Abstract
The formation of light-induced oxygen vacancy (VO) is detected and confirmed on the surface of various metal-oxide-based semiconductors under mild reaction conditions with low cost energy source (sunlight). This self-structural transformation of the materials can bring about new characteristics and functionalities, which has inspired many researchers to explore the applications of light-induced VO in the photochemical conversion. In this perspective, generating and maintaining the light-induced VO are discussed based on some of the important work in the field of photochemical conversion. The effects and utilizations of the light-induced VO are revealed including the models proposed to explain mechanism. Then, the electric current measurements and key challenges of the light-induced VO are also summarized in a comprehensive introduction. Finally, some important aspects and questions in terms of the future research of light-induced VO are emphasized via discussing the potential contribution and development. And the schematic of future developments for light-induced VO is provided based on loop-locked materials design, light engineering and mechanism investigation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metal oxides based photo-responsive materials are the key components of photocatalytic, photoelectrochemical and photo-thermochemical systems that generate useful chemicals from wastes, carbon dioxide and water by using sunlight [1–4]. The in depth research has found that the absorbers are not perfectly crystalline during photochemical conversion. The irradiation treatment could bring about self-structural transformation of metal-oxide-based semiconductors [5–7]. The formation of light-induced oxygen vacancy (VO) is detected and confirmed on the surface of materials such as In2O3, perovskite and TiO2 in many previous studies [8–10]. These findings may raise three relevant questions, i.e. how could the light-induced VO form on the surface? Does the formed Vo has any positive effects on the photochemical conversion? If yes, how can we measure and evaluate these effects? In recent years, the research has been focused on finding answers for these questions; the light-induced VO is being widely studied and becomes more attractive for the photochemical conversion and in the fields of nanomaterial science, catalysis and energy conversion [11–13].
The formation of light-induced VO, same as other techniques of VO formation, will release separate educts and leave the defective substrates behind [14–17]. However, light should be absorbed to generate electron/hole pairs (EHPs) by electron transition in the semiconductors or localized surface plasmons resonance (LSPR) in the noble metal nanoparticles (NPs) to drive the light-induced VO formation on the absorbers at room temperature [12, 18, 19]. Thus, the absorbers as well as the substrates should normally be metal-oxide-based semiconductors and metal oxides decorated by NPs for the light-induced structural transformation to occur, which is different from those VO formations usually driven by thermochemical and/or electrochemical processes.
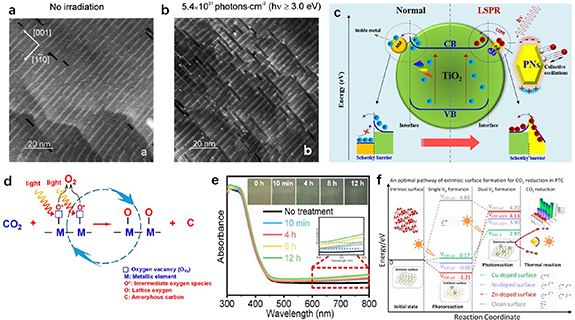
As a non-destructive method, light irradiation can in-situ produce VO during photochemical conversion, and compared to the formation of thermal or electric VO, light-induced VO generation could be driven by solar energy at room temperature without the need of high temperature and other energy input. Vacuum [5, 8, 9, 12, 20, 21], inert atmosphere [7, 10, 13, 17–19, 22–29] or reducing agents [11, 30–50] could be provided to promote exclusion of oxygen and metal ions in the metal-oxide semiconductors and meanwhile, the VO would be created on the surface of metal oxides (see figures 1(a) and (b)). Since light energy is usually weaker than thermal or electric energy, it may not be able to entirely exclude metal ions from the semiconductors. Thus, in most cases, the ions from exsolution are only oxygen ions. The metal ions on the surface of metal oxides could not be totally reduced to metal. For example, Ti4+ ions are usually reduced to Ti3+ [13, 17–19, 24, 27].
Figure 1. Schematic light-induced VO generates in (a) vacuum or inert atmosphere and (b) reductive environment and its applications for photochemical conversion of H2O and CO2 when it acts as a (c) reactant and (d) catalytic center.
Download figure:
Standard image High-resolution imageThe light-induced VO is confirmed to play a very important role in the photochemical conversion based on many studies, which is mostly used in useful fuel and chemicals production. It not only can provide a defective site as well as reaction center for adsorption and activation on the surface of photo-absorbing materials in the process of catalysis, but also can serve as a reactant for subsequent consumption in the cyclic reactions (see figures 1(c) and (d)) [8, 10, 12, 28, 33, 35]. Additionally, the light-induced formation of VO brings about structural transformation, spectral and electromagnetism improvement, so it is widely used for improving performance of light-induced carriers including their lifetime, separation and mobility [21, 32, 37]. It is noted that this function is also utilized in surface detection research such as surface-enhanced Raman spectroscopy [11, 42, 51–53]. However, since this is not a photochemical conversion process, it will not be discussed here.
In this article, we will discuss some of the important works devoted to generating and maintaining the light-induced VO in the photochemical conversion. After a brief description, we will focus on some key researches which have revealed important effects of the light-induced VO on the surface of metal oxide, and present the models proposed to explain its mechanism. Then, we will summarize the measurements and current challenges of the light-induced VO. Finally, we will discuss the potential contribution and development of light-induced VO with emphasis on some important aspects and questions for future research in this field.
