Abstract
Antimicrobial resistance (AMR) poses a huge threat to human health. It is urgent to explore efficient ways to suppress the spread of AMR. Antibacterial nanozymes have become one of the powerful weapons to combat AMR due to their enzyme-like catalytic activity with a broad-spectrum antibacterial performance. However, the inherent low catalytic activity of nanozymes limits their expansion into antibacterial applications. In this regard, a variety of advanced chemical design strategies have been developed to improve the antimicrobial activity of nanozymes. In this review, we have summarized the recent progress of advanced strategies to engineer efficient nanozymes for fighting against AMR, which can be mainly classified as catalytic activity improvement, external stimuli, bacterial affinity enhancement, and multifunctional platform construction according to the basic principles of engineering efficient nanocatalysts and the mechanism of nanozyme catalysis. Moreover, the deep insights into the effects of these enhancing strategies on the nanozyme structures and properties are highlighted. Finally, current challenges and future perspectives of antibacterial nanozymes are discussed for their future clinical potential.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Future perspectives
Nanozymes with intrinsic enzyme-mimicking catalytic activity have shown great potential in combating bacterial antimicrobial resistance. Their antimicrobial activity is extensively dependent on their catalytic ability. To this end, various advanced strategies have been developed to regulate the catalytic activity of nanozymes, aiming to enhance their antibacterial activity and therapeutic efficacy. Based on the key factors related to the intrinsic catalytic activity of nanocatalysts and the basic principles of the nanozyme catalytic mechanism, these enhancing strategies are mainly focused on catalytic activity improvement, external stimuli, bacterial interaction enhancement, and multifunctional nanoplatform construction. To date, hundreds of efficient nanozymes have been explored for antibacterial therapies. Though promising, nanozyme-based antibacterial therapy is still at a very preliminary stage, and there are various key problems to be addressed before clinical translation.
1. Introduction
The spread of antimicrobial resistance (AMR) including multidrug-resistant superbugs and biofilm establishment has emerged as one of the top ten public health threats around the world [1]. Promoted by the long-term overuse or misuse of antibiotics against bacterial infection, bacterial pathogens are evolving and becoming more and more lethal [2, 3]. Currently, new drug development is still the most effective way for combating AMR. However, with gene mutation attributable to the re-abuse of 'new drug', superbugs would evolve into the next generation with stronger antimicrobial-resistance [4, 5]. This initiates the development of alternative antibacterial agents to fight against AMR [6]. Inspired by enzymes in natural self-defense systems for catalytically attacking bacteria, nanozyme-based antibacterial therapy (NABT) has emerged as a timely requirement [7–9]. Nanozymes are a kind of nanomaterial with intrinsic enzyme-like activities [10–12], which have been extensively used in the biomedicine field [12–15]. As for NABT, nanozymes can catalyze the conversion of endogenous substance into highly toxic agents like reactive oxygen species (ROS) to kill bacteria and/or disrupt biofilm irrespective of inherent drug resistance [16]. Moreover, profiting from the merit of nanomaterials, the cost, scale-up production, storage, and stability in harsh environments of nanozymes are superior to natural enzymes [17, 18]. To date, with the rapid development of nanotechnology, a large number of antibacterial nanozymes have been developed, from noble-metal (e.g. Au [19–26], Pt [27], Cu [28], Ir [29], Ag [30], Au–Pt [31, 32], Pd–Cu [33], Pd–Pt [34], Au–Ag [35], etc), transition metal oxide or sulfide (e.g. Fe3O4 [36–38], CuO [39], CeO2 [40], V2O5 [41], Co–V mixed metal oxide (MMO) [42], MoS2 [43–47], NiS2 [48], CuS [49–52], Co4S3 [53], MnO2 [54] etc), and nanocarbon (e.g. boron doped graphdiyne (B-GDY) [8], N-doped sponge-like carbon spheres (N-SCSs) [55], graphene quantum dots [56, 57], etc)-based nanozymes, to recentlyadvanced metal organic framework (MOF) (e.g. nature-inspired MOF@COF nanozyme (NMCTP-TTA) [58], Ce–Au–Fe-MIL-88 [59], etc)-based nanozymes, and single-atom nanozymes (SAzymes) (e.g. hollow mesoporous carbon nanosphere doped with single-atom Fe (Fe-HCMS) [60], Fe–N–C [61–63], Cu–N–C [64, 65], Zn–N–C [66], Pt–C3N4 [27], Pt-PCN [67] single-atom catalysts (SACs), etc), as listed in table 1. Most of these belong to peroxidase (POD) mimics due to their ability of converting low concentration hydrogen peroxide (H2O2) into highly toxic hydroxyl radical (‧OH), which can cause irreversible oxidative damages to the biofilms and bacterial phospholipids, proteins, and DNA, as depicted in scheme 1 [68]. Compared to natural enzymes, however, the intrinsic pitfalls of lacking highly active centers and low active site density on the nanomaterial surface lead to relatively much lower enzymatic activity for nanozymes [69]. Moreover, single mode of nanozyme-based chemodynamic therapy (CDT) has inherent shortcomings due to the short lifetime and diffusion distance of ROS [70]. In this regard, considerable efforts have been focused on developing alternative strategies to promote the antimicrobial capacities of nanozymes, not only by enhancing the catalytic ability of nanozymes but also a combination of multiple antibacterial actions through the construction of multifunctional nanozymes. Although many studies have demonstrated the tremendous potential of NABT for combating AMR, several reviews have clearly indicated the design strategies, surface modification, physicochemical property-based classifications, and antimicrobial therapy applications of nanozymes [16–18, 71, 72]. Nevertheless, to the best of our knowledge, the comprehensive classification of the chemical design strategies for enhancing the antibacterial efficiency of nanozymes is scarcely reported, which is very significant for the guidance of designing efficient antibacterial nanozymes.
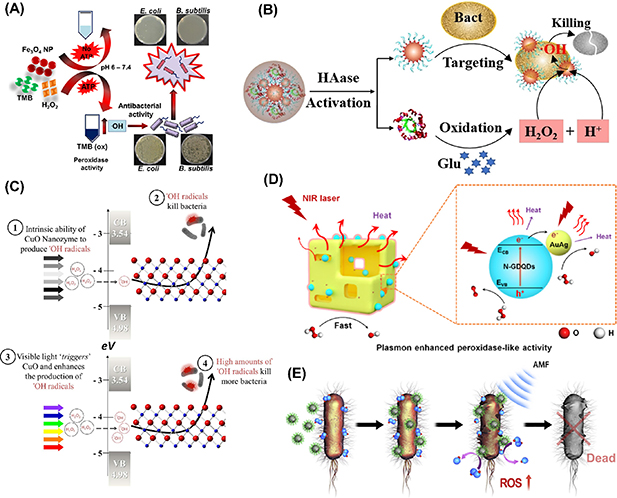
Scheme 1. Illustration of proposed mechanism of nanozyme-based catalytic antibacterial therapy.
