., 2000; Worrall and Thiele, 2001), by rendering affected proteins more susceptible to proteolysis (Nicholls et al., 1994) and/or by inactivating the affected proteins (Chen et al., 2000). Immunohistochemical studies have shown that treatment of rats with ethanol and cyanamide + ethanol increases the amount of malondialdehyde- and acetaldehyde-derived protein adducts in skeletal muscle (Niemela et al., 2002). Greater adduct scores were observed in a type II fibre-predominant muscle (represented by the plantaris) than a type I fibre-predominant muscle (represented by the soleus) (Niemela et al., 2002).
From the aforementioned study, a number of issues have been raised. The first pertains to the fact that the immunohistochemical analysis, although productive for identifying the cellular location of the adducts provided limited information on the type of adduct formed. For example, a number of protein adduct species have been identified elsewhere including hybrid malondialdehyde–acetaldehyde (Worrall et al., 2000a; Thiele et al., 2001) and hydroxyethyl radical (HER)–protein adducts (Iimuro et al., 1996; Worrall et al., 2000a). The formation of malondialdehyde–protein adduct species suggests that increased lipid peroxidation occurs in alcohol-exposed muscle (Singh et al., 2001; Worrall et al., 2001). If malondialdehyde—acetaldehyde (MAA)–protein adducts were found it would suggest that ethanol oxidation and lipid peroxidation occur simultaneously within the same microenvironment for a prolonged time (Thiele et al., 2001). Secondly, two types of acetaldehyde–protein adducts have been identified, depending on whether they are formed under reducing or non-reducing conditions (Worrall et al., 1994, 2000b). It has been suggested that antibodies raised against unreduced adducts may actually recognize reduced adducts (Klassen et al., 1999). This may arise as unreduced acetaldehyde–protein epitopes are reconfigured or transformed to their reduced counterpart epitopes during the raising of the antibodies, and a similar process may be responsible for the appearance of circulating antibodies to reduced-acetaldehyde protein adducts in alcohol-treated animals and patients (Klassen et al., 1999). However, the extent to which reduced-acetaldehyde protein adducts are formed in alcohol-exposed muscle has not been investigated.
To address these issues we measured a variety of protein adducts in alcohol-exposed muscle using a combination of polyclonal and monoclonal antibodies. We compared skeletal muscles with a predominance of either type I fibres (i.e. soleus) or type II fibres (i.e. plantaris). In order to test the effects of elevated levels of acetaldehyde animals were pre-treated with cyanamide, an aldehyde dehydrogenase inhibitor. For comparative purposes, protein–aldehyde adducts were measured in liver.
METHODS
Animals
Male Wistar rats were obtained from Charles River (Margate, Kent, UK) at ∼60 g body weight and fed ad libitum a commercial pelleted diet and housed in an air-conditioned (20–25°C), humidified (40–60%) animal house with a 12 h light/12 h dark cycle starting at 08:00 h.
After ∼1 week of acclimatization, when they weighed ∼110–120 g body weight, the animals were divided into four groups: [A] saline + saline, [B] cyanamide + saline, [C] saline + ethanol, and [D] cyanamide + ethanol.
The dosage used was 75 mmol/kg body weight for alcohol, 0.5 mmol/kg body weight cyanamide, and controls were injected with identical volume of 0.15 mol/l NaCl. The experimental procedure involved i.p. injection (0.5 ml/100 g body weight) ‘pretreatment’ with either saline or cyanamide, followed 30 min later by i.p. injection (1 ml/100 g body weight) ‘treatment’ with either saline or ethanol. Animals were killed by decapitation 2.5 h after the alcohol/saline treatment. The soleus (type I fibre-predominant) and plantaris (type II fibre-predominant) muscles were quickly dissected, and a portion of the liver was also dissected from each rat. Tissues were immediately frozen in liquid nitrogen (−196°C) and then stored at −70°C until analysis.