2. Light-induced VO in the photochemical conversion
2.1. Generation of light-induced VO
According to the previous reports, the light-induced VO is mostly found on different kinds of metal-oxide-based semiconductors. Depending on the circumstance of its generation, it could be divided into three main approaches. Firstly, to avoid reoxidation, the vacuum system should be a natural choice for producing light-induced VO. At the early stage, light-induced VO is observed on the surface of TiO2 in the high vacuum chamber of scanning tunneling microscope (STM) for investigating the super hydrophilicity of TiO2 under UV light (see figures 2(a) and (b)) [5]. Although it is difficult to apply vacuum in the photo-chemical process, some researchers still used vacuum to pre-treated metal-oxide-based semiconductors to generate light-induced VO on the surface for subsequent photo-chemical catalysis. For example, Zu et al fabricated the defective Bi2O2CO3 nanosheets, in which the light-induced VO needed to be regenerated under UV light irradiation in the vacuum in every circle [12].
Figure 2. STM image of TiO2 (110)–(1 × 2) surface (a) with no irradiation and (b) light-induced VO formation under UV irradiation. Reprinted from [5], Copyright (2003), with permission from Elsevier. (c) Reversing transfer direction of energy carrier by LSPR in the light-induced VO formation on the Pd-loaded TiO2. Reprinted with permission from [18]. Copyright (2018) American Chemical Society. (d) The proposed route for splitting CO2 to carbon and O2 by light-induced VO on the α-Zn–Ge–O. Reprinted with permission from [8]. Copyright (2017) American Chemical Society. (e) UV–vis DRS results of WO3 photoanodes under different irradiated time for light-induced VO formation, inset shows the digital images. [37] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (f) Mechanism and DFT guidance of screening doped ions for light-induced VO formation in the photochemical CO2 reduction. Reprinted from [19], Copyright (2017), with permission from Elsevier.
Download figure:
Standard image High-resolution imageSecondly, inert atmosphere without reducing agent could be employed to achieve the similar effect as using vacuum. Inspired by heat-induced VO, Zhang et al extensively studied the light-induced VO in the inert atmosphere such as Ar gas [10, 13, 17–19, 22–24, 28] and have published their research outputs for CO2 reduction and H2O splitting by using pure TiO2 and various decorated TiO2. Since formation energy could be reduced and absorbance of solar light could be expanded from UV to visible light, doping and loading co-catalysts (e.g. Au, Pd, Cu) on the TiO2 have been proven to be the efficient methods for improving generation of light-induced VO on the material surface used in their work. As shown in figure 2(c), Xu et al introduced a LSPR effect by loading Pd NPs on the TiO2 [18]. Since light absorption was enhanced in vis-NIR spectrum, more available charge carriers were generated to induce more VOs on TiO2. Besides TiO2, various metal-oxide-based semiconductors were also found to be good substrates for generating light-induced VO. For instance, amorphous zinc germanate (α-Zn–Ge–O) semiconductor with weak lattice constraint was used by Wang et al to promote the formation of light-induced VO [8]; they also conducted generation and consumption of VO simultaneously to reduce CO2 to C in the CO2 atmosphere (see figure 2(d)).
Thirdly, the light-induced VO could be produced in the solution under some additional conditions such as biased voltages and reducing agents. These additional conditions can create reductive circumstance to promote O exsolution on the surface of metal-oxide-based semiconductors. Normally, the VO could only be maintained when additional conditions were applied. Recently, Sun et al fabricated light-induced VO on the photoanodes (e.g. BiVO4 and WO3) in a neutral solution by applying a reduced potential under AM 1.5 sunlight (see figure 2(e)). They demonstrated that light-induced VO could remarkably enhance the photoelectrochemical performance [37]. Furthermore, during some photocatalytic processes including photocatalytic degradation, dehalogenation and water treatment, it is easy for metal-oxide-based semiconductors (e.g. In2O3, BiOCl crystals) to produce light-induced VO with organic reactants such as rhodamine B under UV light irradiation [36, 40, 41, 54]. Additionally, to promote fabrication of light-induced VO, the Raman reporter such as mercaptobenzoic acid is usually utilized for enhancing signal on the surface in the photo-induced enhanced Raman spectroscopy [51, 53].
Generally, the generation of light-induced VO proceeds with the following steps [8, 17, 27]: in reaction (1) as seen below, the light-induced EHPs are produced as a result of illumination on the Mx
Oy
surface. In reaction (2), the photo-generated excitons are reduced on the Mx
Oy
surface, causing the conversion of  sites to
sites to  sites. In reactions (3) and (4), the excited holes are trapped in the lattice O ions to produce free oxygen radicals (O·) and generate VO on the nonstoichiometric surface. In reaction (5), the two adjacent O· ions combine to form an oxygen molecule in the vacuum and inert atmosphere. If reducing agents or protons are added, the O· ions will combine with reducing agents or protons. reaction (6) shows the overall reaction of light-induced O exsolution [from reactions (3) to (5)]. It should be noted that the A(B) means item A is adsorbed on the item B in the bracket as in the following reactions.
sites. In reactions (3) and (4), the excited holes are trapped in the lattice O ions to produce free oxygen radicals (O·) and generate VO on the nonstoichiometric surface. In reaction (5), the two adjacent O· ions combine to form an oxygen molecule in the vacuum and inert atmosphere. If reducing agents or protons are added, the O· ions will combine with reducing agents or protons. reaction (6) shows the overall reaction of light-induced O exsolution [from reactions (3) to (5)]. It should be noted that the A(B) means item A is adsorbed on the item B in the bracket as in the following reactions.