Download figure:
Standard image High-resolution imageTable 1. Classification of antibacterial nanozymes based on chemical design strategies for enhancing antibacterial efficiency.
| Strategies | Enhanced mechanism | Chemical approaches | Nanomaterials | Catalytic activity | Km -H2O2 (mM) | Vmax-H2O2 (×10−8 M s−1) | Bacteria | Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Catalytic activity improvement | Enzymic active centers mimicking | Pyrolysis and acid-etching of MOFs precursors to create metalloenzyme-like atomically dispersed MNx active centers. | FeN4-SAF NCs | POD-like activity | 11.95 | 22.3 | E. coli, S. aureus | The Fe-N4-SAF and Fe-N5 SA/CNF with active centers similar to HRP and cytochrome P450 shows much higher Vmax than Fe3O4 and CeO2 NPs, respectively. MOF@COF showed enhanced activity as compare to blank MOFs. | [63] |
| ZnN4-PMCSs | POD-like activity | 40.16 | 12.15 | Pseudomonas aeruginosa | [66] | ||||
| CuN4-SASs/NPC | POD-like activity | — | — | E. coli, MRSA | [64] | ||||
| FeN5 SA/CNF | OXD-like activity | 0.148 (TMB) | 75.8 (TMB) | E. coli, S. aureus | [62] | ||||
| Control the growth of COFs to provide spatial microenvironment around active sites (MOFs). | NMCTP-TTA | POD-like activity | 0.458 | 57 | E. coli, S. aureus | [58] | |||
| Downsizing nanoparticles | Ultrathin 2D MOFs confine the growth of ultrasmall Au clusters by in-situ reduction. | UsAuNPs/MOFs | POD-like activity | 7.94 | — | E. coli, S. aureus | The UsAuNPs/MOFs, SA-Pt/g-C3N4-K, Fe-N-C SAzyme with small-sized metal clusters or single atom sites displayed enhanced catalytic activity compared to their large counterparts. | [20] | |
| Pyrolysis of atomically dispersed Pt, Fe supported precursors. | SA-Pt/g-C3N4-K | POD-like activity | 0.002 | 175.0 | E. coli | [27] | |||
| Fe-N-C SAzyme | POD-like activity | 4.84 | 11.8 | E. coli, S. aureus | [61] | ||||
| Engineering defects | Bare Cu NWs, rGO, and Fe3O4 provided nucleation sites for the nucleation and growth of MoS2 nanosheets with defect-rich edges. | R-CMs | POD-like activity | 0.032 | — | E. coli, S. aureus | The defect-rich edges for R-CMs, MoS2/rGO, Fe3O4@MoS2-Ag enhanced their H2O2 affinity as compared to MoS2. | [43] | |
| MoS2/rGO | POD-like activity | 0.26 | 25.6 | E. coli, S. aureus | [81] | ||||
| Fe3O4@MoS2-Ag | POD-like activity | 1.0 | 18.2 | E. coli | [82] | ||||
| Composition regulation | N-doped sponge-like carbon spheres (N-SCSs) were prepared through the pyrolysis of colloidal silica/polyaniline assemblies. Cu-doped phosphate-based glass (Cu-PBG) was prepared by hydrothermal method. | N-SCSs Cu-PBG | POD-like activity POD-like activity | 81.53 — | 23.3 — | E. coli, S. aureus E. coli, S. aureus | N, B, Cu doping endows carbon materials or PBG with multi-enzyme activities. | [55] [83] | |
| CuCo2S4 can be controllably prepared by hydrothermal method, while Pd-Ir cubes can be synthesized by the wet chemical reduction method. | CuCo2S4 | POD-like activity | 209.9 | 23.3 | E. coli, S. aureus MRSA | Bimetallic nanozymes with dual active sites shows remarkably synergistic enhancement in catalytic activity. | [84] | ||
| Ir-Pd cubes | POD-like activity | 0.034 | 5.1 | — | [85] | ||||
| Ultrathin 2D MOFs supported ultrasmall Au nanoparticles were prepared by in situ reduction. | UsAuNPs/MOFs | POD-like activity | 7.94 | — | E. coli, S. aureus | 2D MOFs significantly improved the catalytic activity of UsAuNPs/MOFs as compared to Au NPs. | [20] | ||
| Different shaped Fe3O4 magnetite nanoparticles (MNPs) were synthesized by solvent-thermal method, while bamboo-like nitrogen-doped carbon nanotubes encapsulating cobalt nanoparticles (N-CNTs@Co) were prepared by pyrolysis of cobalt cyanide cobalt at high temperature. | TO-shaped Fe3O4 MNPs Bamboo-like N-CNTs@Co | POD-like activity OXD-like activity | — 0.1 (TMB) | — 130 (TMB) | — E. coli, S. aureus | Different shapes of Fe3O4 MNPs and N-CNTs@Co displayed different catalytic activity | [86] [87] | ||
| External stimuli | pH | The addition of ATP or the microenvironment having glucose can be used as a trigger to induce the changes of pH and even H2O2 concentration, thus resulting in enhanced POD-like activities. A hyaluronic acid capsule functionalized with aptamer-Pt NPs and glucose oxidase (APGH) was constructed to break the limitations of pH and H2O2. | ATP + Fe3O4 NPs APGH + glucose from bacteria | POD-like activity POD-like activity | — — | — — | E. coli, Bacillus subtilis S. aureus | With ATP, the ‧OH generation for Fe3O4 is about 17 folds higher than that without ATP at neutral pH, resulting in enhanced antibacterial efficiency. When bacteria interacted with APGH, the released glucose oxidase could catalyze glucose into glucose acid and H2O2, providing raw-materials for Pt NPs to catalytic the ‧OH generation. | [38] [90] |
| Visible light | Introduction of semiconductor to fabricate nanozymes can be used to enhance the catalytic activity of nanozymes under visible light illumination. | CuO nanorods + light | POD-like activity | 3.4 (in light) 14.8 (in dark) | 10.9 (in light) 0.6 (in dark) | E. coli | With visible light irradiation, the nanozyme showed enhanced catalytic activity due to the improved affinity to H2O2. | [39] | |
| MoS2/rGO + light | POD-like activity | 0.26 (in dark) | 25.6 (in dark) | E. coli, S. aureus | [81] | ||||
| TiO2 NTs@MoS2 + light | POD-like activity | 0.085 (in dark) | 120.5 (in dark) | E. coli, S. aureus | [45] | ||||
| NIR | The activity of nanozymes with absorption in NIR range can be enhanced by NIR irradiation. | Au/CeO2 + NIR | POD-like activity | 0.006 (with NIR) 0.007 (in dark) | 133.4 (with NIR) 82.6 (in dark) | E. coli, S. aureus | The catalytic activities of nanozymes with strong NIR absorption can be significantly enhanced by NIR generated photothermal effect. Moreover, with semiconductor hybrid, the plasmonic effect can also produce hot carrier to further enhance the catalytic activities. | [91] | |
| N-GDQDs/AuAg NC + NIR | POD-like activity | 0.72 (with NIR) 1.24 (in dark) | 10.52 (with NIR) 4.75 (in dark) | E. coli, S. aureus | [35] | ||||
| Au NPTs + NIR | POD-like activity | — | — | MRSA, E. coli | [26] | ||||
| AuPt NDs + NIR | POD-like activity | — | — | E. coli, S. aureus | [32] | ||||
| NiS2 NPs + NIR | POD-like activity | — | — | MRSA, E. coli | [48] | ||||
| WS2 QDs + NIR | POD-like activity | — | — | Mu50, E. coli | [126] | ||||
| MoO3-x NDs +NIR | POD-like activity | 0.26 (in dark) | 15.2 (in dark) | MRSA, E. coli | [127] | ||||
| R-CMs + NIR | POD-like activity | 0.032 (in dark) | — | E. coli, S. aureus | [43] | ||||
| Fe3O4@MoS2-Ag + NIR | POD-like activity | 1.0 (in dark) | 18.2 (in dark) | E. coli | [82] | ||||
| PEG-MoS2 NFs + NIR | POD-like activity | — | — | E. coli, B. subtilis | [106] | ||||
| Cu2MoS4 + NIR | POD/OXD-like activity | 25.46 (in dark) | 42.81 (in dark) | E. coli, S. aureus, MRSA | [111] | ||||
| CuN4-SASs/NPC + NIR | POD-like activity | 11.95 (in dark) | 22.3 (in dark) | E. coli, S. aureus | [63] | ||||