Generation of modified proteins for antisera production
The conditions and verification of adduct formation were defined using radiolabelled [14C] acetaldehyde and [3H] lysine (Tuma et al., 1987). Human plasma protein was modified as described below and used to generate antisera reactive with each type of modification. Once the modification reactions had been carried out the modified proteins were used to raise polyclonal antibodies in rabbits. The antibodies raised against each type of modification were then tested for crossreactivity against unmodified proteins and each type of adduct. These experiments showed there was little or no crossreactivity against the adducts but, as expected, some reactivity against the original carrier protein. This was removed by incubation of small amounts of antiserum with immobilised carrier protein prior to use. At the end of the incubations, the reaction mixtures were extensively dialysed against buffer for 12 h at 4°C before being used as immunogens or in ELISAs. Modified proteins were stored in aliquots at −80°C for <1 month before use.
Acetaldehyde-modified proteins
Acetaldehyde-modified proteins were produced by incubating protein (10 mg/ml) with 1 mmol/l acetaldehyde in phosphate-buffered saline (PBS), pH 7.4. Unreduced-acetaldehyde protein adducts were generated during a 5 h incubation at 37°C. Reduced-acetaldehyde protein adducts were produced by a 1 h incubation at 37°C followed by the addition of 1 ml of 40% (w/v) sodium cyanoborohydride per 10 ml of reaction mixture. A further 30 min incubation at 37°C was then carried out to allow the reduction of Schiff bases.
HER-modified proteins
HER-modified proteins were prepared by incubating 10 mg/ml protein with 50 mmol/l ethanol, 100 μmol/l ferrous ammonium sulphate, 200 μmol/l EDTA and 100 μmol/l hydrogen peroxide, in 50 mmol/l sodium phosphate buffer (pH 7.4) at 37°C for 30 min (Moncada et al., 1994).
Malondialdehyde-modified proteins
Malondialdehyde-modified proteins were produced by incubating (10 mg/ml protein) with 1 mmol/l malondialdehyde for 12 h at 37°C in PBS, pH 7.4. Malondialdehyde was produced immediately before use by the hydrolysis of the dimethylacetal with 1 mol/l HCl for 30 min at 37°C. A portion of the acidic solution was then diluted with water and adjusted to pH 7.4 with 1 mol/l NaOH to prepare neutral malondialdehyde solution for use in the modification reactions.
Malondialdehyde–acetaldehyde modified proteins
It has been shown previously that a mixture of malondialdehyde (MDA) and acetaldehyde react with protein to produce malondialdehyde–acetaldehyde–protein adducts which have been termed MAA adducts. Fluorescence was used to measure the formation of MAA adducts and the number of amino groups was measured to determine the level of adduct formation (Tuma et al., 1996; Xu et al., 1997). Protein, 10 mg/ml in 100 mmol/l sodium phosphate (pH 7.4), was incubated with 1 mmol/l acetaldehyde and 1 mmol/l malondialdehyde for 3 days at 37°C.
Generation of anti-protein adduct antisera
New Zealand white rabbits (8 months old) were immunized with modified human plasma protein in three sets of multi-site injections as described previously (Worrall et al., 1989). After the final booster injection, the rabbits were exsanguinated by cardiac puncture under Nembutal anaesthesia. Blood was collected using heparinized needles and tubes and the resulting plasma was stored at −80°C. To decrease binding to unmodified proteins, antisera were purified as described previously (Worrall et al., 1989; Nicholls et al., 1994). Briefly, 1 ml of rabbit antiplasma and 1 ml of Buffer A (20 mmol/l Tris–HCl, pH 8.0, containing 28 mmol/l NaCl) were mixed and loaded onto a Biogel P-6 DG desalting column (Biorad). The eluate was collected as a single fraction and applied to a DEAE-Affigel blue column (Biorad) pre-equilibrated with Buffer A. The column was eluted with Buffer A and 2 ml fractions collected. Those containing the highest protein concentrations were pooled and applied to an immunoadsorption column for 4 h at 4°C. The eluate from the immunadsorption column was collected as a single fraction and stored at 4°C for <1 week prior to use in the ELISAs described below. The immunoadsorption column was made by reacting 2 ml of rat plasma (from a control rat) with 10 ml of Affigel-10 activated ester gel (Biorad) suspended in coupling buffer (40 mmol/l HEPES, pH 7.5, containing 160 mmol/l CaCl2) for 4 h at room temperature. The gel was poured into a column and washed with 20 bed-volumes of coupling buffer to remove unbound protein. The antibodies raised against each type of modification were then tested for crossreactivity against unmodified proteins and each type of adduct. These experiments showed there was little or no crossreactivity against the adducts but, as expected, some reactivity against the original carrier protein. This was removed by incubation of small amounts of antiserum with immobilized carrier protein prior to use.