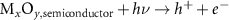
| Light-induced EHPs | (1) |
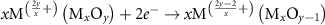
| Metal ion reduction | (2) |
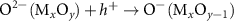
| O exsolution | (3) |
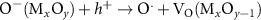
| O exsolution | (4) |
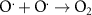
| O exsolution | (5) |

| Total reaction of O exsolution | (6) |
Obviously, the key points are the excitation and separation of EHPs, decreasing formation energy of VO and maintaining VO under the required conditions, which leads to the requirements for both photoabsorbers and circumstances during the generation of light-induced VO. In general, all of these three conditions, vacuum, inert atmosphere and solution, are available to create VO. The light-induced VO generated in vacuum and inert atmosphere is usually consumed in the cycle system since it could not be well maintained when vacuum and inert atmosphere are replaced by reactants. These circumstances are created to avoid re-oxidation which normally reduces the number of VO. Because additional conditions are usually applied to keep reduction, the generated VO could be easily created and maintained in solution and utilized in the continuous system. Researchers should create suitable circumstances in different applications to avoid re-oxidation or keep reduction. While most of works could use UV light to generate VO [27, 55–57], the photoabsorbers need to be further modified for using visible light. Expanding the absorbance of light could provide more available EHPs to drive VO generation. Meanwhile, recombination of EHPs has been found to be the most energy-consumption step in the light conversion [4, 56, 58, 59]. To depress the recombination of EHPs, co-catalysts that could create electron or hole traps and prolong lifetime of carriers will play an important role [4, 60, 61]. But the co-catalysts could also be a recombination center [17, 62], which should be further studied and need delicate adjustment in the fabrication of photoabsorbers. Based on density functional theory (DFT) calculations, Xu et al reported that both interstitial doping and replaced doping are demonstrated to be effective to decrease the formation energy of VO (see figure 2(f)), which could greatly increase the number of VO [19]. However, the easier the generation of VO is, the harder the VO is maintained and used. Thus, the balance between the difficulty of VO formation and utilization needs to be fine-tuned in the experiments. This issue is important but is often neglected by researchers. It should also be noted that the valence of metal could play an important role in the formation of VO. On one hand, because the capacity of losing and obtaining electrons could be much different between the metal ions with single oxidized valence and the metal ions with multiple oxidized valences (e.g. Zn and Ti ions), it leads to different abilities of oxygen evolution in different metal oxides. On the other hand, the valence of metal could also be important even in the same metal oxide. Generating VO is always harder in the metal oxide with low-valence metal ions than that with high-valence metal ions. For example, the formation of VO on the CeO2 could be much easier than on the Ce2O3. Besides, Qi et al also found that the enlarged surface area allows more exposed oxygen atoms on the surface, which is favorable for the generation of VO under the irradiation of light [46]. Constructing efficient nanostructure via light engineering on the photoabsorber to achieve multiple reflection and absorption should be a promising way to increase light-induced VO formation.
2.2. The way to benefit from light-induced VO
Currently, the productions of useful fuels and chemicals such as CO2 reduction [18, 19, 23], H2O splitting [13, 22, 24], nitrogen fixation [63–65], reverse water gas conversion [33, 35, 46, 47] and dry reforming of methane (DRM) [25, 26, 47, 56] are the most studied applications of the light-induced VO for the photochemical conversion. This light-induced VO formation is a unique process whereby the exsolved atoms are initial part of the support crystal lattice of semiconductors, this phenomenon forms the base for the two functionally useful ways used in the photochemical conversion. On one hand, the remaining VO on the non-stoichiometric semiconductor could be utilized as a reactant as well as consumables under a specified condition and regenerated under irradiation. As a result, a completed cycle of VO consumption and regeneration could be established. Taking CO2 reduction and H2O splitting as the examples, they could be used to produce CO and H2 by capturing the oxygen in the CO2 and H2O. Then, the dissociation of CO2 and H2O could be completed by forming a photochemical looping via combining reaction 7 or 8 (see figure 1). Meanwhile, the metal ion  will be oxidized to
will be oxidized to  in reaction (9).
in reaction (9).
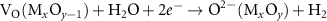
| VO consumption | (7) |
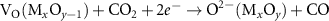
| VO consumption | (8) |
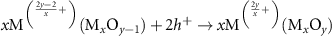
| Metal ion oxidation | (9) |
Zhang et al and Yoon et al have replaced the heat-induced VO with light-induced VO to lower the reaction temperature and improve solar-to-fuel efficiency for solar CO2 reduction and H2O splitting by photo-thermochemical cycles [10, 13, 17–19, 22–28]. The mechanism of photo-thermochemical cycle over TiO2, which achieved CO2 reduction by generating and consuming light-induced VO at a temperature of less than 500 °C, is shown in figure 3(a). As a comparison, the heat-induced VO needs a temperature of higher than 1000 °C [56]. In the CO2 photoreduction work by Zu et al (as shown in figure 3(b)), VOs are generated under fast UV light irradiation on the Bi2O2CO3 nanosheets in near vacuum and reproduced after every 24 h cycling test [12]. Due to light-induced VO, the formation energy of COOH* intermediate can be decreased from 1.64 to 1.13 eV, which was the rate-limiting step in the photochemical CO2 conversion. Finally, a high CO evolution rate of 275 mmol g−1 h−1 using Bi2O2CO3 with VO, which was 120 times higher than that without VO, was achieved for the visible-light-driven CO2 photoreduction during 110 cycling tests. The light-induced VO could also be utilized in photoelectrochemical reactions. As shown in figure 3(c), Sun et al conducted a photocharge/discharge strategy to initiate WO3 photoanode by generating light-induced VO [37]. There was no significant decay in more than 25 cycles with 50 h durability with the photocharged WO3 surrounded by a 8–10 nm overlayer and VOs. It was attributed to the prolonged charge carrier lifetime caused by light-induced VO.