| CuN4-SASs/NPC + NIR | POD-like activity | — | — | E. coli, MRSA | [64] | ||||
| Mesoporous FeN4-C SAzymes + NIR | POD-like activity | 4.84 (in dark) | 11.8 (in dark) | E. coli, S. aureus | [61] | ||||
| RFC + NIRII | POD-like activity | — | — | E. coli, S. aureus, MRSA | [102] | ||||
| N-SCSs + NIR | POD/OXD-like activity | 81.53 (in dark) | 23.27 (in dark) | E. coli, S. aureus | [55] | ||||
| PEG@zirconium-ferrocene MOF + NIR | POD-like activity | — | — | E. coli | [107] | ||||
| ZIF8-PEG@Zn/Pt-CN + NIR | POD-like activity | 0.067 (in dark) | 5.11 (in dark) | E. coli, S. aureus | [128] | ||||
| UiO-66-NH-CO-MoS2 + NIR | POD-like activity | 0.23 (in dark) | 15.7 (in dark) | MRSA, AREC | [129] | ||||
| AMF | Amorphous iron nanoparticles (AIronNPs) were prepared by chemical precipitation with PVP, F-127, and ammonium iron (III) citrate as raw materials | AIronNPs + AMF | POD-like activity | 41.36 (with AMF) 117.23 (without AMF) | 1.19 (with AMF) 1.45 (without AMF) | E. coli, S. aureus | The AMF can significantly improve the catalytic activity of AIronNPs due to the enhanced release of iron ions. | [92] | |
| Bacterial affinity enhancement | Rough surface engineering | Bare Cu NWs, rGO, and Fe3O4 provided nucleation sites for the nucleation and growth of MoS2 nanosheets with defect-rich edges, while RFC was synthesized by a three-step template-carbonization corrosion method. | R-CMs | POD-like activity | 0.032 | — | E. coli, S. aureus | Nanozymes with strong bacterial capture ability due to the rough surfaces can localize the ROS-mediated damages around the bacteria, thus resulting in the enhanced catalytic therapeutic efficiency. | [43] |
| MoS2/rGO | POD-like activity | 0.26 | 25.6 | E. coli, S. aureus | [81] | ||||
| Fe3O4@MoS2-Ag | POD-like activity | 1.0 | 18.2 | E. coli | [82] | ||||
| RFC | POD-like activity | — | — | E. coli, S. aureus, MRSA | [102] | ||||
| Pseudopodia mimicking | The growth of COFs on MOFs form pseudopodia-like shapes, allowing the bacterial capture. | NMCTP-TTA | POD-like activity | 0.458 | 57 | E. coli, S. aureus | [58] | ||
| Multifunctional nanozyme-based platforms | PTT | Nanozymes with NIR absorption can be used for CDT and PTT combined nanoplatforms. | Listed in the part of NIR stimuli | CDT + PTT | — | — | E. coli, S. aureus, MRSA, Mu50, AREC | Nanozymes with strong NIR absorption can be a benign nanoplatform for combining CDT and PTT for synergistic enhanced antibacterial applications. | As listed above |
| Drug release | Using MOF-based nanozymes as nanocarriers to load metal ions or drugs due to the porosity and large surface area of MOFs. | Pd-MOF@PAzo@SNP | CDT + NO | — | — | E. coli, S. aureus, Biofilm | The MOF-nanozyme based nanoplatforms can be used as a good synergistic germicidal system for chronic wound disinfection. | [113] | |
| ZIF8/Au-GOx NPs | CDT + Zn2+ | — | — | E. coli, S. aureus | [114] | ||||
| NH2-MIL-88B(Fe)-Ag | CDT + Ag+ | — | — | E. coli, S. aureus | [112] | ||||
| Fe3O4@MoS2-Ag | CDT + Ag+ | — | — | E. coli | [82] | ||||
| Cascaded reactions | The cascaded nanozymes are generally composed of POD-like nanozymes and GOx, which can be easily loaded on nanomaterials by the electrostatic interaction. | APGH | Glucose oxidation + POD catalysis | — | — | S. aureus | Owing to cascaded reactions, the nanozymes can act even at neutral conditions without H2O2 addition Since GOx catalyze the conversion of glucose into gluconic acid and H2O2. | [90] | |
| Fe3O4-GOx | Glucose oxidation + POD catalysis | — | — | E. coli, MRSA | [116] | ||||
| GOX-on-Fe-iCOF | Glucose oxidation + POD catalysis | — | — | E. coli, S. aureus | [117] | ||||
| Fe2(MoO4)3@GOx | Glucose oxidation + POD catalysis | — | — | E. coli, MRSA | [118] | ||||
| Pro-inflammatory | CuFe5O8 nanocubes can be synthesized by the solvent-thermal method. | CuFe5O8 nanocubes | CDT + Pro-inflammatory | — | — | E. coli, S. aureus, Biofilm | CuFe5O8 nanocubes exhibit space-selective CDT and induce pro-inflammatory macrophage polarization for curing implant-related infection due to the different pH and H2O2 concentration inside and outside the biofilm. | [119] | |
| Hydrogel | Hydrogel can provide a versatile and porous framework of loading different kinds of nanomaterials for constructing a multifunctional platform for antibacterial therapy. | MoS2-hydrogel | POD-like catalysis + bacterial capture | — | — | E. coli | Owing to the multiple functions of the hydrogel, the antibacterial therapeutic effect and wound healing efficacy can be significantly enhanced. | [70] | |
| MoS2@TA/Fe-PVA/Dex hydrogels | POD/CAT like catalysis + PTT + tannic acid anti-inflammatory | — | E. coli, S. aureus | [122] | |||||
| Tannic acid chelated-Ag hydrogel | POD-like catalysis + Ag + tannic acid anti-inflammatory | — | — | Staphylococcus epidermidis, E. coli. | [124] | ||||
| CNT@MoS2 hydrogels | POD/SOD/CAT-like catalysis + PTT | — | — | E. coli, S. aureus | [125] | ||||
| Nanozymes without enhancements | HRP | POD-like activity | 11.63 | 2.758 | — | — | [66] | ||
| Fe3O4 NPs | POD-like activity | 150.5 | 0.292 | [63] | |||||
| Nano-CeO2 | OXD-like activity | 0.420 (TMB) | 0.100 (TMB) | — | — | [130] | |||
| NM-88 | POD-like activity | 0.913 | 21.3 | — | — | [58] | |||
| MSN-AuNPs | POD-like activity | 15.81 | 17.30 | — | — | [78] | |||
| Pt-PCN | POD-like activity | 7.362 | 0.252 | — | — | [131] | |||
| MoS2 | POD-like activity | 60.87 | — | — | — | [43] | |||
| CuS | POD-like activity | 264.1 | 12.6 | — | — | [84] | |||
| CoS | POD-like activity | 114.5 | 5.9 | — | — | [84] | |||
| Pd cubes | POD-like activity | 0.07 | 6.5 | — | — | [85] |
Herein, we summarize the recent advances in the construction of efficient nanozymes for enhanced antimicrobial applications. Subsequently, from the basic principles of designing highly-active nanocatalysts and the mechanism of nanozyme catalysis we classify these engineering strategies into four categories, including catalytic activity improvement by strategies of enzymic center mimicking, downsizing, defect engineering, and composition modulation, catalytic process enhanced by external stimuli such as proper external stimuli such as adenosine triphosphate (ATP), glucose, visible light and near infrared (NIR) light irradiation, and alternative magnetic field (AMF), bacterial affinity enhancement for localizing the damage on bacterial surface through rough surface engineering and pseudopodia mimicking, and multifunctional platform construction for combining other antibacterial actions like photothermal therapy (PTT), drug release, cascade reactions, hydrogel and so on, as shown in scheme 2. Moreover, the deep insights into the effects of these enhancing strategies on the nanozyme structures and properties are highlighted. Finally, the perspective on the current challenges faced by antibacterial nanozymes for clinical applications are outlined. We hope this review can guide researchers to design and construct more efficient nanozyme platforms for enhancing antibacterial therapy.