RT1.1 antibody was a generous gift from Professor Dean Tuma and Associate Professor Geoff Thiele (Department of Veteran's Affairs Alcohol Research Centre and the University of Nebraska Medical Centre, Omaha, Nebraska). This antibody only recognizes epitopes generated by incubating proteins with acetaldehyde in the presence of excess sodium cyanoborohydride (a specific reducing agent for Schiff bases) (Worrall et al., 1994). The archetypal modification formed under these circumstances is N-ethyllysine (i.e. a lysine residue ethylated on the side chain ε-amino group) and will not react against unreduced proteins (Thiele et al., 1994). In general when the proposed modification is unknown it is difficult to state with certain the specificity of the antibody. However, aldehydes can react with proteins containing arginine, histidine, lysine, and tryptophan residues (Esterbauer et al., 1991).
Detection of modified proteins in tissue homogenates by ELISA
Indirect ELISA detected the presence of modified proteins in muscle and liver homogenates, with all samples being analysed in quadruplicate. Briefly, tissues were homogenized in TBS [1:10 (w/v)] using an Ultra-Tarrax homogenizer. Samples were then diluted to 100 ng protein/ml in TBS and 100 μl was added to each well of a 96-well plate. After 2 h at 4°C, the plates were washed with Tris-buffered saline (TBS), pH 7.4. Non-specific binding sites were blocked by incubation with 200 μl of saturated casein solution [2.0% (w/v), pH 7.5–8.0] for 1 h at 4°C. Purified rabbit anti-protein adduct antiplasma (100 μl) was then added to each well and incubated for 4 h at 4°C. The plate was washed with TBS-casein [TBS containing 0.5% (w/v) casein] and 100 μl of biotinylated anti-rabbit immunoglubulin antibody added. After 2 h at 4°C, the plates were again washed with TBS-casein and 100 μl of streptavidin-alkaline phosphatase complex added. After 1 h at 37°C, the plates were again washed with TBS. The final step was to add 100 μl of p-nitrophenyl phosphate (1 mg/ml) in diethanolamine buffer (10 mmol/l diethanolamine, 0.5 mmol/l MgCl2, pH 9.5) and incubate at 37°C. The absorbance of each well at 405 nm was measured using a Titertek multiscan plate reader after 1–3 h incubation depending on the tissue and the type of protein adduct assayed. Appropriate negative and positive controls were carried out for each analysis (Worrall et al., 1989, 2000a, b, 2001; Nicholls et al., 1994). The data displayed refers to assay conditions designed to produce optimum optical densities (OD) and are shown as OD units (at 405 nm) per 10 ng protein.
Statistics
All data are expressed as the mean ± SEM (n = 7–8). The significance of differences between treatments was assessed using two-way ANOVA. Significance was indicated when P < 0.05. Statistics where P > 0.05 are not displayed.