Figure 3. (a) Schematic of the novel photo-thermochemical cycle for CO2 splitting. Reprinted from [10], Copyright (2015), with permission from Elsevier. (b) Schematic of the VO looping on the Bi2O2CO3 nanosheets. [12] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (c) Photocharging/discharging character of WO3 photoanode for light-induced VO regeneration and consumption. [37] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (d) Schematic of the formation mechanism and catalytic process of light-induced VO on the bulk In2O3 and 2D black In2O3 − x nanosheets. (e) Advantages for photothermal CO2 reduction on the In2O3 − x nanosheets with light-induced VO. [46] John Wiley & Sons. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) H2 production rates and (g) CO production rates in the dark and under light irradiation at 600 °C for 20 h in DRM tests. Reprinted from [47], Copyright (2020), with permission from Elsevier. (h) Suggested mechanism of UV-light-induced formation of surface VO of BaSnO+. Photocurrent and persistent photoconductivity increased with slow relaxation which was attributed to the reversible formation of light-induced defects in BaSnO3 films. [9] John Wiley & Sons. © 2021 Wiley-VCH GmbH.
Download figure:
Standard image High-resolution imageOn the other hand, the light-induced VO could act as the activated sites for photochemical conversion on the surface [6, 30, 31, 33, 35, 38, 40–42, 44–46, 48, 66, 67]. Different from the cyclic utilization, all the steps are completed under the same condition. Taking H2 production for example, the mechanism could be shown as follows [6, 17, 56, 67]:
According to the electronegativity of ions, H+ and OH− could be generated and adsorbed on the reduced metal ions ( ) and VO in the water ionization, respectively (reaction 10). The VO and metal ions could be the activated sites for further reactions of O2− and H2 (reactions 11 and 12). The O2− could react with holes and VO could be unoccupied in the O2 production (reaction 13). Different from the application in the photochemical looping, the light-induced VO will not be consumed, but act as an activation center in the photocatalysis. Zhang et al found that light-induced VO could be sustainable on the BiOBr nanoflower [30]. And reaction rates were found to be a greatly increased which was attributed to the VO-induced improvement for transfer efficiency of photoinduced carriers and the light absorption capacity in photocatalytic water splitting. In addition, Qi et al fabricated a large quantity of dual-functional light-induced VO on the 2D In2O3 nanostructures, where local heat was more efficiently produced to achieve a high photothermal conversion and provide active sites for the photothermal CO2 reduction reaction simultaneously (see figures 3(d) and (e)) [46]. A great CO production rate of 103.21 mmol gcat
−1 h−1 could be obtained. The work indicates that the light (acted as an ideal external stimulus) could efficiently produce a large number of VOs on the 2D nanostructures. Besides, some reactions which do not need light could be assisted by light-induced VO under light. Pan et al integrated photocatalysis and thermocatalysis for DRM on MgO/Pt/Zn-CeO2 [47]. As shown in figures 3(f) and (g), they maintained the light illumination to generate light-induced VO on CeO2 by photoinduced electrons, which could avoid deactivation to stabilize the thermochemical DRM process. It has also been found that CO disproportionation as the major side-reaction could be restrained with the light-induced VO, therefore the rate of carbon deposition was decreased during the process of DRM. Interestingly, Lee et al generated light-induced VO in 2D BaSnO3 epitaxial films to reversibly control photocurrent responsivity and persistent photoconductivity (see figure 3(h)). It indicates that light-induced VO could be applied to develop even 2D optoelectronic devices by optically controllable manipulation of surface defect states, which should be another interesting application for light-induced VO [9]. Additionally, the stability of light-induced VO needs to be considered in two cases according to its application. The first one is the application of VO consumption for reduction reaction, which requires that oxygen vacancies could be steadily decreased and easily participate in the reduction reaction. In the second case, the environment where the oxygen vacancies perform catalytic reactions mostly exists in the presence of oxygen, so how to maintain VO is challenging. Especially, light-induced VOs are mostly formed on the surface, which are less stable than those in the bulk. Some modification methods have been employed, such as nitrogen doping and nanostructure design to stabilize valance state of metal ions to keep VOs stable [68, 69]. Besides, starting from the reaction circumstances, utilizing some reaction conditions to maintain a reducing environment, such as reducing gas, sacrificial agent and biased voltages, can also maintain oxygen vacancies. Both ways of utilizing light-induced VO have been demonstrated to be efficient in improving the photochemical conversion. The production and utilization of light-induced VO are simple, precise, nondestructive and easy to be expanded. It is foreseeable that more and more photochemical conversions including solar-driven water splitting, CO2 reduction, synthesis gas conversion, seawater desalinization and organic pollutant disposal could benefit from the activation and catalysis of light-induced VO.