Scheme 2. Illustration of the types of antibacterial nanozymes resembling different natural enzymes and the enhancing strategies for improving the catalytic antibacterial activity of nanozymes.
Download figure:
Standard image High-resolution image2. Activity improvement of nanozymes
To date, POD nanozymes have been extensively developed for NABT. Although most are promising, their therapeutic efficiency remains far from satisfactory because of the low catalytic activity. Since the enzyme-like activity of nanozymes actually originates from the chemical catalytic ability, strategies for enhancing the intrinsic catalytic ability of nanozymes, including mimicking enzymic active centers, downsizing nanoparticles, engineering defects, and composition modulations, have been effectively developed to enhance the bacterial eradication efficacy of nanozymes.
2.1. Mimicking enzymic active centers
As mentioned, many kinds of nanomaterials have been found to possess POD-like characteristics. However, the active centers of these POD mimics, especially for noble metal and carbon-based nanomaterials, are remarkably different from natural enzymes, thus leading to relatively low activity and selectivity compared with natural enzymes. Fortunately, the emerging of SACs bridges the gap between nanozymes and enzymes, not only because they possess atomically isolated metal sites with ultrahigh activity, but more importantly, they possess similar active centers to metalloenzymes composed of metal–nitrogen coordination (M–Nx ). By integrating advanced single-atom technology with intrinsic active centers of metalloenzymes, a variety of bioinspired single-atom nanozymes (SAzymes) resembling the active centers in metalloenzymes have been designed and explored for boosting the enzyme-like activity [62–66, 73].
Benefiting from the MOFs pyrolysis strategy for SACs synthesis, a series of POD SAzymes with FeN4 [61, 63], ZnN4 [66], CuN4 [64, 73] sites, which simulate the active centers of horseradish peroxidase (HRP) [74], Zn-cytochrome c [75], and type-2 Cu depleted laccase [76], respectively, have been atomically dispersed and anchored on the surfaces of N doped porous carbon, as shown in figure 1. Owing to the elaborately mimicking the structures of the active site of metalloenzyme, taking the atomically isolated FeN4 sites on nitrogen-doped amorphous carbon (SAF NCs) for example, the active FeN4 site resembles the active center of HRP composed of Fe atom coordinated with four iminazole groups of histidine residues (figure 1(A-i)). The transmission electron microscope (TEM) image shows no copper nanoparticles present on the NCs surfaces (figure 1(A-ii)), while the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image demonstrates the Fe atoms densely dispersed on the NCs surface (figure 1(A-iii)), demonstrating the successful synthesis of single Fe atoms, which endows the SAF NCs with a Vmax value of 2.23 × 10−7 M s−1, about 76.4 folds higher than one of the most favorable POD-like Fe3O4 NPs. This can be attributed to the FeN4 sites mimicking the active center of HRP (figure 1(A-ii)) [63]. Consequently, the SAF NCs could produce abundant ‧OH for noticeably eliminating Escherichia coli and Staphylococcus aureus by critical cell membrane destruction, and in vivo the SAF NCs displayed highly effective eradication of E. coli and S. aureus pathogens and achieved better wound healing efficacy [63]. More interestingly, inspired by the active centers of cytochrome P450, Dong's group built a novel SAzymes (single-atom nanozyme with carbon nanoframe–confined FeN5 active centers (FeN5 SA/CNF)) with axial FeN5 as active sites based on a bottom-up strategy [62]. The FeN5 SA/CNF exhibited extremely high oxidase (OXD)-like activity, about 17 times higher than that of square planar FeN4 catalyst. Thus, it directly converted O2 into abundant ‧OH for effectively killing bacterial pathogens and promoting the healing of the infected wound [62]. Shi's team also predicted by density functional theory (DFT) calculation, as compared to the CuN4–C sites, CuN5 sites incorporated with a fifth nitrogen atom could enhance the adsorption of single *OH, largely reducing the apparent barrier energy, thus they believed the CuN5 would be a more reactive POD-like center [73]. Based on these, it can be seen that rationally mimicking the enzymic active centers by the booming SAC technology can obtain highly active catalytic sites for boosting the catalytic performance of nanozymes.
Figure 1. Bioinspired synthesis of SAzymes with enzyme-like active centers. (A) Atomically isolated Fe–N4 sites on nitrogen-doped amorphous carbon (SAF NCs) mimicking the active center of HRP. (i) HRP structure with corresponding active center. (ii) Schematic illustration of SAF NCs model and its catalytic decomposition of H2O2 to ‧OH via the Fe–N4 single sites. (iii) TEM image of SAF NCs. (iv) HAADF-STEM image of SAF NCs. The bright dots as some marked by yellow circles are single iron atoms. Reprinted with permission from [63] John Wiley & Sons [© 2019 Wiley-VCH GmbH]. (B) Monodispersed ZIF-8 derived carbon nanospheres with a zinc-centered porphyrin-like structure (PMCS). (i) Cytochrome c structure with corresponding active center. (ii) PMCS model mimicking the active center of cytochrome c. (iii) TEM image of PMCS. (iv) HAADF-STEM image of Cu SASs/NPC. Reprinted with permission from [66] John Wiley & Sons [© 2019 Wiley-VCH GmbH]. (C) Hollow N-doped carbon sphere doped with a single-atom copper species (Cu-HNCS) mimicking the active center of laccase. (i) Laccase structure with corresponding active center. (ii) Model of Cu-HNCS mimicking the active center of laccase. (iii) TEM image of Cu-HNCS. (iv) HAADF-STEM image of Cu-HNCS. Reprinted with permission from [73] John Wiley & Sons [© 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageMoreover, for enzyme simulation, apart from merely resembling the enzymic active centers, simultaneously creating a spatial microenvironment for the active centers similar to that in natural enzymes is also crucial for designing highly active nanozymes, since the high catalytic efficiency of enzymatic active centers is derived from its nearby favorable hydrophobic spatial microenvironment offered by the assembly hierarchical structure of the enzyme itself [77]. Recently, Qu's group has designed a highly efficient metal–organic frameworks and covalent organic frameworks hybrid material (MOF@COF) nanozyme, in which the MOF of NH2-MIL-88B (Fe) was used as the POD mimic, and the superficial COFTP-TTA was constructed with the ligands possessing phenol and triazine functional groups [58]. The hierarchical nanocavities with tailored surface functional groups from COFTP-TTA offered the enzyme-like binding pockets around the active sites, remarkably enriching H2O2 via non-covalent interactions. Owing to this unique bioinspired design, the MOF@COF nanozyme exhibited a significant enhancement in bacterial inhibition as compared to the parent NH2-MIL-88B (Fe) [58].