RESULTS
Unreduced-acetaldehyde–protein adducts
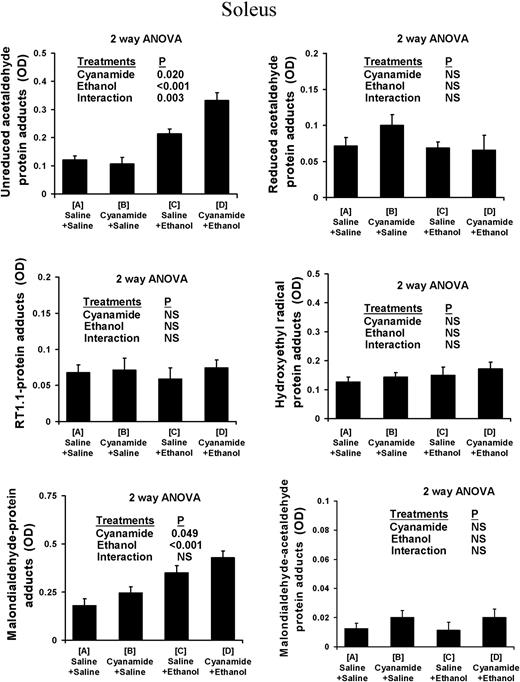
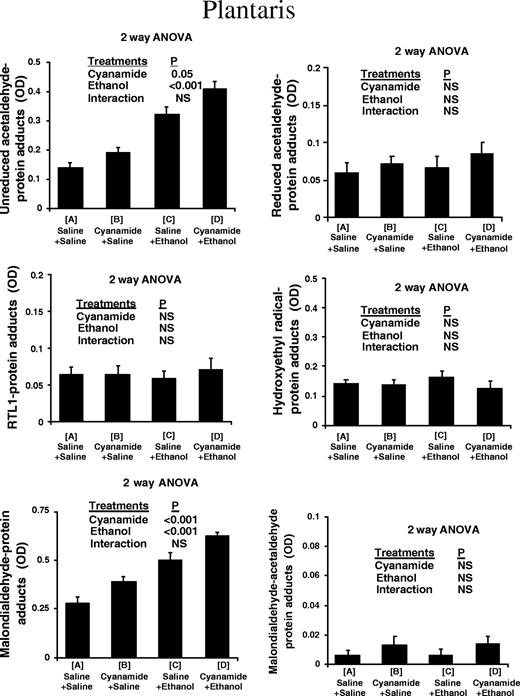
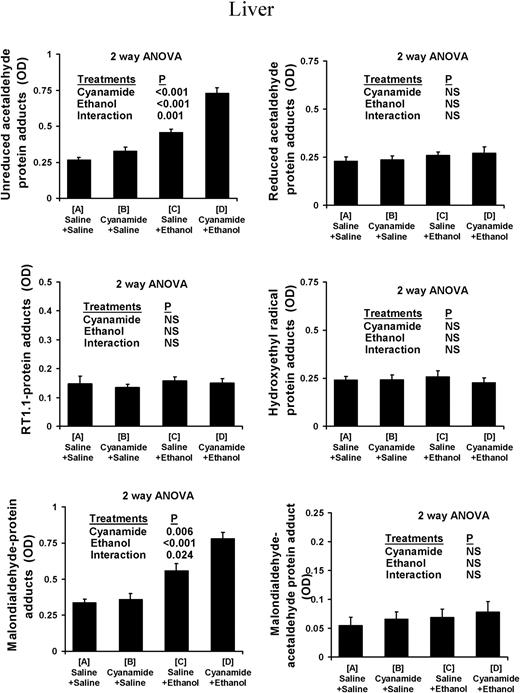
In the type I fibre-predominant soleus muscle (Fig. 1) alcohol treatment caused a highly significant increase in the levels of unreduced acetaldehyde adducts. There was also a highly significant interaction with cyanamide pretreatment, which potentiated the effect of alcohol treatment. Alcohol treatment also caused a highly significant increase in the levels of unreduced acetaldehyde adducts in the type II fibre-predominant plantaris muscle (Fig. 2), and cyanamide pretreatment also caused a significant increase in levels of unreduced acetaldehyde adducts that was independent of alcohol treatment. In the liver (Fig. 3) alcohol treatment again caused a highly significant increase, which was potentiated by cyanamide pretreatment.
Protein adduct species in soleus of rats subjected to ethanol and cyanamide treatments. Data are OD units per 10 ng protein, shown as mean ± SEM of n = 7–8 observations in each group.
Protein adduct species in plantaris of rats subjected to ethanol and cyanamide treatments.
Protein adduct species in liver of rats subjected to ethanol and cyanamide treatments.