) and VO in the water ionization, respectively (reaction 10). The VO and metal ions could be the activated sites for further reactions of O2− and H2 (reactions 11 and 12). The O2− could react with holes and VO could be unoccupied in the O2 production (reaction 13). Different from the application in the photochemical looping, the light-induced VO will not be consumed, but act as an activation center in the photocatalysis. Zhang et al found that light-induced VO could be sustainable on the BiOBr nanoflower [30]. And reaction rates were found to be a greatly increased which was attributed to the VO-induced improvement for transfer efficiency of photoinduced carriers and the light absorption capacity in photocatalytic water splitting. In addition, Qi et al fabricated a large quantity of dual-functional light-induced VO on the 2D In2O3 nanostructures, where local heat was more efficiently produced to achieve a high photothermal conversion and provide active sites for the photothermal CO2 reduction reaction simultaneously (see figures 3(d) and (e)) [46]. A great CO production rate of 103.21 mmol gcat
−1 h−1 could be obtained. The work indicates that the light (acted as an ideal external stimulus) could efficiently produce a large number of VOs on the 2D nanostructures. Besides, some reactions which do not need light could be assisted by light-induced VO under light. Pan et al integrated photocatalysis and thermocatalysis for DRM on MgO/Pt/Zn-CeO2 [47]. As shown in figures 3(f) and (g), they maintained the light illumination to generate light-induced VO on CeO2 by photoinduced electrons, which could avoid deactivation to stabilize the thermochemical DRM process. It has also been found that CO disproportionation as the major side-reaction could be restrained with the light-induced VO, therefore the rate of carbon deposition was decreased during the process of DRM. Interestingly, Lee et al generated light-induced VO in 2D BaSnO3 epitaxial films to reversibly control photocurrent responsivity and persistent photoconductivity (see figure 3(h)). It indicates that light-induced VO could be applied to develop even 2D optoelectronic devices by optically controllable manipulation of surface defect states, which should be another interesting application for light-induced VO [9]. Additionally, the stability of light-induced VO needs to be considered in two cases according to its application. The first one is the application of VO consumption for reduction reaction, which requires that oxygen vacancies could be steadily decreased and easily participate in the reduction reaction. In the second case, the environment where the oxygen vacancies perform catalytic reactions mostly exists in the presence of oxygen, so how to maintain VO is challenging. Especially, light-induced VOs are mostly formed on the surface, which are less stable than those in the bulk. Some modification methods have been employed, such as nitrogen doping and nanostructure design to stabilize valance state of metal ions to keep VOs stable [68, 69]. Besides, starting from the reaction circumstances, utilizing some reaction conditions to maintain a reducing environment, such as reducing gas, sacrificial agent and biased voltages, can also maintain oxygen vacancies. Both ways of utilizing light-induced VO have been demonstrated to be efficient in improving the photochemical conversion. The production and utilization of light-induced VO are simple, precise, nondestructive and easy to be expanded. It is foreseeable that more and more photochemical conversions including solar-driven water splitting, CO2 reduction, synthesis gas conversion, seawater desalinization and organic pollutant disposal could benefit from the activation and catalysis of light-induced VO.
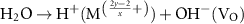
| H2O dissociation | (10) |
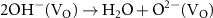
| OH− catalysis on the VO | (11) |
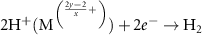
| H2 generation | (12) |
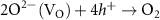
| O2 generation | (13) |
2.3. Measurements of light-induced VO
Revealing the relationship between the light-induced VO formation and activities is very important for designing high-performance defective catalysts. Therefore, measurements and evaluations of light-induced VO need to be carefully handled by using various characterizations. In that perspective, the measurements could be generally divided into electron microscopy techniques and spectroscopy techniques.
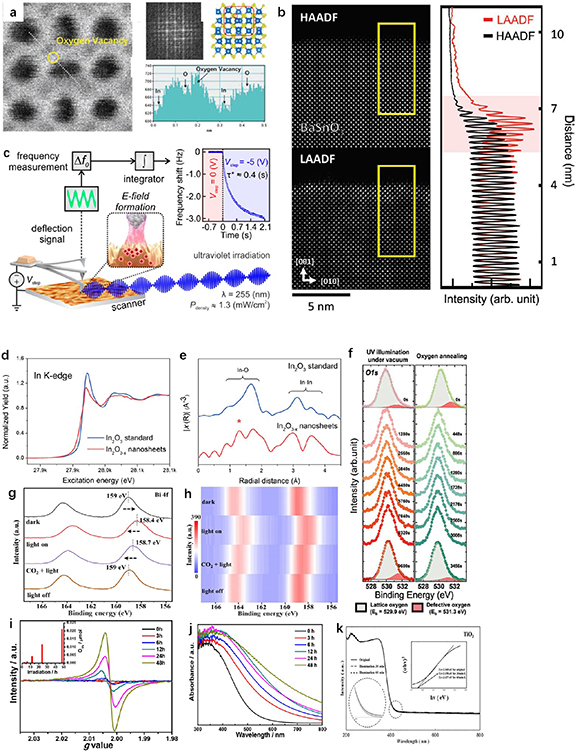
The atomic structure of imaging materials could be shown by electron microscopy technology directly. Due to the low resolution, some normal microscopy techniques, including scanning electron microscopy and transmission electron microscopy, are not able to accurately measure VO [33, 41, 43, 54]. As mentioned before, STM could directly observe and demonstrate the VO formation (see figures 1(a) and (b)) [5]. And scanning transmission electron microscopy (STEM) which owns a resolution of atomic level is a very useful tool for observing the VO on the surface of materials [9, 15, 40, 54, 63, 65, 70]. Light-induced VO was well confirmed by Qi et al in the magnified image obtained by an annular bright-field scanning transmission electron microscopy (ABF-STEM), which could show the atom arrangements of the 2D In2O3 − x layers (see figure 4(a)) [46]. Furthermore, as shown in figure 4(b), Lee et al found that it was better to use the low-angle annular dark-field (LAADF) signal of STEM, which was more sensitive to the strain field from point defects (i.e. VO), than the high-angle annular dark field signal to measure light-induced VO [9]. It shows that LAADF-STEM is a good direct way to observe light-induced VO optically. Besides, Dagdeviren et al employed a time-resolved atomic force microscopy with fast-detection electronics and high-frequency cantilevers to demonstrate the effect of the light-induced surface VO on charge carrier dynamics (see figure 4(c)) [21]. They measured the resonance frequency shift (Δf0) of the oscillating cantilever to assess the time-dependent variation in the tip-sample interaction force, which could further obtain the effective activation energy related to the migration of holes, Ea*, and the migration barrier for a single hole motion, Ea. The Ea in dark and that under light could be measured and detected to uncover the change of hole migration dynamics by this method. It has been proven to be effective to study the deep mechanism in the formation of light-induced VO.