Overall, mimicking enzymatic active centers integrated with spatial microenvironments pave a new way to the design and construction of highly active nanozymes for antibacterial therapy applications.
2.2. Downsizing nanoparticles
Besides lacking highly-active catalytic centers, due to the large and solid spatial structures, active atoms available on the nanozyme surface are only a very small fraction. So, the low atomic utilization efficiency is another important reason for causing the low catalytic activity of nanozymes. Downsizing the nanoparticles to ultrasmall clusters with size of less than 2 nm can be an effective strategy to improve the catalytic activity of nanozymes due to significantly increased specific surface area and quantum confinement effects of clusters as compared to the larger counterparts. For example, the Michaelis–Menten constant (Km ) to the H2O2 substrate for silica-supported Au NPs (4.5 nm) toward the POD enzymatic reaction was about 15.8 mM [78], while for ultrathin 2D MOFs supported Au clusters (1.96 nm), the Km was determined as 7.94 mM, indicating a much stronger affinity to H2O2 [20]. Consequently, the UsAuNPs/MOFs nanozyme could convert low dosage of H2O2 into abundant ‧OH for killing both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria, and exhibit an enhanced wound healing efficacy. Interestingly, further downsizing the metal clusters to atomic-level to form the SACs, such as Fe [61, 63], Zn [66], Cu [64], Pt [27, 67], and Pd [79]-based SAzymes, can even boost their catalytic performance comparable to natural enzymes by virtue of the maximum atom utilization efficiency. Moreover, the well‐defined active centers of SAzymes allows us to interpret their catalytic mechanism more precisely based on DFT calculations. During the catalytic decomposition of H2O2 into ‧OH over SAzymes, the H2O2 molecule is first adsorbed on the active M–N4 sites (M = Fe, Co, Zn), then the activated H2O2 molecule is homogeneously dissociated into two absorbed OH*, one of them will finally desorb from the M–N4 site, generating the active ‧OH, while the other reacts with H+ under acidic conditions to form an H2O molecule (figure 2(A-i)). Compared to CoN4 and ZnN4 sites, the FeN4 exhibited less electron transfer to the adsorbed OH*, which is beneficial for FeN4–C SAzymes reducing the energy barrier of ‧OH formation (figure 2(A-ii, iii)), and therefore showing enhanced PDO-like activities [80]. However, due to the easy migration of high-surface-energy single metal atoms to form metal nanoparticles, the metal loading on SACs is extremely low (∼1%), obviously hindering their catalytic activities. To address this issue, Jiao et al have explored a universal salt-template strategy for fabricating 2D MN4–C SAzymes (M = Zn, Co, and Fe) with highly dense metal atoms, of which the Fe concentration of FeN4–C SAzymes was up to 13.5 wt%, showing a far exceeding POD-like activity as compared to its counterpart SAzymes with a Fe mass of 1.3 wt%, as shown in figure 2(A-iv) [80]. In general, downsizing nanoparticles to atom levels can not only allow us to obtain high-efficiency antibacterial nanozymes but also to investigate the catalytic mechanism in depth, and in turn rationally design more powerful nanozymes.
Figure 2. (A) (i) Catalytic mechanisms of decomposition of H2O2 into ‧OH over Fe–N4–C SAzymes; (ii) energy diagrams of Fe, Co, Zn–N–C SAzymes in proposed reaction process; (iii) EPR signals of ‧OH for different M–N–C SAzymes; (iv) time-dependent absorbance of 3,3–,5,5–-Tetramethylbenzidine (TMB) catalyzed by Fe–C–N SAzymes with Fe-atom loading of 1.3 and 13.5 wt %, respectively. Reprinted with permission from [80]. © 2020, American Chemical Society. (B) (i) TEM image of R-CMs; (ii and iii) HRTEM image of RCMs; (iv) time-dependent absorbance changes at 652 nm of TMB for different MoS2; (v) TA signal for different MoS2 nanozymes under different conditions; (vi) proposed catalytic mechanism of decomposition of H2O2 into ‧OH over S-defect MoS2; (vii) energy diagrams of S-defect MoS2 in catalytic process. Reprinted with permission from [43]. John Wiley & Sons [© 2019 Wiley-VCH GmbH].
Download figure:
Standard image High-resolution image2.3. Engineering defects
Despite small amounts, the active sites, mainly derived from some coordinatively unsaturated atoms in the sites of steps, edges, kinks and defects; play the key role in offering nanozymes with inherent enzymatic properties. Therefore, besides mimicking enzymatic active centers, creating more edges or defects on the nanomaterial surface can be another promising approach to improve the intrinsic catalytic ability of nanozymes due to more highly active centers available, which is particularly crucial for the large-sized metal oxide/sulfide-based nanozymes. Inspired by the defect-rich engineering protocol for catalytic activity improvement, Qu's group fabricated a unique MoS2 POD nanozyme (R-CMs (R-CMs have MoS2 nanosheets wrapped around the Cu nanowires)) with rough surfaces and defect-rich active edges (figure 2(B-i, iii)) through the control growth of MoS2 nanosheets on the copper nanowires by a one-pot hydrothermal approach [43]. The reaction energy of MoS2, S-defect MoS2, and MoS2 edge in the whole reaction (H2O2 → 2OH* → OH* → ‧OH) was 1.92, ‒0.82, and ‒0.26 eV, respectively (figure 2(B-vi)), suggesting both S-defect MoS2 and MoS2 edge facilitate the production of ‧OH radical in the H2O2 decomposition. As a result, the MoS2 with defect rich edges endowed the nanozymes with higher intrinsic POD-like activity than pristine structure (figure 2(B-iv, v)). Moreover, the rough surface originated from MoS2 nanosheets is beneficial for trapping the bacteria. Therefore, increased antibacterial activity against both drug resistant E. coli and S. aureus and wound healing efficacy was obviously observed due to the enhanced production of ‧OH localizing on the bacterial surfaces [43]. Subsequently, Wang et al developed a microwave-assisted hydrothermal method for constructing a defect-rich adhesive MoS2/rGO vertical heterostructure (VHS) [81]. This MoS2/rGO VHS could obtain much more active edge sites, allowing them with triple OXD-, POD-, CAT-like activities, thereby showing efficiently catalytic disruption of drug-resistant E. coli and S. aureus in vitro and in vivo. More recently, Wei et al have also taken advantage of defect-rich-edge MoS2 to construct recoverable Fe3O4@MoS2-Ag nanozyme for enhanced catalytic antibacterial ability [82]. The combination of Fe3O4 and MoS2 could further increase the intrinsic POD-like properties. In addition, the rough MoS2 shell favored the capture of bacteria, amplifying the synergistic attack of ‧OH and Ag+ on bacteria surface. Meanwhile, the magnetism of Fe3O4 could be used to recycle the nanozyme after antibacterial treatment [82].