Reduced-acetaldehyde protein adducts
Use of both the rabbit polyclonal and the mouse monoclonal RT1.1 antibody showed that there was no measurable increase in levels of reduced-acetaldehyde adducts and in particular N-ethylated lysine residues, in the soleus, plantaris, or liver in response to cyanamide, ethanol, or cyanamide + ethanol (Figs 1–3).
Malondialdehyde–protein adducts
In both the soleus and the plantaris muscles, malondialdehyde–protein adduct scores increased in response to both cyanamide and ethanol with no significant interaction (Figs 1 and 2). In the liver, malondialdehyde–protein adducts also increased in response to both cyanamide and ethanol, although in this case there was some interaction as cyanamide appeared to have a much greater effect in combination with alcohol than on its own (Fig. 3).
HER– and MAA adducts
There was no measurable increase in these adducts in the soleus, plantaris, or liver in any of the experimental conditions (Figs 1–3).
Differences between the soleus and plantaris
Because soleus and plantaris were taken from the same animals and assayed under the same analytical conditions direct comparisons between these two muscles were made. The calculated ratio of the amounts of adducts in the two muscles for each animal were for plantaris/soleus 1.69 (95% CI 1.17–2.20; n = 30) for unreduced-acetaldehyde adducts and 1.65 (95% CI 1.45–1.84) for MDA adducts. Thus in both cases the average level of adducts was greater in the plantaris than the soleus. However, when the ratios were subjected to analysis of variance there were no significant effects of either alcohol or cyanamide and no significant interactions. It appears that neither muscle was significantly more sensitive to either alcohol or cyanamide treatment in terms of adduct formation.
DISCUSSION
In these studies, we sought to investigate the nature of the protein adducts formed in skeletal muscle as a consequence of alcohol exposure. The experimental design also allowed us to assess the effects of markedly raising circulating acetaldehyde levels, by administering cyanamide to some of the rats that were subsequently dosed with ethanol (Hillbom et al., 1983; Pron'ko et al., 1999; Adachi et al., 2001). Levels of acetaldehyde in the blood of cyanamide plus ethanol treated rats at the end of 2.5 h are 500-fold higher than levels achieved with ethanol alone (Adachi et al., 2001). Thus the failure to detect increased levels of reduced-acetaldehyde adducts with both the rabbit polyclonal and mouse monoclonal (RT1.1) antibodies in the present series of data must reflect the fact that these adducts are not formed acutely rather than the possibility that acetaldehyde levels were not sufficiently elevated.
Low levels of acetaldehyde adducts were seen in all tissues of untreated rats, in line with previous observations in the liver (Nicholls et al., 1994). These adducts may arise from endogenous acetaldehyde generation by intestinal bacteria (Salaspuro, 1997; Seitz and Poschl, 1997) or serine hydroxy-methyltransferase activity which catalyses the conversion of threonine to glycine (Nicholls et al., 1994). Rat skeletal muscle also contains aldehyde dehydrogenase (Nilsson, 1988; Stewart et al., 1996), so it would not be unreasonable to expect to see small increases in acetaldehyde adducts in control rats injected with cyanamide alone. However, the main effect of cyanamide on acetaldehyde adducts was to potentiate the effect of alcohol, which was particularly apparent in the soleus and the liver, where the interaction term in the analysis of variance was highly significant.
Cyanamide also had an effect on MDA adduct levels in both muscles, and this effect was independent of the effect of ethanol. We have recently found based on immunohistochemical staining a similar effect of cyanamide on MDA adduct levels in the gastrocnemius muscle, which has a mixed fibre-type composition (Niemela O., Parkkila S., Emery P. W. and Preedy V. R., unpublished data). These data suggest that cyanamide imposes oxidative stress in muscle, as has previously been shown in the pancreas (Altomare et al., 1996) and liver (Kera et al., 1988; Kato et al., 1990; Vendemiale et al., 1998).