Figure 4. (a) ABF-STEM images of In2O3 − x nanosheets with light-induced VO and magnified area of the region in the blue square. [46] John Wiley & Sons. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) HAADF-STEM and LAADF-STEM image of BaSnO3 epitaxial films along the [1 0 0] zone axis under illumination in vacuum. Contrasting intensity profiles in the yellow rectangles in HAADF-STEM (black line) and LAADF-STEM (red line) images. [9] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (c) Schematic of the local measurement of hole migration time constants under UV irradiation. Reprinted with permission from [21]. Copyright (2021) American Chemical Society. (d) Normalized In K-edge XANES spectra and (e) EXAFS spectra of In2O3 standard and nanosheets with VO. [46] John Wiley & Sons. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) In situ XPS O 1s spectra of BaSnO3 epitaxial films under UV illumination in vacuum at room temperature (left) and without illumination in oxygen atmosphere at 350 °C (right). [9] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (g) Quasi in-situ XPS spectra of Bi 4f for the Bi2O2CO3 nanosheets. (h) Heat map corresponded to figure 4(g). [12] John Wiley & Sons. © 2021 Wiley-VCH GmbH. (i) EPR and (j) UV–vis DRS results for formation of light-induced VO on the α-Zn–Ge–O under light in vacuum at different irradiation time. The inset in (i) shows the concentration of VOs. Reprinted with permission from [8]. Copyright (2017) American Chemical Society. (k) UV–vis DRS spectra for formation of light-induced VO on the TiO2 with illumination in Ar gas at different time. Reprinted from [13], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn regards to the x-ray spectroscopy techniques, resolution of x-ray diffraction is usually too low to detect light-induced VO, but x-ray photoelectron spectroscopy (XPS) [12, 13, 22–24, 30–34, 36–42, 71–73] and x-ray absorption spectroscopy (XAS) [1, 2, 24, 27, 33, 46, 50, 54, 60, 62, 63, 65, 71, 74] are effective. XAS, including x-ray absorption near-edge structure spectroscopy and extended x-ray absorption fine structure, could assess oxidation state in bulk and average coordination environment of elements in materials. As shown in figure 4(d), Qi et al studied the In K-edge of In2O3 − x nanosheets and standard In2O3 and found the existence of lower average oxidation states of In species in In2O3 − x nanosheets [46]. According to results in the R space (see figure 4(e)), the light-induced VO led to the decrease of the In–O distance, which caused a split between 1 and 2 Å of In2O3 − x nanosheets. XAS could show ample information of the defects in bulk, however, the light-induced VO is normally generated on the surface only. Thus, use of XPS gains more popularity than XAS in the study of light-induced VO, since the former only detects the surface defects. Especially, time-resolved in-situ XPS could be more powerful to clarify the formation and function of light-induced VO. As shown in figure 4(f), the process of light-induced VO formation could be easily observed in Lee et al's study [9]. To reveal the interaction between light-induced VO and metal ions, Zu et al employed a synchrotron-radiation quasi in-situ XPS to study the Bi ions near the VO in figures 4(g) and (h) [12]. Their XPS results indicated that the Bi3+ sites could easily receive the photoexcited electrons with the presence of light-induced VO and then adsorb CO2 molecules, i.e. not only CO2 was efficiently activated, the partially reduced Bi sites were returned into the original Bi3+ sites as well.