2.4. Composition regulation
Composition regulations including hetero atom doping, multi-active sites design, and support hybridization, and so forth are promising ways to take advantage of the synergy between different components or alter the lattice structure of nanozymes to tune the catalytic activities. For instance, a B-GDY nanosheet shows enhanced POD-like activity [8]. N-SCSs displayed enhanced bacterial disruption and accelerated infected wound recovery due to the synergistic effect between its excellent multiple enzyme-like activities [55]. Copper doping endows the degradable phosphate-based glass (PBG) bio-glass with excellent POD-like activity to achieve high antibacterial effects [83]. Owing to the synergy between binary metals, the bimetallic CuCoS4 NPs nanozymes possessed higher POD-like activity than monometallic CuS and CoS, and even offered high activity in neutral pH [84]. As for bimetallic Pd–Ir nanocubes nanozyme, the introduction of more active Ir component into Pd nanocubes significantly improved the catalytic efficiency, giving a 20- and 400-fold higher activity than monometallic Pd nanocubes and HRP, respectively [85]. The ultrathin 2D MOFs supported ultrasmall AuNPs (UsAuNPs/MOFs) hybrid exhibited a remarkable POD-like catalytic antibacterial performance and could effectively facilitate wound healing at a low dosage of H2O2 [20]. Moreover, besides active centers, the enzyme-mimicking activity of nanozymes is also strongly related to their own morphology. For example, the POD mimetic activity of truncated octahedron shaped Fe3O4 NPs was superior to that of spherical-shaped ones [86]. The bamboo-like nitrogen-doped carbon nanotubes encapsulating Co nanoparticles (N-CNTs@Co NPs) possessed higher OXD-like activity than its spherical precursor CoHCF due to that the bamboo-like structure could prevent Co NPs leakage and improve its dispersion and stability [87]. Notably, the chemical state of copper in carbon supported copper nanohybrids also plays an important role in regulating the enzyme-mimicking activity of nanozymes [28]. Overall, rationally regulating the compositions of nanozymes can improve their catalytic and antibacterial efficiency.
3. External stimuli enhancing catalytic processes
As for the catalytic mechanism of POD nanozyme, the initial H2O2 absorption by nanozyme is a crucial factor for activating the decomposition of H2O2. Meanwhile, the presence of proton (H+) is also very vital for nanozyme to recover its catalytic ability through reacting with the absorbed ‧OH to form H2O [43, 80]. Besides, the Arrhenius equation demonstrates the chemical reaction rate significantly dependent on temperature [88]. Thus, starting from these basic principles, proper external stimuli such as ATP [40, 89], glucose [90], visible light irradiation [39], and NIR light irradiation [91], AMF [92] can enhance the catalytic reaction process of nanozymes through H+, H2O2, H2O2 affinity, thermal, and active ion concentration modulations, thereby resulting in significantly enhanced bactericidal efficiency of nanozymes under low H2O2 concentration and even neutral pH.
3.1. ATP/glucose stimulation
In general, the activities of most POD nanozymes are pH dependent. As for Fe3O4 nanozymes, better catalytic performance is obtained in an acidic medium (pH = ∼4) [93], whereas in a neutral environment the PDO activity can be dramatically reduced and even changed [94]. Studies have shown some chronic wound infections are around 6.5–8.5 [95], which hinders the effective antibacterial activity of Fe3O4 NPs due to the near neutral pH. In this regard, Vallabani et al have recently developed an alternative ATP-triggering strategy to enhance the Fe3O4 NPs catalytic activity and antibacterial efficiency at neutral pH [89]. With the aid of ATP, owing to its ability of participation in single electron transfer reaction through complexation with Fe3O4 NPs [96, 97], the ‧OH generation catalyzed by Fe3O4 NPs could be significantly accelerated, resulting in enhanced bactericidal efficiency in presence of H2O2 at neutral pH [38] (figure 3(A)). To break the limitations of local pH and H2O2 under physiological conditions, instead of ATP, Chen et al have constructed a versatile glucose-stimulated nanozymes composed of aptamer-functionalized platinum nanozymes (Apt-PtNZ), glucose oxidase (GOX) and hyaluronic acid (HA) (denoted as APGH) for treating diabetic infections (pH = 7.0–8.0) [90]. With aptamer recognition, APGH could first adhere to bacterial surface, then glucose oxidase would catalyze the glucose oxidation to generate H2O2 and lower the local pH, and finally the POD-mimicking Pt NPs in-situ converted the H2O2 into ‧OH on bacteria surfaces, subsequently enhancing the antibacterial effect in vitro and in diabetic wounds [90] (figure 3(B)).
Figure 3. (A) Schematic illustration of ATP stimulation for enhancing the catalytic decomposition of H2O2 over Fe3O4 NPs for antibacterial application. Reprinted with permission from [38] © 2020 Elsevier. (B) Schematic illustration of glucose stimulation for enhancing the catalytic decomposition of H2O2 over APGH nanozymes. Reprinted with permission from [90] John Wiley and Sons [© 2021 Wiley‐VCH GmbH]. (C) Schematic illustration of visible light stimulation for enhancing the catalytic decomposition of H2O2 over CuO NPs. Reprinted with permission from [39] © 2018, American Chemical Society. (D) Schematic illustration of NIR stimulation for enhancing the catalytic decomposition of H2O2 over N-GDQDs/AuAg NC. Reprinted with permission from [35] © 2022 American Chemical Society. (E) Schematic illustration of AMF stimulation for enhancing the catalytic decomposition of H2O2 over AIron NPs. Reprinted with permission from [92] © 2020 Elsevier B.V.
Download figure:
Standard image High-resolution image3.2. Visible light stimulation
For most antibacterial nanozymes, their continuously generating ROS-mediated antibacterial action may allow opportunistic bacteria to generate resistance, similar to antibiotics. Therefore, nanozymes with 'on-demand' antimicrobial activity could be highly desirable. Considering light possessing great tenability and spatial controllability along with no physical contact, Karim et al have developed visible-light-active CuO nanorods (NRs) using a highly basic tertiary amine [39]. The visible light illumination obviously facilitated the binding affinity of CuO NRs to H2O2, 4.35 times higher than that in dark, and activated the CuO NRs with a band gap of 1.44 eV, remarkably accelerating the ROS production (figure 3(C)). As a result, the photoactive CuO NRs exhibited a significant enhanced antibacterial performance against E. coli even at a low H2O2 concentration [39]. Similarly, Chen's work also demonstrated visible light illumination improved ROS generation for MoS2/rGO VHS, thereby resulting in high bactericidal efficiency and wound healing promotion with the assistant of H2O2 and light [81]. A TiO2NTs@MoS2 nanozyme composed of TiO2 nanotubes coated with MoS2 nanoflowers was also reported to display visible light-stimulated enhancement in the POD-like activity of MoS2 due to the combination with the semiconductor TiO2NTs [45]. Accordingly, the as-obtained TiO2NTs@MoS2 could produce much more ‧OH, showing outstanding antibacterial effect against drug-resistant bacteria under the visible light and excellent wound healing efficacy.