Reduced-acetaldehyde adducts
To investigate the nature of the adducted protein species formed in muscle of alcohol-dosed rats we employed an array of rabbit polyclonal antibodies and one mouse monoclonal antibody. The purpose of using both a mono- and polyclonal-antibody reacting against reduced-acetaldehyde adducts relates to the phenomena of epitope interconversion. It has been observed that when unreduced protein adducts are used to raise antibodies, antibodies reacting against both reduced- and unreduced-acetaldehyde protein adducts are detected (Klassen et al., 1999). Use of reduced-acetaldehyde protein adducts, however, raises antibodies that react only with the reduced adduct species (Klassen et al., 1999). The conversion of unreduced-acetaldehyde protein adducts to reduced species may be mediated by macrophages via a mechanism that has yet to be elucidated (Klassen et al., 1999). Nevertheless, the presence of antibodies reacting against reduced-acetaldehyde adducts in man (Worrall et al., 1990; Klassen et al., 1999) and experimental animals (Worrall et al., 2000a) has been used to suggest that reduced protein adducts are formed as a consequence of alcohol exposure.
In the present study no significant increase in reduced-acetaldehyde adducts was identified using either the RT1.1 monoclonal or polyclonal antibody in types I or II tissues. The same observation was made even in the presence of supra-physiological levels of acetaldehyde in the cyanamide plus ethanol group. However, in a previous study when the polyclonal antibody was used for immunohistochemical analysis a slight increase (P < 0.05) was observed in reduced acetaldehyde adducts following cyanamide plus alcohol treatment in type II tissue. This observation is in contrast to the present study where no increased levels of reduced-acetaldehyde adduct was identified using either the RT1.1 or polyclonal antibody following cyanamide plus alcohol treatment. It is possible that by using immunohistochemistry subtle localized changes are identified whereas with ELISA these site-specific effects are diluted out.
Unreduced-acetaldehyde adducts
Comparisons were made between different types of skeletal muscle fibres, i.e. type I anaerobic, glycolytic, fast-twitch, and type II aerobic, oxidative, slow twitch, as represented by the soleus and plantaris, respectively. Such an approach of analysing anatomically distinct skeletal muscles with different fibre types have been used before, for example, in studies on malnutrition (Ardawi et al., 1989), hypoxia (Bigard et al., 2000) and exercise (Hildebrandt and Neufer, 2000) as well as alcohol exposure (Adachi et al., 2000; Smith et al., 2000). It is well known that alcoholic myopathy affects type II fibres more severely than type I fibres (Preedy et al., 2001a, b, c, d). In the present experiment there was a significant increase in the levels of unreduced acetaldehyde adducts and this effect was potentiated by cyanamide. However, there was no difference between the two muscles in the amounts of unreduced adducts formed in response to alcohol treatment, thus each muscle type displays equal sensitivity to alcohol dosing. This observation is in line with our previous finding in rats treated chronically with alcohol for 6 weeks (Worrall et al., 2001). We therefore conclude that, after acute alcohol exposure, unreduced adducts are preferentially formed than reduced adducts in muscle. Similar conclusions can be applied to the liver, where the results were similar to those in muscle. These results also emphasize the importance of oxidative stress as a mechanism by which alcohol may damage tissues.
MDA adducts
In a previous study using immunohistochemistry, the intensity of staining for MDA adducts in cyanamide plus ethanol treated animals was 1.5 and 2.5 for soleus and plantaris, respectively, (Niemela et al., 2002) suggesting a preferential increase in type II tissues. In the current study a similar response was observed whereby mean absorbance values were higher in plantaris than in soleus, but the calculated ratio of the amount of adducts in the two muscles was not significantly different. Thus although there appears to be preferential formation of MDA adducts in type II tissue, the evidence is not conclusive. The formation of MDA adducts observed following acute alcohol dosing may reflect a transient phenomenon to which animals may be able to adapt over time as following chronic alcohol administration no statistical difference was observed (Worrall et al., 2001).
MAA adducts
Following acute ethanol exposure MAA adducts were not detected in the liver but are significantly elevated following chronic alcohol administration (Worrall et al., 2001). In muscle, however, MAA levels were not altered following either acute or chronic alcohol administration (Worrall et al., 2001) in either types I or II tissue. This suggests that the formation of acetaldehyde and MDA by lipid peroxidation possibly occurs in separate cellular compartments, preventing any interaction.