As an effective method for investigating unpaired electrons in materials, electron paramagnetic resonance (EPR) is also often used for qualitative and quantitative characterizations of light-induced VO [13, 24, 30, 32, 34, 35, 37–39, 41, 42, 71, 75]. As shown in figure 4(i), Wang et al detected an EPR signal of VO at g = 2.003 after α-Zn–Ge–O was illuminated by a 300 W Xe lamp in vacuum, which demonstrated the process of light-induced VO formation [8]. They also conducted a rough quantitative EPR analysis by reference to the spin numbers of lone electrons of natural coal. Their results indicated that the VO concentration of α-Zn–Ge–O continuously increased from 0 to 0.21 μmol g−1 as the irradiation time was increased from 0 to 48 h. However, the EPR could not distinguish the surface VO and bulk VO. Thus, it was still hard to know the number of light-induced VOs on the surface, which were the real effective sites. Besides, Raman spectroscopy, photoluminescence and UV-visible diffuse reflectance spectroscopy (UV–vis DRS) could also detect the VO indirectly [4, 9, 11, 22–24, 33, 36–38, 41, 43, 44, 46, 48–51, 54, 56]. Nonetheless, the accuracy of results highly depends on the VO concentration, materials property and conditions of measurements, making these methods not very universal. For example, the changes in the results of UV–vis DRS when producing light-induced VO on the TiO2 in Ar gas were much smaller than those on the α-Zn–Ge–O in vacuum (figures 4(j) and (k)) [8, 13]. In addition, the production in the VO formation (such as O2) could also be analyzed by using gas chromatography and traced by mass spectrum with isotopic gas [13, 24, 27, 35, 60, 63, 67]. The materials, methods and corresponding measurements of the representative studies of photo-induced VO are summarized in table 1, which shows that various materials could be utilized to generate light-induced VO in different kinds of circumstances. While most measurements are similar, in-situ methods and advanced electron microscope could be rare and powerful in the study of light-induced VO. Conclusively, although the measurements and evaluations of light-induced VO are either very complex and expensive or lack of accuracy, the development of in-situ techniques combined with electron microscopy and spectroscopy techniques is expected to bring more valuable information and knowledge about light-induced VO.
Table 1. Materials, methods and corresponding measurements of the representative studies of light-induced VO.
| Materials | Light source | Circumstances | Main measurements | References |
|---|---|---|---|---|
| TiO2 | UV | Vacuum | STM, TR-AFM | [5, 21] |
| Bi2O2CO3 | UV | Vacuum | XPS, EPR, GC | [12] |
| α-Zn–Ge–O | Xe lamp | Vacuum | XPS, EPR, MS, UV–vis | [8] |
| BaSnO3 | UV | Vacuum | STEM, XPS | [9] |
| Doped TiO2 | Hg lamp | He gas | XPS, EPR | [17–19] |
| Doped TiO2 | Xe lamp | Ar gas | XPS, EPR, MS, UV–vis | [13, 22–24] |
| Cu2O | Hg lamp | i-propanol | XPS | [31] |
| BiOCl | UV | RhB | XPS, EPR | [6, 34, 38] |
| WO3 | AM 1.5G | Biased potential | XPS, EPR, UV–vis, HRTEM | [65] |
| In2O3 | Xe lamp | H2 | XPS, EPR, XAFS, HRTEM, CO2-TPD | [46] |
2.4. Challenges of light-induced VO
The light-induced VO can be implemented under mild reaction conditions with low cost energy source (sunlight). Since the reaction conditions of photochemical conversion are very similar to that for light-induced VO formation, it is convenient to combine materials fabrication (via in situ light-induced VO formation) with the subsequent reactions of photochemical conversion. Thus, this method demonstrates a great potential of its much broader applications for the photochemical conversion. However, achieving an effective light-induced VO formation could face three challenges, i.e. suitable semiconductor support, measurement of VO, mechanism of the effect of light-induced VO on the photochemical conversion.
Firstly, in order to enable light-induced VO formation, the semiconductor support should meet two conditions: (a) the bandgap of semiconductor should be suitable to generate enough EHPs, (b) the formation energy of VO should be low enough to be driven by EHPs. In the previous studies, many approaches have been taken to adjusting the bandgap and VO formation energy with some promising results, including doping with transition metal ions and loading noble metals [13, 18, 22–24, 51, 56, 76, 77]. However, more efficient semiconductor supports should be developed and fabricated to produce more light-induced VO. Besides, many studies have shown that the formation of VO could also be the recombination of EHPs [9, 21, 30, 39, 56]. Although it is important and should be well adjusted in the fabrication, no detailed information is available and no discussion and research have been conducted yet.
Secondly, it is difficult to evaluate the performance of light-induced VO without the accurate information of the numbers of VO. However, on the one hand, due to the formation of light-induced VO occurs on the surface of metal oxide and its concentration is quite low in terms of the whole material, making it difficult to detect and measure it accurately. On the other hand, since the light-induced VO is usually not stable in the atmosphere and tends to be transient during the photochemical conversion, critical conditions should be applied to be able to detect and evaluate it [39, 54, 56]. Therefore, in-situ techniques may need to be combined with above measuring techniques to make a detailed and real-time evaluation of light-induced VO during photochemical conversion. In-situ tests combined with STM, STEM, EPR and XPS could be employed to observe the light-induced VO formation directly. However, the very strict requirements of these tests are hardly to be practical, and the complete quantitative VO measurements also remain challenging.
Thirdly, although the mechanism of the effects of light-induced VO has been extensively investigated and many possible reasons have been proposed, there is lack of a clear picture in regards to the contributions of each effect, e.g. the synergetic effect and the effect of interaction between VO and reduced metal ions in the process of photochemical conversion. Additionally, in addition to the number of exsolved sites, the qualities of VO and metal ions are also important since some low-quality VO may not be the activated sites and would not participate in the reaction. It is worth noting that some recent calculation work has noticed this point. The DFT calculations of Li et al indicate that the balance between the formation and reactivity of light-induced VO is the key factor for the pathway preference for water splitting, which should be taken into consideration for catalyst design and screening [77, 78]. Currently, there is still no reported experimental work focusing on this. The three issues mentioned above could greatly affect the final efficiency of reactions which would dictate the future development and potential applications of the light-induced VO for the photochemical conversion.