3.3. NIR stimulation
As mentioned above, light has become an attracting trigger to tailor the enzyme-like activity. Comparing with visible light, NIR with wavelength ranging from 780 to 2500 nm is featured with deeper tissue penetration and invasiveness [98]. Thus, NIR-based PTT has been extensively explored for anti-infective therapy [99–101]. The photothermal nanoagents can absorb the NIR and convert the optical energy into heat, which then initiates the cell membrane breakage and protein denaturation in bacteria, finally leading to their thermal ablation [102–104]. Based on this, NIR-responsive nanozymes, such as Au nanoplates [26], Au@Pt nanodots [32], Au NRs/CeO2 hybrid [91], rough carbon/Fe3O4 nanohybrids [102], cationic chitosan@RuO2 hybrid [105], MoS2 nanozymes [43, 82, 106], N-doped carbon nanozyme [55], PEG@Zr-Fc MOF hydrogel [107], and Fe- and Cu–N–C SAzymes [61, 63, 64], have been developed. Under the NIR laser irradiation, these NIR-active nanozymes can not only produce local photothermal effect, but more importantly the elevated temperature certainly accelerates catalytic reaction rate, thereby realizing the enhanced synergic CDT and PTT for effectively treating drug-resistant bacterial infections. Actually, for NIR responsive metal-semiconductor hybrid nanozymes, the NIR excitation can not only induce local photothermal effect but also transfer of hot electrons simultaneously, of which the enzyme-like function is dominantly dependent on the hot electrons' transfer. Taking the gold–silver nanocages loaded nitrogen-doped graphdiyne quantum dots (N-GDQDs/AuAg NC) heterostructures as an example [35], as shown in figure 3(D), under NIR excitation, the N-GDQDs was induced to produce heat and hot carriers (e‒), which was then injected into AuAg, remarkably accelerating the decomposition of H2O2 into ‧OH. Taking advantage of NIR-enhanced POD-like activity, the N-GDQDs/AuAg NC nanozyme possessed efficient broad-spectrum antibacterial activity under NIR irradiation. Similar enhanced effect can be found for the Au/CeO2 plasmonic nanohybrids [91] and graphdiyne nanowalls wrapped hollow copper sulfide nanocubes (CuS@GDY) [50] with strong LSPR absorption and fast hot electrons transfer efficiency. Overall, the NIR stimuli will be a promising spatiotemporal way to enhance the catalytic process of nanozymes.
3.4. AMF modulation
Iron-based nanozymes are one of the most promising POD mimics for antimicrobial therapy. However, their unsatisfactory catalytic efficacy is still limiting their practical applications. Thus, developing proper external stimuli to modulate the catalytic activity of iron-based nanozymes may give an opportunity in enhancing their antibacterial treatment. To address this issue, Zhao's recent work reported an alternative strategy of utilizing alternating magnetic field (AMF) to regulate the activity of amorphous iron nanoparticles (AIronNPs) in POD-like catalytic reaction [92]. Due to the rapidly ionized AIronNPs and the AMF augmented chemodynamic effect, abundant ferrous iron ions were released from AIronNPs with AMF exposure (figure 3(E)), which significantly accelerated the catalytic reaction rate of converting H2O2 to ‧OH as compared to AIronNPs alone, thus exhibiting excellent broad-spectrum antibacterial properties. Moreover, for in vivo infected model the AIronNPs combined with AMF synergistically promoted the formation of granulation tissue and facilitated the wound healing. This work paves a new way for spatiotemporally modulating the catalytic activity of magnetic nanozymes in the antibacterial field.
4. Bacterial affinity enhancement
Nanozymes have extensive potential in biomedical applications. As for antibacterial therapy, although many above mentioned nanozymes possess excellent POD-like activity, their weak interaction with bacteria severely hinders their practical antibacterial efficiency due to the short lifetime and diffusion distance of ROS [70]. In this case, nanozymes with strong bacterial adhesion is necessary for disinfection. Only then can the catalytic attack of ROS be localized around the bacterial surfaces, resulting in maximum antibacterial therapeutic effect and minimal side effects.
4.1. Rough surface engineering
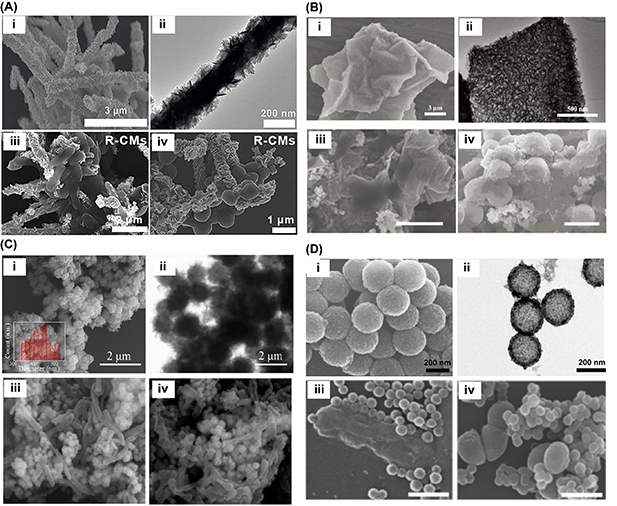
Rough surface engineering has been recognized as a powerful tool to enhance the bacterial adhesion of nanozymes. This is because bacteria covered with plenty of flagella and pili are easily trapped by the rough nanostructures, but normal cells will not be attracted [108, 109]. Moreover, benefiting from rough surfaces, larger surface area and more edge-defect active sites are available on the nanozyme surface, resulting in enhanced intrinsic catalytic activity [43, 70, 81, 82, 102]. Remarkable works have been done by fabricating defect-rich MoS2 nanosheets on the surface of one-dimension Cu nanowires (R-CMs) (figure 4(A-i, ii)) [43], two-dimension rGO (MoS2/rGO VHS) (figure 4(B-i, ii)) [81], and zero-dimension Fe3O4 nanoparticles (Fe3O4@MoS2-Ag) (figure 4(C-i, ii)) [82] to develop rough-surface nanozymes. As for 1D R-CMs hybrid, it possessed dual antibacterial actions of POD-like catalytic attack and NIR-based photothermal lysis [43], while 2D MoS2/rGO VHS exhibited triple POD, OXD, and CAT-like properties with visible light enhanced ROS generation [81], and the 0D Fe3O4@MoS2-Ag could generate dual antibacterial actions of ‧OH and Ag+ [82]. Therefore, the rough MoS2 nanosheets facilitated the enhanced bacterial adhesion (figures 4(A-iii, iv), (B-iii, iv) and (C-iii, iv)) due to special interaction between roughness of MoS2 nanosheets and flagella and pili of bacteria, which made the generated antibacterial actions surrounding the bacterial surface rather than normal cells, resulting in the enhanced catalytic therapeutic effect without potential hazards. Lately, Xu et al reported a carbon–iron oxide hybrid nanozymes with rough surfaces (RCF) (figure 4(D-i, ii)), which also provided increased bacterial adhesion for the nanozyme (figure 4(D-iii, iv)), thereby benefiting the catalytic and photothermal therapeutic outcome for wound infection [102]. Similarly, engineering sea urchin-like PdCu nanoparticles can also produce rather rough surfaces for enhancing the bacterial adhesion ability of the nanozymes and achieve improved antibacterial activity [33]. In general, rough surface engineering paves a new way to design strong bacteria-adhesive nanozymes with enhanced catalytic activity.
Figure 4. (A) Scanning electron microscope (SEM) (i) and TEM (ii) images of R-CMs, and SEM images of interaction between R-CMs and bacteria (iii and iv). Reprinted with permission from [43] John Wiley & Sons [© 2019 Wiley-VCH GmbH]. (B) SEM (i) and TEM (ii) images of MoS2/rGO VHS, and SEM images of interaction between MoS2/rGO VHS and bacteria (iii and iv). Reprinted with permission from [81] John Wiley & Sons [© 2020 Wiley-VCH GmbH]. (C) SEM (i) and TEM (ii) images of Fe3O4@MoS2-Ag, and SEM images of interaction between Fe3O4@MoS2-Ag and bacteria (iii and iv). Reprinted with permission from [82] © 2020 Elsevier B.V. (D) SEM (i) and TEM (ii) images of RCF, and SEM images of interaction between RCF and bacteria (iii and iv). Reprinted with permission from [102] © 2021, American Chemical Society.