CONCLUSION
Protein adducts are formed in skeletal muscle of rats acutely exposed to alcohol and pertain to unreduced acetaldehyde and malondialdehyde species.
Author to whom reprint requests should be addressed at: Alcohol Research Unit, Department of Biochemistry and Molecular Biology, The University of Queensland, Brisbane, Queensland, QLD 4072, Australia. Tel: +61 7 3365 4626; Fax: +61 7 3365 4699; E-mail: s.worrall@uq.edu.au
We are grateful to Professors John de Jersey, Tom A. B. Sanders and Timothy J. Peters for encouragement and support.
REFERENCES
Adachi, J., Asano, M., Ueno, Y. et al. (
Adachi, J., Asano, M., Ueno, Y. et al. (
Altomare, E., Grattagliano, I., Vendemiale, G. et al. (
Ardawi, M. S., Majzoub, M. F., Masoud, I. M. et al. (
Bigard, A. X., Sanchez, H., Birot, O. et al. (
Chen, J., Petersen, D. R., Schenker, S. et al. (
Esterbauer, H., Schaur, R. J. and Zollner, H. (
Estruch, R., Nicolas, J. M., Villegas, E. et al. (
Fernandez-Sola, J., Sacanella, E., Estruch, R. et al. (
Hildebrandt, A. L. and Neufer, P. D. (
Hillbom, M. E., Sarviharju, M. S. and Lindros, K. O. (
Iimuro, Y., Bradford, B. U., Gao, W. et al. (
Kato, S., Kawase, T., Alderman, J. et al. (
Kera, Y., Ohbora, Y. and Komura, S. (
Klassen, L. W., Jones, B. L., Sorrell, M. F. et al. (
Moncada C., Torres V., Varghese G. et al. (
Nicholls, R. M., Fowles, L. F., Worrall, S. et al. (
Niemela, O., Parkkila, S., Pasanen, M. et al. (
Niemela, O., Parkkila, S., Koll, M. et al. (
Nilsson, G. E. (
Preedy, V. R., Adachi, J., Peters, T. J. et al. (
Preedy, V. R., Adachi, J., Ueno, Y. et al. (
Preedy, V. R., Paice, A., Mantle, D. et al. (
Preedy, V. R., Peters, T. J. and Adachi, J. (
Pron'ko, P. S., Kuz'mich, A. B. and Abakumov, G. Z. (
Rolla, R., Vay, D., Mottaran, E. et al. (
Sacanella, E., Fernandez-Sola, J., Cofan, M. et al. (
Salaspuro, M. (
Seitz, H. and Poschl, G. (
Singh, R., Leuratti, C., Josyula, S. et al. (
Smith, C., Stamm, S. C., Riggs, J. E. et al. (
Stewart, M. J., Malek, K and Crabb, D. W. (
Thiele, G. M., Wegter, K. M., Sorrell, M. F. et al. (
Thiele, G. M., Worrall, S., Tuma, D. J. et al. (
Tuma, D. J., Newman, B. S., Donohue, T. M. Jr et al. (
Tuma, D. J., Thiele, G. M., Xu, D. et al. (
Vendemiale, G., Grattagliano, I., Signorile, A. et al. (
Worrall, S. and Thiele, G. M. (
Worrall, S., De Jersey, J., Shanley, B. C. et al. (
Worrall, S., De Jersey, J., Shanley, B. C. et al. (
Worrall, S., de Jersey, J., Nicholls, R. M. et al. (
Worrall, S., De Jersey, J., Shanley, B. C. et al. (
Worrall, S., de Jersey, J. and Wilce, P. A. (
Worrall, S., Richardson, P. J. and Preedy, V. R. (
Worrall, S., Niemela, O., Parkkila, S. et al. (
Author notes
1Department of Biomedical Sciences, University of Westminster, 115 New Cavendish Street, London W1W 6UW, UK
2Alcohol Research Unit, Department of Biochemistry and Molecular Biology, The University of Queensland, Brisbane, Queensland, QLD 4072, Australia
3Nutrition, Food and Health Research Centre, King's College London, 150 Stamford Street, London SE1 9NN, UK