3. Future perspectives
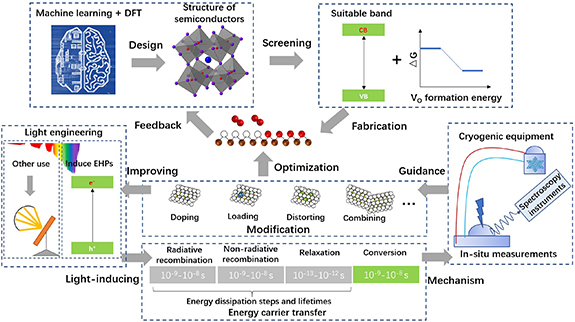
According to the particular requirements of light-induced VO, the exciting light is the driven force but, despite its importance, remains much less studied so far. The light-induced VO could be greatly enhanced by increasing the power density of incident light. Furthermore, if the photons with different frequencies could be applied to the processes of light-induced VO formation and the subsequent photochemical conversions, the whole light energy will be fully utilized. To adjust the wavelength and power density of the incident light, specific light equipment including mirrors, beam splitter and filters needs to be designed based on the latest techniques developed in field of advanced optic devices manufacture.
To fabricate suitable semiconductors, the bandgap and VO formation energy are critical since they are the preconditions for absorbing light energy and generating exsolution, respectively. The ideal bandgap and VO formation energy of semiconductors could be obtained by calculating the density of states and Gibbs free energy based on DFT before the experiments (see figure 2(f)), but the screening speed and range are low and narrow. The recent developments in the field of machine learning (ML) have further broadened the range of theoretical calculations to efficiently screen suitable semiconductor supports [79]. The much larger screening range of ML can greatly narrow down the ranges of DFT calculations and experimental verifications, thus forming an effective iterative looping to design efficient semiconductor supports. The ML applicable fabrication methods include doping, loading and compositing materials.
Besides light energy absorbance and final conversion, the energy transfer (including carrier's mobility rate and transfer direction) is also important to reduce the energy dissipation in the bulk of semiconductors, but it is difficult to control and study this process due to its very short time span (usually from nanoseconds to microseconds). The mobility of energy carrier could be slowed down at ultra-low temperature, thus to enlarge the time scale and magnify the signals of energy transfer by combining specially-made exsolution cells, spectroscopy instruments and cryogenic equipment in the light-induced VO formation. Accordingly, the mechanism of energy transfer could be clarified to guide the design of semiconductors. In addition, some ingenious methods based on the optic characterizations of certain materials could be applied to control and smooth the energy transfer process. For example, the light-induced hot electrons generated by the LSPR of some special metal particles (e.g. Ag, Cu, Pd...) could exceed the Schottky barrier (see figure 2(c)), in which the direction of energy transfer could be reversed to promote the exsolution [4, 18, 56].
In terms of the measurement of VO, a range of material spectroscopy techniques, especially the techniques at synchrotron, would be highly desirable. The customized in-situ cell could be designed to cooperate with the XPS, diffraction, absorption spectroscopy. Because the irradiation of synchrotron is not in the absorbance range of semiconductor, these spectroscopy techniques would not affect the generation of light-induced VO. Thus, time-resolved, even space-resolved measurements could be achieved to study the generation, disappearance and distribution of VO and exsolved sites in the semiconductor supports. Besides the in-situ techniques of materials structure, the in-situ tests for measuring chemical groups could be conducted to investigate the mechanism of the effects of light-induced VO on the photochemical conversion. In-situ Fourier transform infrared spectroscopy and EPR are among the good choices [12, 35, 36, 44, 50, 60]. Different from the measurements of materials structure, the intermediate chemical groups are easily to be affected by the conditions of measurements. Therefore, a stable and precise in-situ reaction cell is more important in this regard. Lastly, the theoretical calculations could also be employed as a powerful tool in support of the in-situ measurement results and provide deeper insights into the reaction mechanism [76–78, 80].
Additionally, there are a lot of other methods for VO formation in the metal oxide including hydrogenation, hydride reaction, mixed gas reduction, high-energy particle reaction, metal reaction, organic reactant, electrochemical reduction and so on [68, 69]. Although these approaches are different from light-induced VO in the step of VO formation, the applications of VO are similar, which could be well studied to inspire researchers to expand the utilization of light-induced VO. Light-induced VO is a unique method for photochemical conversion and serves as an alternative way for material design, catalysis and energy conversion. With the mild reaction conditions, low cost energy source (sunlight) and the benefits of photo reactions, the light-induced VO is suitable for a wide range of applications and shows a great potential in the photochemical conversion. Figure 5 shows the schematic of potential future developments for light-induced VO. To tackle the current challenges of (a) suitable semiconductor support, (b) the measurement of VO, (b) the mechanism of the effects of light-induced VO, the novel research approaches and creative ways of light engineering, materials design, coupled with the advanced theoretical calculations and in-situ spectroscopy techniques, would bring breakthroughs in the field of light-induced VO for the photochemical conversion in the foreseeable future.
Figure 5. Schematic of potential future developments for light-induced VO.
Download figure:
Standard image High-resolution imageAcknowledgments
This work is financially supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant [GRPIN-2016-05494]. As part of the University of Alberta's Future Energy Systems research initiative, this research was made possible in part thanks to funding from the Canada First Research Excellence Fund.
Data availability statement
No new data were created or analyzed in this study.