Download figure:
Standard image High-resolution image4.2. Pseudopodia mimicking
Inspired by the branch-like pseudopodia mechanism of neutrophil trapping bacteria in the human immune system [110], Qu's group have designed a MOF@COF nanozyme with a pseudopodia-like surface and tailored microenvironment (figure 5(A)). The spiky COFs (figure 5(B)) offer multivalent topological interactions for the nanozyme, thus allowing them to strongly capture bacteria (figure 5(C)). Then, integrating with active sites generated by the hierarchical nanocavities of COFs for binding substrate molecules, the ROS could be in-situ produced around the bacteria, significantly amplifying the catalytic and therapeutic efficiency (figure 5(D)) [58]. Overall, this bioinspired strategy provides an alternative way to design novel strong bacteria-adhesive nanozymes.
Figure 5. Pseudopodia-like MOF@COF nanozyme for antibacterial therapy. (A) (i) TEM image of NMCTP-TTA and (ii–vi) corresponding element mappings of O, N, Fe, C, and Merge, respectively. (B) Morphologies of E. coli (i) and S. aureus (iv), and treated with NM-88 + H2O2 (ii and v) and NMCTP-TTA + H2O2 (iii and vi). (C) In vivo catalytic therapy of S. aureus wound-infection. (i) Schematic illustration of treatment strategy; (ii) photographs of wound-healing performance treated by different nanozyme catalysts; (iii) corresponding photographs of bacterial colonies isolated from different treated wound; (iv) H&E staining and immunohistochemical analysis of skin tissues. Reprinted with permission from [58] John Wiley and Sons [© 2020 Wiley‐VCH GmbH].
Download figure:
Standard image High-resolution image5. Multifunctional nanozyme-based platforms
As mentioned, catalytic activity enhancement is a benign way to improve the antibacterial performance of nanozymes for fighting against multidrug-resistant bacteria. However, owing to the intrinsic drawbacks of ROS (short life time and diffusion distance), single mode of nanozyme-based CDT is usually unsatisfactory for complex practical antibacterial therapy. Fortunately, benefiting from the unique physicochemical properties of nanomaterials such as magnetic, optical, thermal and electrical properties, a variety of multifunctional nanozyme-based platforms combining POD-like catalysis with cascade enzymatic catalysis, PTT, nitric oxide (NO) and ionic silver/zinc release, anti-inflammatory, pro-inflammatory immunity, and so on, have been explored to promote bacteria-infected wound healing, enteritis and diabetic wound recovery, and implant-related infections. Among them, Au nanoplates [26], MoS2-nanozymes [106, 111], carbon-nanozymes [55, 102] and M–N–C SAzymes [63, 64] with high photothermal conversion efficiency are inherent nanoplatforms for synergic CDT/PTT treating bacteria-infected wounds. MOFs-nanozymes with high loading capacity can be good candidate for multiple drugs delivering to realize CDT and drug (i.e. NO, Ag+, Zn2+, carvacrol) release for enhancing chronic wound healing [112–115]. Cascaded nanozymes that can trigger POD-, catalase-, and glucose oxidase-like catalysis have shown great potential for diabetic wound recovery due to the coupling of glucose consumption, antibacterial and anti-inflammatory activity [90, 116–118]. Moreover, the MOF2.5Au–Ce nanozymes with dual POD and deoxyribonuclease (DNase) and POD mimetic activities displayed significantly enhanced biofilm disruption efficiency [59]. Smart nanozymes like CuFe5O8 nanocubes can exhibit space-selective CDT and induce pro-inflammatory macrophage polarization for curing implant-related infection due to the different pH and H2O2 concentration inside and outside the biofilm [119]. Owing to their injectable, degradable, biocompatible, adhesive, and anti-inflammatory properties of hydrogel [120], a series of nanozyme-based multifunctional hydrogels have been constructed as alternative unique platforms for the chronic infected wound treatments [70, 121–125].
6. Conclusion and outlook
In this review, we summarized the recent progress of various chemical design strategies to construct efficient nanozymes for combating microbial superstrains. Strategies including enzyme active center mimicking, downsizing, defect engineering, catalytic processes enhanced by external stimulations, and bacterial capture enhancement can efficiently improve the antimicrobial activity of nanozymes as it is highly dependent on their catalytic efficiency. Besides, benefiting from the features of nanomaterials, nanozymes can be a unique footstone for building multifunctional nanoplatforms combining multiple antibacterial actions for overcoming the shortcomings of CDT alone in practical antibacterial therapies. Overall, advanced chemical design strategies have paved new ways to engineering efficient nanozymes with enhanced antibacterial performance.
Although these well chemically designed nanozymes have shown great success in treating bacteria-related diseases in many in vitro or in vivo models, they still have a long, long way to go before clinical translation due to the rather limited studies at the current stage. First, so far, the existing antibacterial nanozymes are almost exclusively POD, OXD, or hydrolase mimics, while other enzymes that may cooperate and participate in the life dense of microbial infections are less studied. In this case, it is urgent to explore new types of antibacterial nanozymes and interpret their antibacterial mechanisms. Secondly, various advanced strategies have been attempted to regulate the catalytic activity of nanozymes to obtain highly efficient antibacterial nanozymes such as SAzymes, while attempts on the modulation of substrate selectivity and product selectivity are rather few. The unique specificity and activity of natural enzymes are not only derived from their high density of active centers, but more importantly from their special 3D spatial structures. To this end, the purposive design of enzyme-comparable nanozymes still requires the collaborative interplay of mimicking enzymatic active centers and their spatial microenvironment construction, so as to acquire highly active and specific nanozymes. Additionally, taking advantage of the advanced SAC technologies and theoretical stimulations, simple enzyme-like active sites can be made more controllable and definite, and the corresponding catalytic mechanisms can be well clarified in vitro. Nevertheless, it is challenging to explore and fully understand the in vivo antibacterial mechanism of nanozymes due to the complexity of biological microenvironments. Finally, although nanozymes have potentiated a brilliant future in antibacterial therapy, their biological safety, potential toxicity, along with the in vivo translocation, biodistribution, degradation, and metabolic pathways, and so on remain challenging before real disease treatment.
In conclusion, the development of advanced chemical design strategies has undoubtedly provided new opportunities to break through the inherent limitations of nanozymes and acquire enhanced antibacterial therapeutic effects. Even if NABT is still at its primary stage and many unsolved issues are required to be tackled, the antibacterial nanozymes do provide an alternative powerful weapon for combating AMR and will usher in a bright prospect for future clinical translations.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 82160421), Natural Science Foundation of Jiangsu Province (BK20211322), China Postdoctoral Science Foundation (No. 2021M691331), and Postdoctoral Fund of Jiangsu Province (No. 2021K371C). This work was also supported by Research Fellow (Grant No. 328933), Solution for Health Profile (336355), and InFLAMES Flagship (337531) grants from Academy of Finland; Finland China Food and Health International Pilot Project funded by the Finnish Ministry of Education and Culture.